Greenhouse Photoluminescent PMMA Panels Improve the Agronomical and Physiological Performances of Lettuce (Lactuca sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Plant Material and Crop Management
2.2. Photoluminescent PMMA Panels for Greenhouse Covering
2.3. Light Spectra, Total Irradiance, PAR, and Temperature Measurements
2.4. Plant Growth and Yield Measurements
2.5. Chroma Meter Measurements and Pigments Determinations
2.6. Hydrophilic and ABTS Antioxidant Activities, Total Ascorbic Acid, Total Phenols and Nitrate Content
2.7. Chlorophyll Fluorescence Measurements
2.8. Statistical Analysis
3. Results
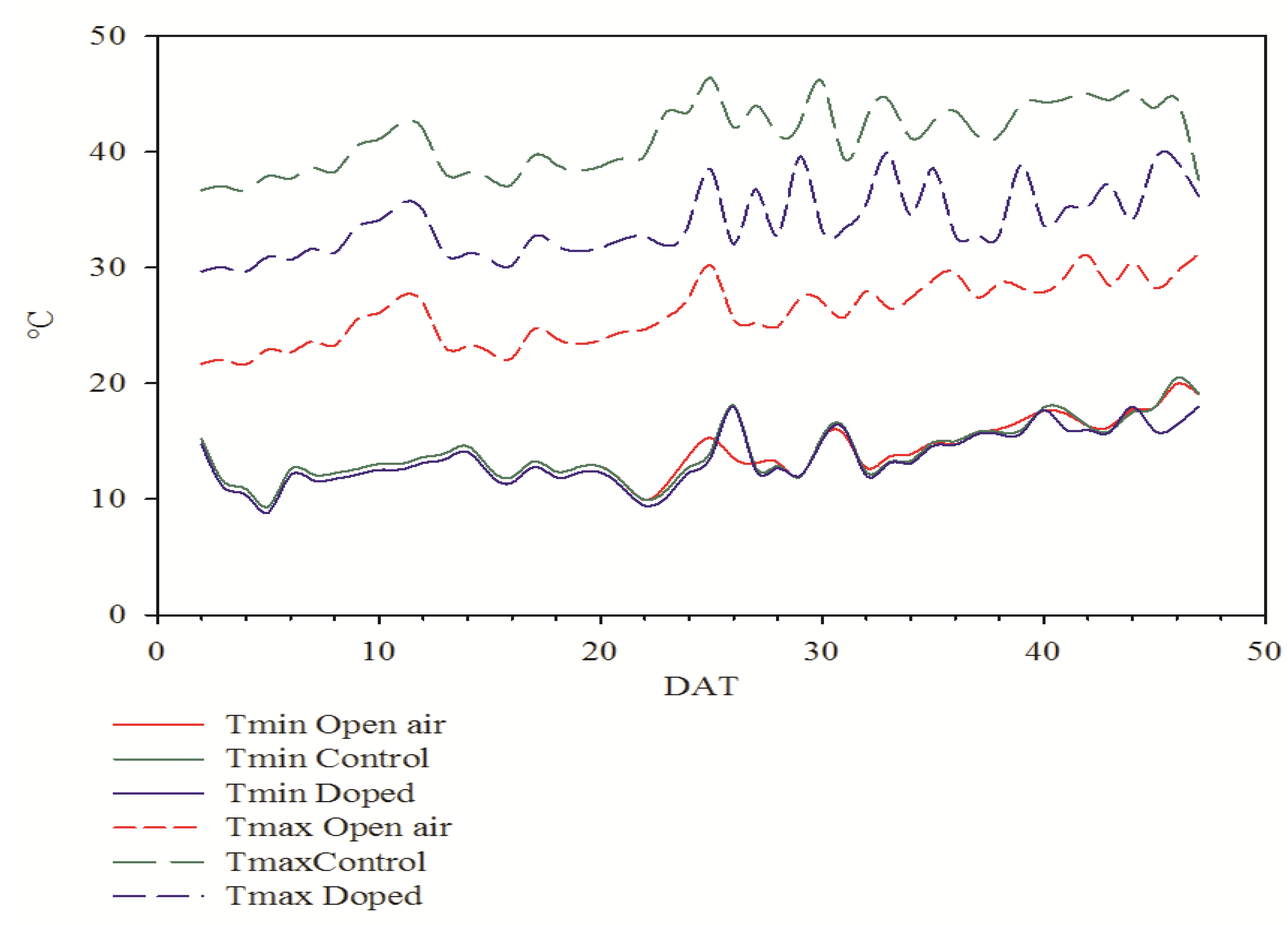
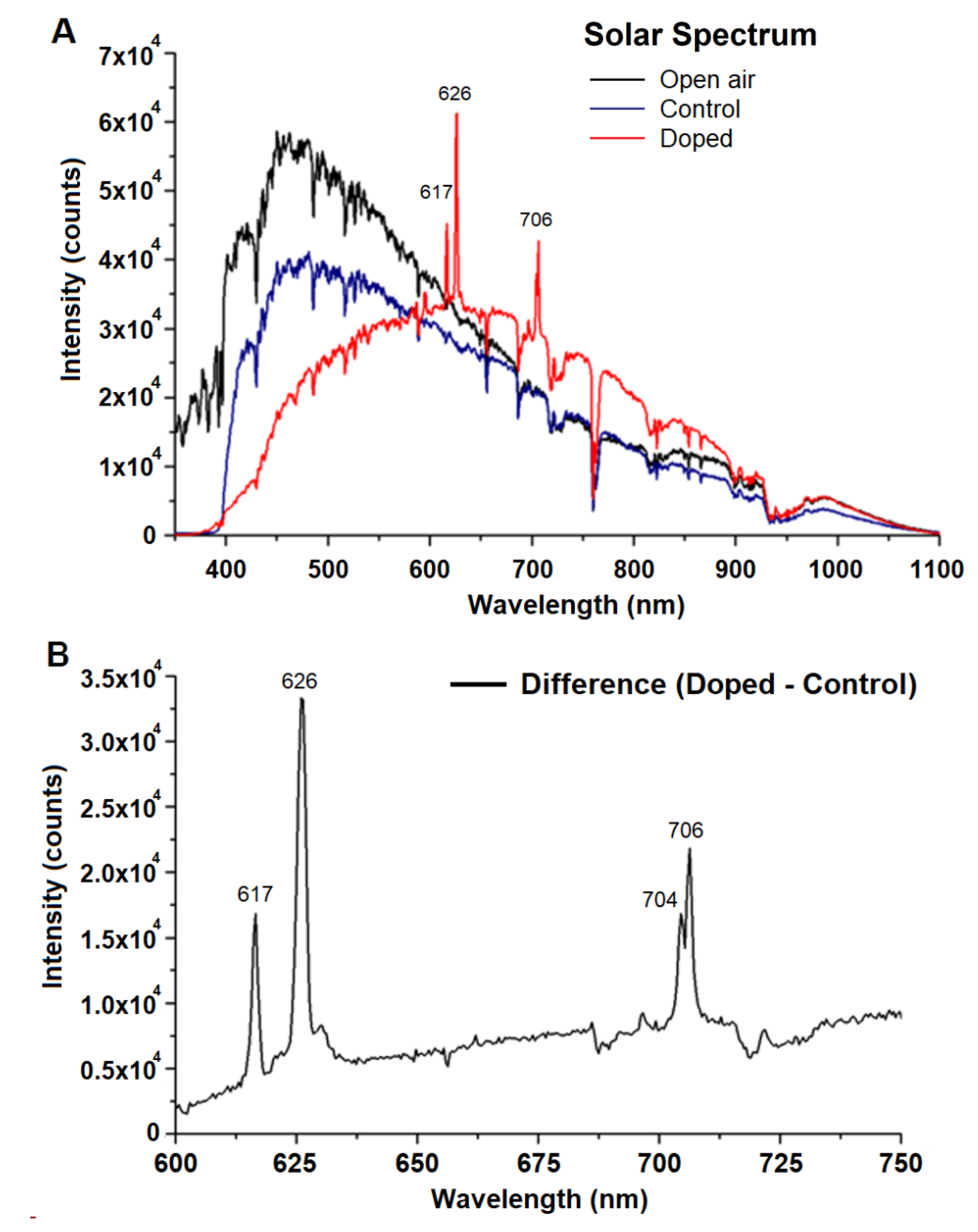
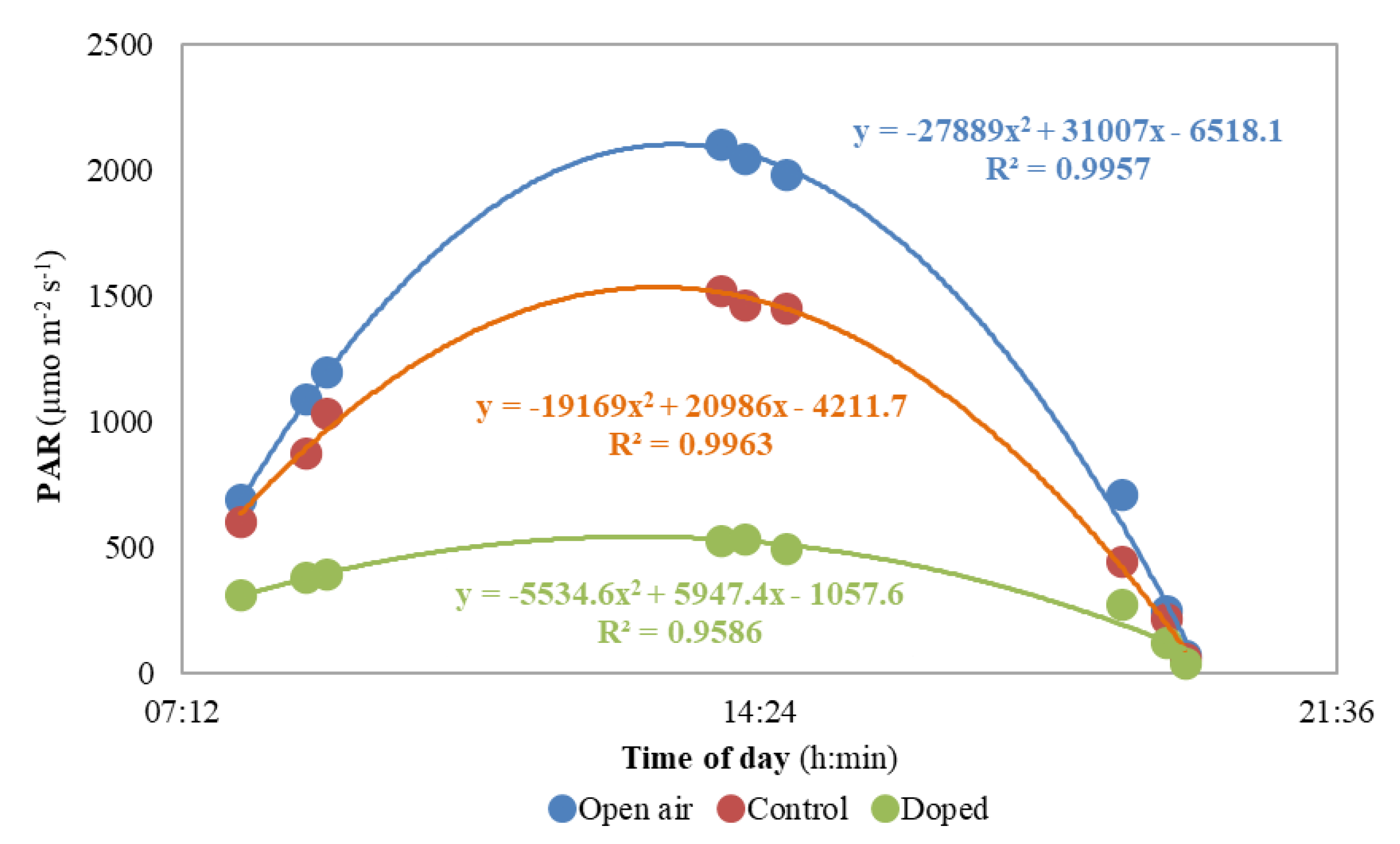
3.1. Air Temperature and Light Parameters
3.2. Lettuce Yield, and Growth Parameters
3.3. Color Parameters, Pigments and Nitrate Content of Lettuce Leaves
3.4. Antioxidant Activities, Total Phenols, and Total Ascorbic Acid of Lettuce Leaves
