Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Olive Leaf Sampling
2.2. Chemicals
2.3. High-Performance Liquid Chromatography (HPLC)
2.4. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.5. Statistical Analysis
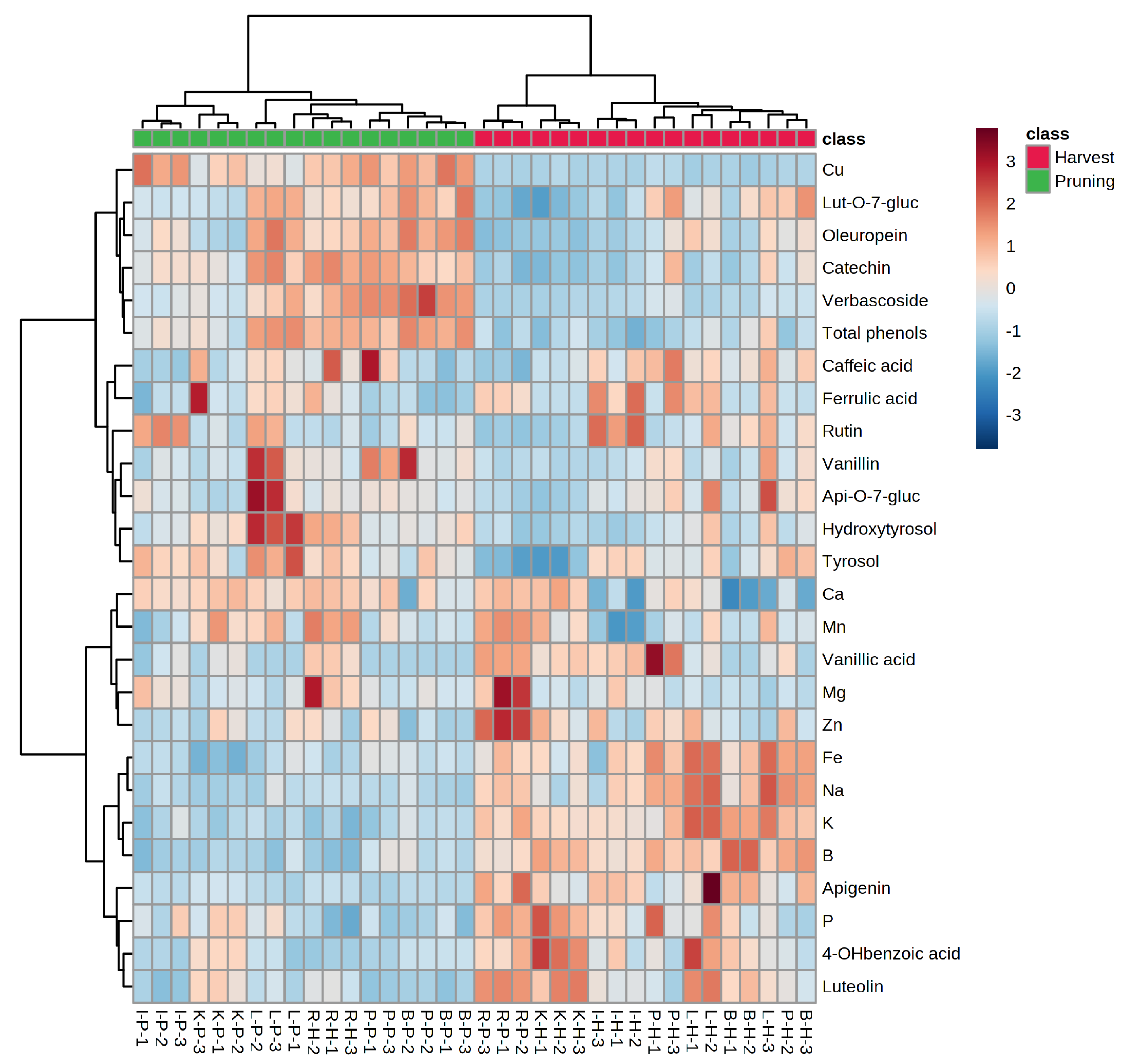
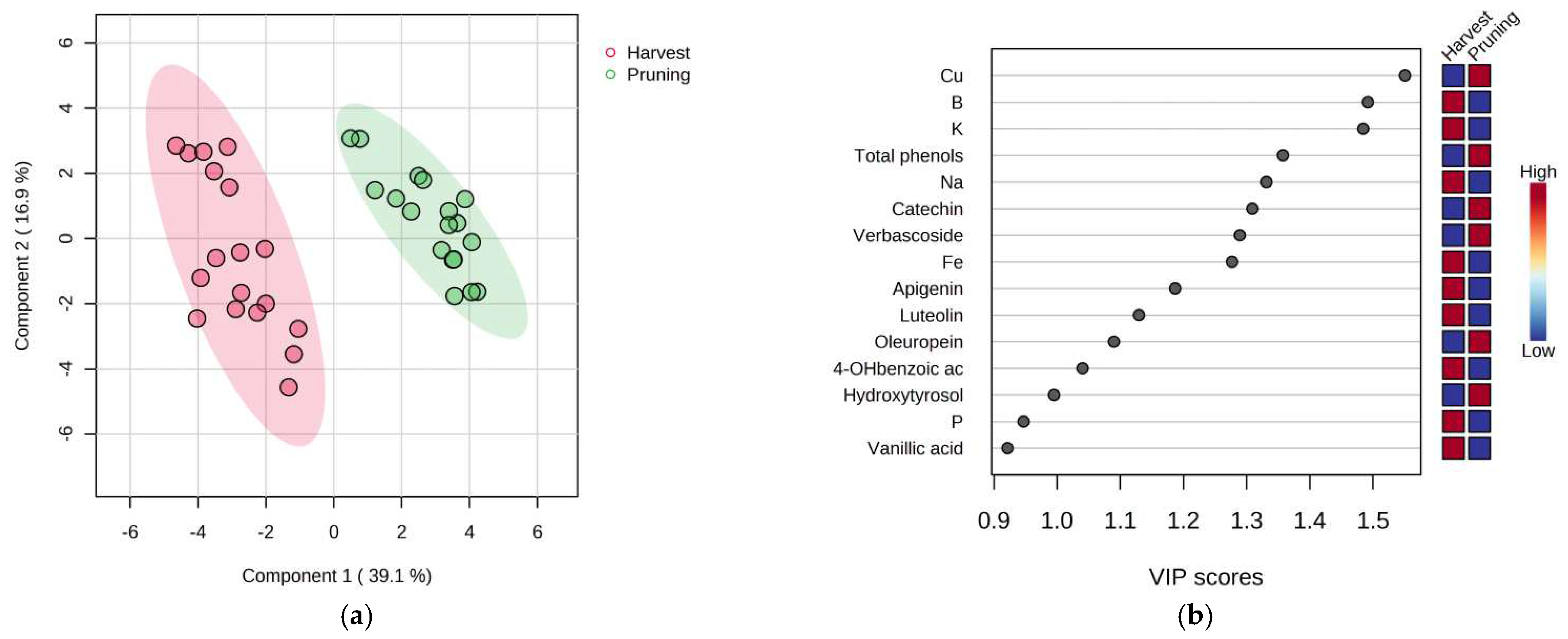
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New Possibilities for the Valorization of Olive Oil By-Products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef]
- Vidović, N.; Pasković, I.; Lukić, I.; Žurga, P.; Germek, V.M.; Grozić, K.; Cukrov, M.; Marcelić, Š.; Ban, D.; Talhaoui, N.; et al. Biophenolic Profile Modulations in Olive Tissues as Affected by Manganese Nutrition. Plants 2021, 10, 1724. [Google Scholar] [CrossRef]
- Cukrov, M.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Ban, D.; Lukić, I.; Goreta Ban, S.; Pasković, I. Effect of Olive (Olea Europaea L.) Variety on Leaf Biophenolic Profile. Agric. Conspec. Sci. 2021, 86, 277–282. [Google Scholar]
- Pasković, I.; Soldo, B.; Talhaoui, N.; Palčić, I.; Brkljača, M.; Koprivnjak, O.; Majetić Germek, V.; Ban, D.; Klanjac, J.; Franić, M.; et al. Boron Foliar Application Enhances Oleuropein Level and Modulates Volatile Compound Composition in Olive Leaves. Sci. Hortic. 2019, 257, 108688. [Google Scholar] [CrossRef]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial Activity of Commercial Olea Europaea (Olive) Leaf Extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and Antigrowth Action of Peracetylated Oleuropein in Thyroid Cancer Cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea Europaea) Leaf Extract Effective in Patients with Stage-1 Hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Han, J.; Talorete, T.P.N.; Yamada, P.; Isoda, H. Anti-Proliferative and Apoptotic Effects of Oleuropein and Hydroxytyrosol on Human Breast Cancer MCF-7 Cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Gondim, S.; Gómez-García, R.; Ribeiro, T.; Pintado, M. Olive Leaf Phenolic Extract from Two Portuguese Cultivars –Bioactivities for Potential Food and Cosmetic Application. J. Environ. Chem. Eng. 2021, 9, 106175. [Google Scholar] [CrossRef]