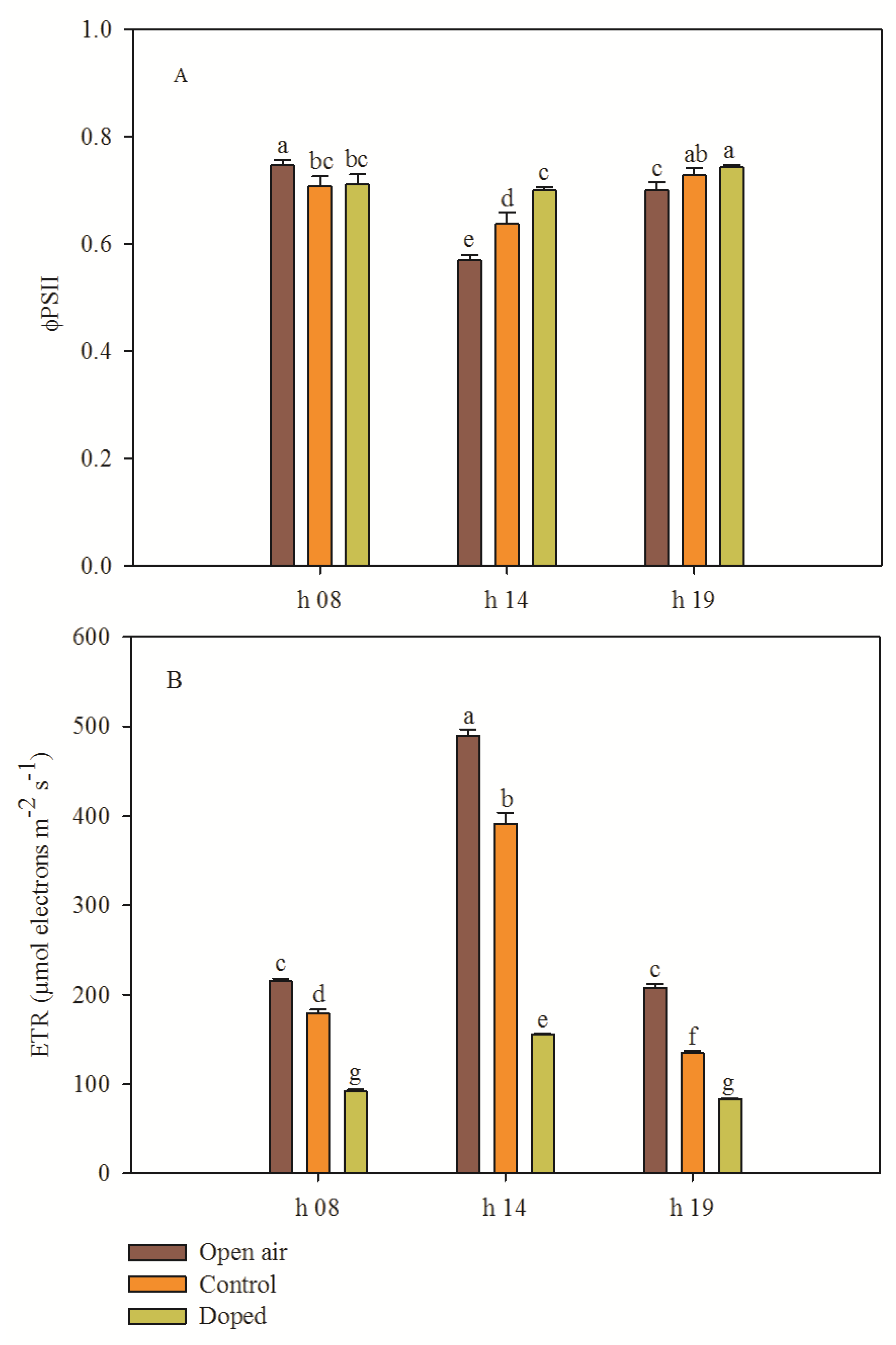
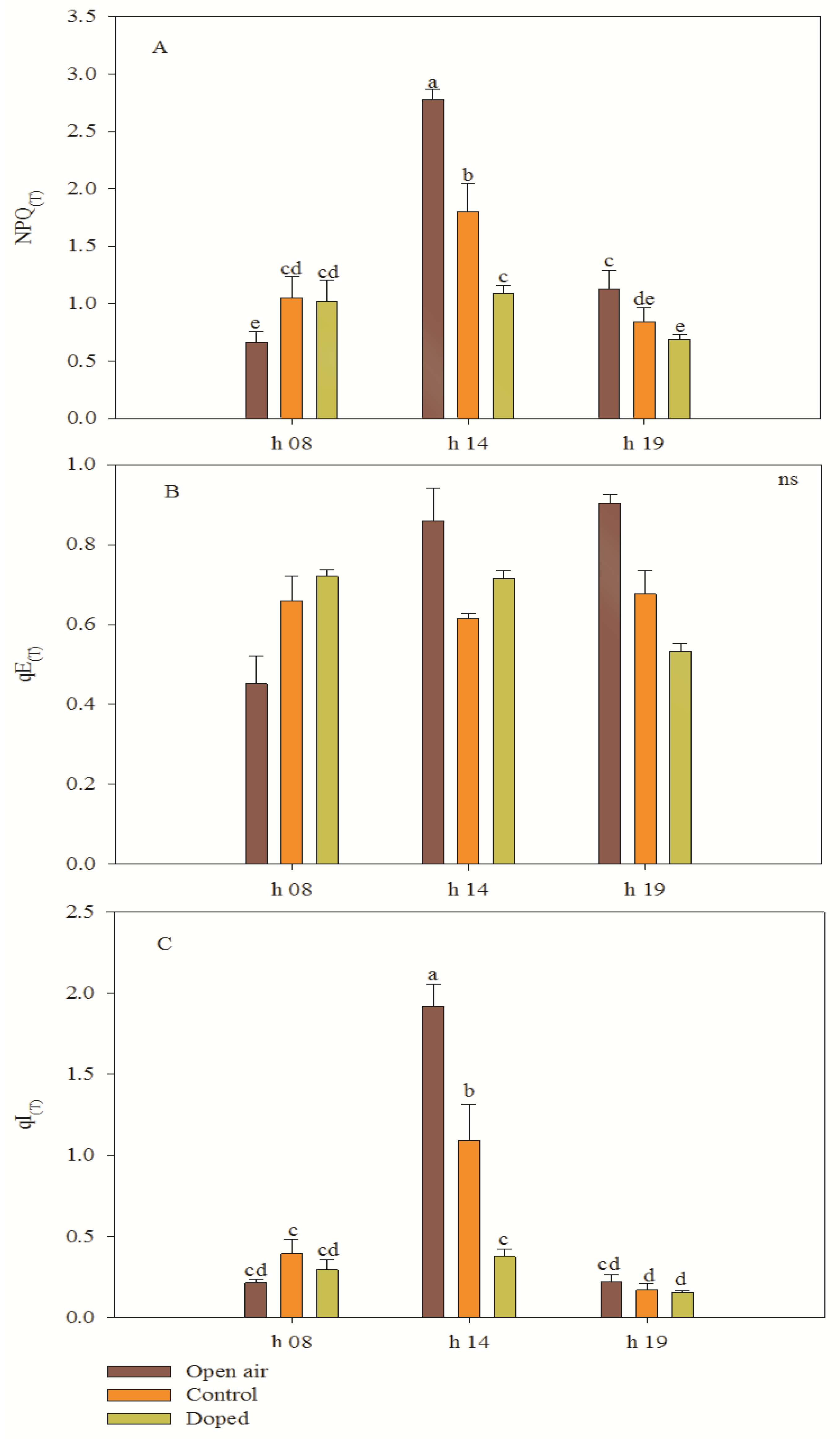
3.5. Chlorophyll Fluorescence Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Good Agricultural Practices for Greenhouse Vegetable Crops—Principles for Mediterranean Climate Areas; Baudoin, W., Nono-Womdim, R., Lutaladio, N., Hodder, A., Castilla, N., Leonardi, C., De Pascale, S., Qaryouti, M., Duffy, R., Eds.; Food And Agriculture Organization of the United Nations: Rome, Italy, 2013; p. 616. ISBN 978-92-5-107649-1. Available online: https://www.fao.org/docrep/018/i3284e/i3284e.pdf (accessed on 5 May 2022).