- Di Meo, M.C.; De Cristofaro, G.A.; Imperatore, R.; Rocco, M.; Giaquinto, D.; Palladino, A.; Zotti, T.; Vito, P.; Paolucci, M.; Varricchio, E. Microwave-Assisted Extraction of Olive Leaf from Five Italian Cultivars: Effects of Harvest-Time and Extraction Conditions on Phenolic Compounds and In Vitro Antioxidant Properties. ACS Food Sci. Technol. 2022, 2, 31–40. [Google Scholar] [CrossRef]
- Özcan, M.M.; Fındık, S.; AlJuhaimi, F.; Ghafoor, K.; Babiker, E.F.E.; Adiamo, O.Q. The Effect of Harvest Time and Varieties on Total Phenolics, Antioxidant Activity and Phenolic Compounds of Olive Fruit and Leaves. J. Food Sci. Technol. 2019, 56, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Monteleone, J.I.; Spagna, G.; Restuccia, C.; Raffaele, M.; Vanella, L.; Li Volti, G.; Barbagallo, I. Olive Leaf Extract from Sicilian Cultivar Reduced Lipid Accumulation by Inducing Thermogenic Pathway during Adipogenesis. Front. Pharmacol. 2016, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Lukić, I.; Pasković, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z.; et al. Determination of the Variability of Biophenols and Mineral Nutrients in Olive Leaves with Respect to Cultivar, Collection Period and Geographical Location for Their Targeted and Well-Timed Exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef]
- Majetić Germek, V.; Žurga, P.; Koprivnjak, O.; Grozić, K.; Previšić, I.; Marcelić, Š.; Goreta Ban, S.; Pasković, I. Phenolic Composition of Croatian Olive Leaves and Their Infusions Obtained by Hot and Cold Preparation. Czech J. Food Sci. 2021, 39, 393–401. [Google Scholar] [CrossRef]
- FLOS OLEI 2023. A Guide to the World of Extra Virgin Olive Oil. Available online: https://www.flosolei.com/ (accessed on 23 April 2023).