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. CRC Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Sabatino, L.; Sifola, M.I.; Mormile, P.; El-Nakel, C.; Rouphael, Y.; Mori, M. Optical Characteristics of greenhouse plastic films affect yield and some quality traits of spinach (Spinacia oleracea L.) Subjected to Different Nitrogen Doses. Horticulturae 2021, 7, 200. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Wei, H.; Liu, C.; Hu, J.; Jeong, B.R. Quality of supplementary morning lighting (SML) during propagation period affects physiology, stomatal characteristics, and growth of strawberry plants. Plants 2020, 9, 638. [Google Scholar] [CrossRef]
- Wei, H.; Hu, J.; Liu, C.; Wang, M.; Zhao, J.; Kang, D.I.; Jeong, B.R. Effect of supplementary light source on quality of grafted tomato seedlings and expression of two photosynthetic genes. Agronomy 2018, 8, 207. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, J.; Hu, J.; Jeong, B.R. Effect of supplementary light intensity on quality of grafted tomato seedlings and expression of two photosynthetic genes and proteins. Agronomy 2019, 9, 339. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Agarwal, A. Artificial Lighting System for Plant Growth and Development: Chronological Advancement, Working Principles, and Comparative Assessment. In Light Emitting Diodes for Agriculture; Springer Nature: Berlin/Heidelberg, Germany, 2017; Chapter 1. [Google Scholar] [CrossRef]
- Liu, Y.; Gui, Z.; Liu, J. Research Progress of Light Wavelength Conversion Materials and Their Applications in Functional Agricultural Films. Polymers 2022, 14, 851. [Google Scholar] [CrossRef]
- Roslan, N.; Ya’acob, M.E.; Radzi, M.A.M.; Hashimoto, Y.; Jamaludin, D.; Chen, G. Dye Sensitized Solar Cell (DSSC) greenhouse shading: New insights for solar radiation manipulation. Renew. Sust. Energ. Rev. 2018, 92, 171–186. [Google Scholar] [CrossRef]
- Li, S.; Rajapakse, N.C.; Young, R.E.; Oi, R. Growth responses of chrysanthemum and bell pepper transplants to photoselective plastic films. Sci. Hortic. 2000, 84, 215–225. [Google Scholar] [CrossRef]
- Kido, J.; Hayase, H.; Hongawa, K.; Nagai, K.; Okuyama, K. Bright red light-emitting organic electroluminescent devices having a europium complex as an emitter. Appl. Phys. Lett. 1994, 65, 2124–2126. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Cao, H.; Guan, Y.; Ma, Y. Synthesis of novel europium complexes and their photoluminescence properties. J. Rare Earths 2012, 30, 17–20. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Feng, Z.; Jiang, S.; Lin, J.; Pang, Y.; Li, L.; Xiang, G. Color tunable emission and low-temperature luminescent sensing of europium and terbium carboxylic acid complexes. Inorg. Chim. Acta 2018, 469, 576–582. [Google Scholar] [CrossRef]
- Ming, C.; Song, F.; An, L.; Ren, X. Research phosphate glass on turning sunlight into red light for glass greenhouse. Mater. Lett. 2014, 137, 117–119. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Wang, B.; Liang, R.; Li, Y.; Kang, L.; Liu, P. Effects of impurity doping on the luminescence performance of Mn4+-doped aluminates with the magnetoplumbite-type structure for plant cultivation. Materials 2019, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Zhu, H.; Gao, P.; Zhou, C.; Kong, Z.; Molokeev, M.S.; Qi, Z.; Zhou, Z.; Xia, M. Structure analysis, tuning photoluminescence and enhancing thermal stability on Mn4+-doped La2-xYxMgTiO6 red phosphor for agricultural lighting. Ceram. Int. 2020, 46, 20173–20182. [Google Scholar] [CrossRef]