- Poljuha, D.; Sladonja, B.; Šetić, E.; Milotić, A.; Bandelj, D.; Jakše, J.; Javornik, B. DNA Fingerprinting of Olive Varieties in Istria (Croatia) by Microsatellite Markers. Sci. Hortic. 2008, 115, 223–230. [Google Scholar] [CrossRef]
- HAPIH. Official Croatian Olive Cultivar List. Available online: https://www.hapih.hr/wp-content/uploads/2021/07/2.1.-Popis-sorti-vocnih-vrsta.pdf (accessed on 23 April 2023).
- Šegota, T.; Filipčić, A. Köppen’s Classification of Climates and the Problem of Corresponding Croatian Terminology. Geoadria 2003, 8, 17–37. [Google Scholar] [CrossRef]
- Pasković, I.; Soldo, B.; Goreta Ban, S.; Radić, T.; Lukić, M.; Urlić, B.; Mimica, M.; Brkić Bubola, K.; Colla, G.; Rouphael, Y.; et al. Fruit Quality and Volatile Compound Composition of Processing Tomato as Affected by Fertilisation Practices and Arbuscular Mycorrhizal Fungi Application. Food Chem. 2021, 359, 129961. [Google Scholar] [CrossRef]
- MetaboAnalyst. Available online: http://www.metaboanalyst.ca (accessed on 24 April 2023).
- Seabra, R.M.; Andrade, P.B.; Valentão, P.; Faria, M.; Paice, A.G.; Oliveira, M.B.P.P. Phenolic Profiles of Portuguese Olives: Cultivar and Geographics; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Filgueira-Garro, I.; González-Ferrero, C.; Mendiola, D.; Marín-Arroyo, M.R. Effect of Cultivar and Drying Methods on Phenolic Compounds and Antioxidant Capacity in Olive (Olea Europaea L.) Leaves. AIMS Agric. Food 2022, 18, 250–264. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors Influencing Phenolic Compounds in Table Olives (Olea Europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Urbani, E.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Seasonal Variations in Antioxidant Compounds of Olea Europaea Leaves Collected from Different Italian Cultivars. J. Appl. Bot. Food Qual. 2016, 89, 202–207. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Liao, J. Seasonal Variations in the Chemical Composition of Liangshan Olive Leaves and Their Antioxidant and Anticancer Activities. Foods 2019, 8, 657. [Google Scholar] [CrossRef]
- Orak, H.H.; Karamać, M.; Amarowicz, R.; Orak, A.; Penkacik, K. Genotype-Related Differences in the Phenolic Compound Profile and Antioxidant Activity of Extracts from Olive (Olea Europaea L.) Leaves. Molecules 2019, 24, 1130. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. Phenylalanine Ammonia-Lyase, Polyphenol Oxidase, and Phenol Concentration in Fruits of Olea Europaea L. Cv. Picual, Verdial, Arbequina, and Frantoio during Ripening. J. Agric. Food Chem. 2009, 57, 10331–10340. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. The Response of Phenylalanine Ammonia-Lyase, Polyphenol Oxidase and Phenols to Cold Stress in the Olive Tree (Olea Europaea L. Cv. Picual). J. Sci. Food Agric. 2009, 89, 1565–1573. [Google Scholar] [CrossRef]
- Pongrac, P.; Kelemen, M.; Vogel-Mikuš, K.; Vavpetič, P.; Pelicon, P.; Žurga, P.; Vidović, N.; Polić Pasković, M.; Smiljana, G.B.; Lukić, I.; et al. Tissue-Specific Calcium and Magnesium Allocation to Explain Differences in Bulk Concentration in Leaves of One-Year-Old Seedlings of Two Olive (Olea Europaea L.). Cultivars. Plant Physiol. Biochem. 2023, 194, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Alonso, G.L.; Salinas, M.R. Determination of the Variability of Bioactive Compounds and Minerals in Olive Leaf along an Agronomic Cycle. Agronomy 2021, 11, 2447. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; de Pava Folletto-Filipe, M.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- El-Gohary, F.A.; Badawy, M.I.; El-Khateeb, M.A.; El-Kalliny, A.S. Integrated treatment of olive mill wastewater (OMW) by the combination of Fenton’s reaction and anaerobic treatment. J. Hazard. Mater. 2009, 162, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Ji, X.; Zhao, M.; He, F.; Wang, X.; Wang, Y.; Liu, P.; Niu, L. The Influence of Light Quality on the Accumulation of Flavonoids in Tobacco (Nicotiana Tabacum L.) Leaves. J. Photochem. Photobiol. B 2016, 162, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escobar, R. Olive Nutritional Status and Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Stateras, D.C.; Moustakas, N.K. Seasonal Changes of Macro- and Micro-Nutrients Concentration in Olive Leaves. J. Plant Nutr. 2018, 41, 186–196. [Google Scholar] [CrossRef]
- Dabbaghi, O.; Tekaya, M.; Flamini, G.; Zouari, I.; El-Gharbi, S.; M’barki, N.; Laabidi, F.; Cheheb, H.; Attia, F.; Aïachi Mezghani, M.; et al. Modification of Phenolic Compounds and Volatile Profiles of Chemlali Variety Olive Oil in Response to Foliar Biofertilization. J. Am. Oil Chem. Soc. 2019, 96, 585–593. [Google Scholar] [CrossRef]
- Treutter, D. Managing Phenol Contents in Crop Plants by Phytochemical Farming and Breeding-Visions and Constraints. Int. J. Mol. Sci. 2010, 11, 807–857. [Google Scholar] [CrossRef]
- Ortuño, A.; Gómez, P.; Báidez, A.; Frías, V.; Del Río, J.A. Citrus Sp.: A Source of Flavonoids of Pharmaceutical Interest. ACS Symp. Ser. 2006, 936, 175–185. [Google Scholar]
- Del Río, J.A.; Báidez, A.G.; Botía, J.M.; Ortuño, A. Enhancement of Phenolic Compounds in Olive Plants (Olea Europaea L.) and Their Influence on Resistance against Phytophthora Sp. Food Chem. 2003, 83, 75–78. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Soares, M.E.; Bastos, M.L.; Pereira, J.A. Antioxidant Activity and Phenolic Contents of Olea Europaea L. Leaves Sprayed with Different Copper Formulations. Food Chem. 2007, 103, 188–195. [Google Scholar] [CrossRef]
- Saha, D.; Mandal, S.; Saha, A. Copper Induced Oxidative Stress in Tea (Camellia Sinensis) Leaves. J. Environ. Biol. 2012, 33, 861. [Google Scholar]
- Beutel, J.; Uriu, K.; Lilleland, O. Leaf Analysis for California Deciduous Fruits. Bulletin; Division of Agricultural Sciences, University of California: Oakland, CA, USA, 1976; pp. 11–14. [Google Scholar]
- Fernández-Escobar, R.; Moreno, R.; García-Creus, M. Seasonal Changes of Mineral Nutrients in Olive Leaves during the Alternate-Bearing Cycle. Sci. Hortic. 1999, 82, 25–45. [Google Scholar] [CrossRef]
- Karioti, A.; Chatzopoulou, A.; Bilia, A.R.; Liakopoulos, G.; Stavrianakou, S.; Skaltsa, H. Novel Secoiridoid Glucosides in Olea Europaea Leaves Suffering from Boron Deficiency. Biosci. Biotechnol. Biochem. 2006, 70, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, G.; Karabourniotis, G. Boron Deficiency and Concentrations and Composition of Phenolic Compounds in Olea Europaea Leaves: A Combined Growth Chamber and Field Study. Tree Physiol. 2005, 25, 307–315. [Google Scholar]
| Source of Variability | Simple phenols | Secoiridoids | |||
|---|---|---|---|---|---|
| Hydroxytyrosol | Tyrosol | Vanillin | Oleuropein | Total Phenols | |
| Cultivar (cv.) | |||||
| Puntoža | 28.78 ± 1.58 cd | 7.91 ± 0.8 abc | 1.56 ± 0.29 ab | 4919.26 ± 722.13 a | 3747.69 ± 590.23 bcd |
| Buža | 32.89 ± 4.36 bc | 6.59 ± 0.89 bc | 1.34 ± 0.46 ab | 5258.78 ± 1332.27 a | 4373.40 ± 548.45 ab |
| I. bjelica | 22.51 ± 3.23 d | 9.24 ± 0.30 ab | 0.63 ± 0.10 b | 2733.04 ± 653.41 b | 3045.22 ± 364.60 cd |
| Karbonaca | 29.61 ± 6.20 bcd | 4.76 ± 1.50 c | 0.56 ± 0.08 b | 1149.39 ± 249.34 c | 3178.26 ± 273.08 cd |
| Leccino | 68.90 ± 10.26 a | 10.36 ± 1.20 a | 1.87 ± 0.48 a | 6194.95 ± 627.58 a | 4687.47 ± 461.50 a |
| Rošinjola | 39.37 ± 9.19 b | 5.73 ± 1.54 c | 0.75 ± 0.13 ab | 2782.62 ± 1028.16 b | 3956.97 ± 524.53 abc |
| Collecting period (CP) | |||||
| Harvest (CP1) | 24.91 ± 2.88 b | 6.00 ± 0.78 b | 0.78 ± 0.12 b | 2228.51 ± 416.30 b | 2883.34 ± 158.04 b |
| Pruning (CP2) | 49.11 ± 5.18 a | 8.86 ± 0.58 a | 1.45 ± 0.24 a | 5450.84 ± 543.68 a | 4779.67 ± 205.33 a |
| Cv. × CP | |||||
| Puntoža × Harvest | 25.93 ± 2.09 b | 8.09 ± 1.40 a | 1.18 ± 0.22 | 3389.23 ± 452.35 ab | 2453.63 ± 140.18 ab |
| Puntoža × Pruning | 31.62 ± 0.19 CD | 7.73 ± 1.08 B | 1.93 ± 0.49 | 6449.28 ± 248.56 AB | 5041.76 ± 218.15 A |
| Buža × Harvest | 24.95 ± 4.04 b | 6.69 ± 1.88 ab | 0.77 ± 0.31 | 2453.51 ± 964.06 bc | 3192.12 ± 259.01 ab |
| Buža × Pruning | 40.84 ± 3.94 CD | 6.49 ± 0.65 B | 1.90 ± 0.79 | 8064.04 ± 275.26 A | 5554.67 ± 203.69 A |
| I. bjelica × Harvest | 15.99 ± 1.48 b | 8.98 ± 0.17 a | 0.58 ± 0.10 | 1394.17 ± 219.83 bc | 2270.87 ± 206.15 b |
| I. bjelica × Pruning | 29.03 ± 2.73 D | 9.50 ± 0.60 AB | 0.69 ± 0.19 | 4071.90 ± 542.06 C | 3819.57 ± 150.21 B |
| Karbonaca × Harvest | 16.13 ± 2.38 b | 1.85 ± 0.74 b | 0.45 ± 0.07 | 671.04 ± 139.62 c | 2790.83 ± 351.68 ab |
| Karbonaca × Pruning | 43.09 ± 2.25 C | 7.68 ± 1.47 B | 0.66 ± 0.11 | 1627.73 ± 250.10 D | 3565.69 ± 314.75 B |
| Leccino × Harvest | 47.07 ± 6.35 a | 8.03 ± 0.75 a | 1.21 ± 0.54 | 4962.29 ± 346.07 a | 3765.28 ± 456.85 a |
| Leccino × Pruning | 90.73 ± 3.10 A | 12.69 ± 1.11 A | 2.53 ± 0.67 | 7427.62 ± 574.51 A | 5609.66 ± 76.06 A |
| Rošinjola × Harvest | 19.38 ± 4.00 b | 2.36 ± 0.51 b | 0.51 ± 0.09 | 500.80 ± 167.15 c | 2827.28 ± 299.65 ab |
| Rošinjola × Pruning | 59.37 ± 2.58 B | 9.09 ± 0.49 AB | 0.99 ± 0.12 | 5064.45 ± 225.64 BC | 5086.67 ± 98.31 A |
| (Cv.) p-value | <0.001 | <0.001 | 0.010 | <0.001 | <0.001 |
| (CP) p-value | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 |
| (Cv. × CP) p-value | <0.001 | 0.003 | 0.567 | <0.001 | 0.018 |
| Source of Variability | Phenolic Acids | ||||
|---|---|---|---|---|---|
| 4-Hydroxybenzoic Acid | Caffeic Acid | Ferulic Acid | Vanillic Acid | Verbascoside | |
| Cultivar (cv.) | |||||
| Puntoža | 0.74 ± 0.17 b | 1.94 ± 0.39 | 0.97 ± 0.35 ab | 3.10 ± 1.20 a | 572.09 ± 170.49 a |
| Buža | 1.15 ± 0.25 b | 1.04 ± 0.21 | 0.64 ± 0.11 b | 0.80 ± 0.00 c | 470.53 ± 170.77 ab |
| I. bjelica | 0.84 ± 0.28 b | 1.05 ± 0.24 | 1.49 ± 0.47 ab | 2.4 ± 0.53 ab | 136.59 ± 34.06 c |
| Karbonaca | 2.66 ± 0.40 a | 1.14 ± 0.20 | 1.32 ± 0.49 ab | 2.43 ± 0.39 ab | 121.98 ± 49.95 c |
| Leccino | 1.61 ± 0.60 b | 1.61 ± 0.12 | 1.85 ± 0.11 a | 1.44 ± 0.30 bc | 316.77 ± 115.50 b |
| Rošinjola | 1.12 ± 0.42 b | 1.11 ± 0.39 | 1.62 ± 0.17 ab | 3.84 ± 0.29 a | 326.57 ± 144.41 b |
| Collecting period (CP) | |||||
| Harvest (CP1) | 2.00 ± 0.26 a | 1.33 ± 0.14 | 1.55 ± 0.18 a | 3.21 ± 0.42 a | 81.59 ± 18.34 b |
| Pruning (CP2) | 0.71 ± 0.13 b | 1.30 ± 0.19 | 1.08 ± 0.21 b | 1.46 ± 0.24 b | 556.58 ± 72.69 a |
| Cv. × CP | |||||
| Puntoža × Harvest | 0.97 ± 0.25 | 1.89 ± 0.42 | 1.48 ± 0.59 a | 5.40 ± 1.40 a | 205.47 ± 28.48 a |
| Puntoža × Pruning | 0.51 ± 0.14 | 1.99 ± 0.77 | 0.46 ± 0.14 | 0.80 ± 0.00 B | 938.70 ± 100.61 A |
| Buža × Harvest | 1.49 ± 0.44 | 1.44 ± 0.19 | 0.80 ± 0.00 ab | 0.80 ± 0.00 c | 94.47 ± 34.65 ab |
| Buža × Pruning | 0.80 ± 0.00 | 0.64 ± 0.16 | 0.48 ± 0.18 | 0.80 ± 0.00 B | 846.59 ± 56.48 AB |
| I. bjelica × Harvest | 1.30 ± 0.43 | 1.53 ± 0.24 | 2.44 ± 0.38 ab | 3.43 ± 0.20 a−c | 66.69 ± 12.85 ab |
| I. bjelica × Pruning | 0.39 ± 0.08 | 0.58 ± 0.05 | 0.55 ± 0.25 | 1.36 ± 0.54 B | 206.49 ± 27.39 C |
| Karbonaca × Harvest | 3.51 ± 0.30 | 0.99 ± 0.08 | 0.80 ± 0.00 ab | 3.10 ± 0.25 a–c | 22.30 ± 14.83 b |
| Karbonaca × Pruning | 1.81 ± 0.06 | 1.30 ± 0.40 | 1.84 ± 0.96 | 1.75 ± 0.48 B | 221.66± 48.14 C |
| Leccino × Harvest | 2.67 ± 0.80 | 1.72 ± 0.20 | 2.09 ± 0.01 a | 2.07 ± 0.19 bc | 80.21 ± 56.92 ab |
| Leccino × Pruning | 0.55 ± 0.25 | 1.49 ± 0.12 | 1.62 ± 0.10 | 0.80 ± 0.00 B | 553.32 ± 86.63 BC |
| Rošinjola × Harvest | 2.03 ± 0.25 | 0.44 ± 0.09 | 1.72 ± 0.09 b | 4.45 ± 0.05 ab | 20.43 ± 3.47 b |
| Rošinjola × Pruning | 0.21 ± 0.04 | 1.79 ± 0.53 | 1.52 ± 0.35 | 3.24 ± 0.23 A | 632.72 ± 102.67 AB |
| (Cv.) p-value | <0.001 | 0.072 | 0.036 | <0.001 | <0.001 |
| (CP) p-value | <0.001 | 0.853 | 0.037 | <0.001 | <0.001 |
| (Cv. × CP) p-value | 0.101 | 0.031 | 0.019 | 0.002 | <0.001 |
| Source of Variability | Flavonoids | |||||
|---|---|---|---|---|---|---|
| Apigenin | Apigenin-7-O-Glucoside | Luteolin | Luteolin-7-O-Glucoside | Rutin | Catechin | |
| Cultivar (cv.) | ||||||
| Puntoža | 2.00 ± 0.56 | 53.42 ± 3.43 b | 19.19 ± 3.13 c | 537.28 ± 24.17 a | 31.36 ± 3.55 cd | 30.03 ± 4.34 a |
| Buža | 6.00 ± 2.02 | 40.60 ± 5.03 b | 25.80 ± 5.73 bc | 538.28 ± 69.17 a | 58.78 ± 5.17 bc | 23.99 ± 4.64 ab |
| I. bjelica | 5.46 ± 1.61 | 41.14 ± 2.99 b | 21.71 ± 3.94 c | 299.82 ± 22.15 b | 114.83 ± 5.03 a | 17.24 ± 3.51 b |
| Karbonaca | 4.32 ± 0.82 | 14.26 ± 2.89 b | 46.00 ± 4.50 a | 231.22 ± 37.75 b | 27.93 ± 4.59 d | 13.99 ± 3.89 b |
| Leccino | 6.49 ± 3.67 | 105.16 ± 20.17 a | 36.48 ± 7.57 ab | 516.62 ± 39.54 a | 77.15 ± 12.86 b | 28.70 ± 5.67 a |
| Rošinjola | 6.86 ± 2.25 | 30.78 ± 6.03 b | 41.85 ± 6.40 a | 313.83 ± 62.60 b | 23.48 ± 5.34 d | 24.62 ± 7.52 ab |
| Collecting period (CP) | ||||||
| Harvest (CP1) | 8.58 ± 1.22 a | 42.68 ± 7.78 | 42.14 ± 3.43 a | 341.16 ± 41.20 b | 55.03 ± 9.55 | 13.44 ± 2.10 b |
| Pruning (CP2) | 1.80 ± 0.20 b | 52.44 ± 8.96 | 21.53 ± 2.29 b | 471.19 ± 31.06 a | 56.15 ± 7.80 | 32.75 ± 1.97 a |
| Cv. × CP | ||||||
| Puntoža × Harvest | 3.06 ± 0.52 | 57.34 ± 5.78 | 24.35 ± 4.50 | 550.16 ± 39.08 | 33.51 ± 4.02 | 23.30 ± 6.20 |
| Puntoža × Pruning | 0.94 ± 0.40 | 49.50 ± 3.17 | 14.03 ± 1.49 | 524.40 ± 35.05 | 29.21 ± 6.51 | 36.77 ± 3.19 |
| Buža × Harvest | 10.51 ± 0.17 | 40.87 ± 10.48 | 37.01 ± 5.94 | 456.18 ± 113.78 | 63.96 ± 5.82 | 15.20 ± 5.09 |
| Buža × Pruning | 1.48 ± 0.11 | 40.33 ± 4.09 | 14.59 ± 1.73 | 620.39 ± 65.05 | 53.60 ± 8.53 | 32.78 ± 2.15 |
| I. bjelica × Harvest | 9.03 ± 0.44 | 39.41 ± 4.13 | 30.15 ± 1.45 | 265.90 ± 35.65 | 120.89 ± 8.15 | 9.81 ± 1.65 |
| I. bjelica × Pruning | 1.88 ± 0.25 | 42.86 ± 4.90 | 13.26 ± 2.08 | 333.74 ± 5.70 | 108.77 ± 4.84 | 24.66 ± 1.98 |
| Karbonaca × Harvest | 5.58 ± 1.34 | 9.37 ± 3.98 | 54.00 ± 5.57 | 155.91 ± 36.25 | 20.72 ± 4.10 | 5.81 ± 0.98 |
| Karbonaca × Pruning | 3.06 ± 0.07 | 19.14 ± 1.47 | 37.99 ± 2.46 | 306.53 ± 11.85 | 35.15 ± 6.03 | 22.18 ± 2.79 |
| Leccino × Harvest | 11.87 ± 6.19 | 90.46 ± 28.12 | 51.36 ± 7.62 | 440.29 ± 43.97 | 94.54 ± 29.56 | 18.44 ± 6.17 |
| Leccino × Pruning | 1.12 ± 0.45 | 119.86 ± 32.07 | 21.61 ± 2.70 | 592.96 ± 7.53 | 93.48 ± 33.02 | 38.96 ± 4.20 |
| Rošinjola × Harvest | 11.40 ± 2.16 | 18.62 ± 4.25 | 55.99 ± 0.86 | 178.54 ± 31.20 | 12.95 ± 2.17 | 8.09 ± 2.51 |
| Rošinjola × Pruning | 2.32 ± 0.15 | 42.94 ± 4.02 | 27.71 ± 1.99 | 449.13 ± 17.67 | 34.01 ± 5.18 | 41.14 ± 1.81 |
| (Cv.) p-value | 0.173 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| (CP) p-value | <0.001 | 0.212 | <0.001 | <0.001 | 0.847 | <0.001 |
| (Cv. × CP) p-value | 0.164 | 0.695 | 0.119 | 0.076 | 0.468 | 0.128 |
| Source of Variability | Macronutrients (g/kg DW) | Micronutrients (mg/kg DW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phosphorus | Pottasium | Calcium | Magnesium | Sodium | Iron | Zinc | Manganese | Copper | Boron | |
| Cultivar (cv.) | ||||||||||
| Puntoža | 1.53 ± 0.08 ab | 4.99 ± 0.42 b | 19.36 ± 0.45 ab | 0.93 ± 0.03 b | 0.5 ± 0.1 ab | 76.16 ± 5.11 a | 24.96 ± 0.77 ab | 38.78 ± 1.4 cd | 56.27 ± 13.39 ab | 19.66 ± 1.3 ab |
| Buža | 1.47 ± 0.04 b | 5.49 ± 0.42 ab | 14.81 ± 0.96 c | 0.88 ± 0.02 b | 0.44 ± 0.08 bc | 72.20 ± 4.44 ab | 20.55 ± 0.56 c | 38.83 ± 0.44 cd | 61.40 ± 18.29 a | 21.22 ± 2.08 a |
| I. bjelica | 1.57 ± 0.04 ab | 4.86 ± 0.31 b | 17.20 ± 1.20 bc | 1.07 ± 0.05 b | 0.37 ± 0.06 bc | 64.72 ± 4.33 bc | 21.86 ± 1.16 bc | 32.08 ± 1.87 d | 62.01 ± 17.79 a | 16.85 ± 1.27 c |
| Karbonaca | 1.72 ± 0.06 a | 4.87 ± 0.33 b | 20.81 ± 0.31 a | 0.89 ± 0.02 b | 0.31 ± 0.05 c | 60.32 ± 5.22 c | 24.24 ± 1.14 abc | 46.73 ± 1.88 ab | 43.82 ± 10.32 bc | 18.9 ± 1.74 b |
| Leccino | 1.6 ± 0.05 ab | 5.88 ± 0.68 a | 18.47 ± 0.97 ab | 0.86 ± 0.03 b | 0.60 ± 0.13 a | 79.41 ± 8.23 a | 22.96 ± 1.21 bc | 44.58 ± 2.36 bc | 36.37 ± 7.39 c | 18.12 ± 1.44 bc |
| Rošinjola | 1.55 ± 0.09 ab | 4.94 ± 0.52 b | 20.77 ± 0.15 a | 1.48 ± 0.14 a | 0.46 ± 0.06 abc | 68.09 ± 4.20 abc | 27.95 ± 2.52 a | 53.27 ± 0.61 a | 51.42 ± 13.07 ab | 16.48 ± 1.35 c |
| Collecting period (CP) | ||||||||||
| Harvest (CP1) | 1.66 ± 0.04 a | 6.12 ± 0.17 a | 17.6 ± 0.78 b | 1.02 ± 0.07 | 0.61 ± 0.04 a | 80.30 ± 2.86 a | 25.44 ± 1.07 a | 41.50 ± 1.96 b | 22.87 ± 0.78 b | 21.84 ± 0.55 a |
| Pruning (CP2) | 1.49 ± 0.03 b | 4.23 ± 0.09 b | 19.54 ± 0.40 a | 1.02 ± 0.05 | 0.29 ± 0.01 b | 60.00 ± 1.46 b | 22.07 ± 0.55 b | 43.26 ± 1.72 a | 80.90 ± 4.94 a | 15.24 ± 0.39 b |
| Cv. × CP | ||||||||||
| Puntoža × Harvest | 1.63 ± 0.14 | 5.83 ± 0.37 b | 18.75 ± 0.71 ab | 0.91 ± 0.04 | 0.72 ± 0.03 ab | 86.75 ± 3.56 ab | 26.13 ± 0.72 ab | 38.16 ± 1.63 | 27.31 ± 2.13 | 22.38 ± 0.71 |
| Puntoža × Pruning | 1.44 ± 0.03 | 4.15 ± 0.18 | 19.97 ± 0.39 A | 0.95 ± 0.05 | 0.28 ± 0.01 | 65.57 ± 2.36 A | 23.80 ± 1.06 | 39.41 ± 2.59 | 85.23 ± 7.28 AB | 16.95 ± 0.78 |
| Buža × Harvest | 1.52 ± 0.07 | 6.39 ± 0.20 ab | 13.11 ± 0.44 c | 0.85 ± 0.02 | 0.60 ± 0.08 bc | 80.97 ± 4.35 ab | 21.59 ± 0.45 b | 38.50 ± 0.75 | 20.85 ± 2.34 | 25.71 ± 0.78 |
| Buža × Pruning | 1.42 ± 0.05 | 4.58 ± 0.17 | 16.52 ± 1.24 B | 0.91 ± 0.01 | 0.29 ± 0.05 | 63.43 ± 1.62 A | 19.50 ± 0.50 | 39.17 ± 0.53 | 101.96 ± 4.79 A | 16.73 ± 0.87 |
| I. bjelica × Harvest | 1.59 ± 0.04 | 5.44 ± 0.08 b | 14.76 ± 1.10 bc | 1.04 ± 0.08 | 0.47 ± 0.10 bc | 68.88 ± 8.72 b | 22.86 ± 2.37 b | 29.21 ± 2.13 | 22.84 ± 0.83 | 19.61 ± 0.28 |
| I. bjelica × Pruning | 1.55 ± 0.07 | 4.28 ± 0.37 | 19.64 ± 0.29 A | 1.11 ± 0.07 | 0.27 ± 0.03 | 60.57 ± 0.55 A | 20.87 ± 0.29 | 34.94 ± 2.19 | 101.18 ± 6.91 A | 14.08 ± 0.56 |
| Karbonaca × Harvest | 1.83 ± 0.06 | 5.60 ± 0.08 b | 21.02 ± 0.54 a | 0.88 ± 0.03 | 0.39 ± 0.07 c | 71.37 ± 3.56 b | 25.27 ± 1.53 b | 45.59 ± 2.85 | 23.27 ± 1.70 | 22.76 ± 0.41 |
| Karbonaca × Pruning | 1.62 ± 0.06 | 4.14 ± 0.14 | 20.60 ± 0.37 A | 0.89 ± 0.05 | 0.24 ± 0.01 | 49.27 ± 1.08 B | 23.20 ± 1.76 | 47.87 ± 2.87 | 64.38 ± 10.33 BC | 15.05 ± 0.30 |
| Leccino × Harvest | 1.66 ± 0.09 | 7.39 ± 0.11 a | 17.16 ± 1.67 a-c | 0.84 ± 0.05 | 0.89 ± 0.02 a | 97.45 ± 0.43 a | 23.52 ± 2.28 b | 44.47 ± 3.62 | 20.35 ± 1.31 | 21.13 ± 0.35 |
| Leccino × Pruning | 1.54 ± 0.04 | 4.36 ± 0.11 | 19.78 ± 0.42 A | 0.89 ± 0.05 | 0.31 ± 0.05 | 61.37 ± 3.64 A | 22.40 ± 1.35 | 44.69 ± 3.85 | 52.40 ± 3.83 C | 15.10 ± 1.08 |
| Rošinjola × Harvest | 1.74 ± 0.03 | 6.04 ± 0.28 b | 20.81 ± 0.24 a | 1.58 ± 0.20 | 0.59 ± 0.02 bc | 76.37 ± 3.78 ab | 33.27 ± 0.86 a | 53.08 ± 0.72 | 22.58 ± 0.84 | 19.44 ± 0.33 |
| Rošinjola × Pruning | 1.36 ± 0.05 | 3.84 ± 0.21 | 20.73 ± 0.22 A | 1.38 ± 0.20 | 0.33 ± 0.00 | 59.80 ± 2.29 A | 22.63 ± 1.63 | 53.46 ± 1.15 | 80.27 ± 4.56 A-C | 13.51 ± 0.42 |
| (Cv.) p-value | 0.025 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| (CP) p-value | <0.001 | <0.001 | <0.001 | 0.925 | <0.001 | <0.001 | <0.001 | 0.205 | <0.001 | <0.001 |
| (Cv. × CP) p-value | 0.208 | 0.004 | 0.015 | 0.713 | 0.002 | 0.027 | 0.022 | 0.844 | <0.001 | 0.051 |
| Verbascoside | Luteolin-7-O-Glucoside | Oleuropein | Total Phenols | |
|---|---|---|---|---|
| Phosphorus | r = −0.654 * | r = −0.636 | r = −0.621 | r = −0.617 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Potassium | r = −0.617 | r = 0.202 | r = 0.352 | r = 0.533 |
| p < 0.001 | p = 0.236 | p = 0.035 | p < 0.001 | |
| Calcium | r = 0.110 | r = 0.233 | r = 0.034 | r = 0.125 |
| p = 0.524 | p = 0.171 | p = 0.842 | p = 0.467 | |
| Magnesium | r = −0.098 | r = 0.371 | r = 0.250 | r = 0.113 |
| p = 0.568 | p = 0.026 | p = 0.142 | p = 0.510 | |
| Sodium | r = −0.492 | r = 0.016 | r = 0.201 | r = 0.462 |
| p = 0.002 | p = 0.927 | p = 0.239 | p = 0.005 | |
| Iron | r = −0.392 | r = 0.044 | r = 0.121 | r = 0.413 |
| p = 0.018 | p = 0.797 | p = 0.482 | p = 0.012 | |
| Zinc | r = −0.402 | r = 0.470 | r = 0.476 | r = 0.474 |
| p = 0.015 | p = 0.004 | p = 0.003 | p = 0.004 | |
| Manganese | r = 0.009 | r = 0.121 | r = 0.055 | r = 0.208 |
| p = 0.960 | p = 0.483 | p = 0.751 | p = 0.223 | |
| Copper | r = 0.710 | r = 0.342 | r = 0.592 | r = 0.657 |
| p < 0.001 | p = 0.041 | p = <0.001 | p = <0.001 | |
| Boron | r = −0.536 | r = 0.194 | r = 0.456 | r = 0.614 |
| p < 0.001 | p = 0.257 | p = 0.005 | p = <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polić Pasković, M.; Vidović, N.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Goreta Ban, S.; Kos, T.; Čoga, L.; Tomljanović, T.; Simonić-Kocijan, S.; et al. Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia. Horticulturae 2023, 9, 594. https://doi.org/10.3390/horticulturae9050594
Polić Pasković M, Vidović N, Lukić I, Žurga P, Majetić Germek V, Goreta Ban S, Kos T, Čoga L, Tomljanović T, Simonić-Kocijan S, et al. Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia. Horticulturae. 2023; 9(5):594. https://doi.org/10.3390/horticulturae9050594
Chicago/Turabian StylePolić Pasković, Marija, Nikolina Vidović, Igor Lukić, Paula Žurga, Valerija Majetić Germek, Smiljana Goreta Ban, Tomislav Kos, Lepomir Čoga, Tea Tomljanović, Sunčana Simonić-Kocijan, and et al. 2023. "Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia" Horticulturae 9, no. 5: 594. https://doi.org/10.3390/horticulturae9050594
APA StylePolić Pasković, M., Vidović, N., Lukić, I., Žurga, P., Majetić Germek, V., Goreta Ban, S., Kos, T., Čoga, L., Tomljanović, T., Simonić-Kocijan, S., Ban, D., Godena, S., & Pasković, I. (2023). Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia. Horticulturae, 9(5), 594. https://doi.org/10.3390/horticulturae9050594