- Whitelam, G.C.; Halliday, K.J. Annual Plant Reviews, Light and Plant Development; Blackwell Publishing: Oxford, UK, 2007; Volume 30, ISBN 9781405145381. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence—A Signature of Photosynthesis; Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Roháček, K.; Soukupová, J.; Barták, M. Chlorophyll fluorescence: A wonderful tool to study plant physiology and plant stress. In Plant Cell Compartments—Selected Topics; Schoefs, B., Ed.; Research Signpost: Kerala, India, 2008; pp. 41–104. ISBN 978-81-308-0104-9. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and drymatter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.D.; Guo, Y.P.; Liu, J.R.; Mattson, N. Midday depression of photosynthesis is related with carboxylation efficiency decrease and D1 degradation in bayberry (Myrica rubra) plants. Sci. Hortic. 2009, 123, 188–196. [Google Scholar] [CrossRef]
- Panda, D. Diurnal variations in gas exchange and chlorophyll fluorescence in rice leaves: The cause for midday depression in CO2 photosynthetic rate. J. Stress Physiol. Biochem. 2011, 7, 175–186. [Google Scholar]
- Bacarin, M.A.; Garbin Martinazzo, E.; Cassol, D.; Falqueto, A.R.; Moura Silva, D. Daytime variations of chlorophyll a fluorescence in pau d’alho seedlings. Rev. Árvore. 2016, 40, 1023–1030. [Google Scholar] [CrossRef]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; and Kramer, D.M. NPQ(T): A chlorophyll fluorescence parameter for rapid estimation and imaging of non-photochemical quenching of excitons in photosystem-II-associated antenna complexes. Plant Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, G.H.; Samani, Z.A. Reference crop evapotranspiration from temperature. Appl. Eng. Agrc. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of phtosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar] [CrossRef]
- Di Mola, I.; Rouphael, Y.; Colla, G.; Fagnano, M.; Paradiso, R.; Mori, M. Morphophysiological traits and nitrate content of greenhouse lettuce as affected by irrigation with saline water. HortScience 2017, 52, 1716–1721. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Fukumoto, Y.; Aruga, H.; Shimoi, Y. Growth and developmental characteristics of vegetables grown under spectrum conversion film. Hortic. Environ. Biotechnol. 2009, 50, 416. [Google Scholar]
- Kwon, J.K.; Park, K.S.; Choi, H.H.; Lee, S.Y.; Bekhod, K.; Hwang, M.R.; Kang, N.J. Growth and developmental characteristics of lettuce, tomato and melon grown under spectrum conversion greenhouse films. J. Agric. Life Sci. 2013, 47, 57–63. [Google Scholar]
- Wu, W.B.; Zhang, Z.B.; Dong, R.Y.; Xie, G.N.; Zhou, J.; Wu, K.; Zhang, H.; Cai, Q.; Lei, B. Characterization and properties of a Sr2Si5N8:Eu2+-based light-conversion agricultural film. J. Rare Earths 2020, 38, 539–545. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; Cirillo, V.; Pannico, A.; El-Nakhel, C.; Cristofano, F.; Duri, L.G.; Giordano, M.; Rouphael, Y.; De Pascale, S. Divergent leaf morpho-physiological and anatomical adaptations of four lettuce cultivars in response to different greenhouse irradiance levels in early summer season. Plants 2021, 10, 1179. [Google Scholar] [CrossRef]
- Stagnari, F.; Galieni, A.; Pisante, M. Shading and nitrogen management affect quality, safety and yield of greenhouse-grown leaf lettuce. Sci. Hortic. 2015, 192, 70–79. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quench-ing and photoprotection of Photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 760–769. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Zhang, A.; Lu, C. Non-Photochemical Quenching: From Light Perception to Photoprotective Gene Expression. Int. J. Mol. Sci. 2022, 23, 687. [Google Scholar] [CrossRef] [PubMed]
- Malnoë, A.; Schultink, A.; Shahrasbi, S.; Rumeau, D.; Havaux, M.; Niyogi, K.K. The plastid lipocalin LCNP is required for sustained photoprotective energy dissipation in Arabidopsis. Plant. Cell 2017, 30, 196–208. [Google Scholar] [CrossRef]
- Bartucca, L.M.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue:Red LED Light Proportion Affects Vegetative Parameters, Pigment Content, and Oxidative Status of Einkorn (Triticum monococcum L. ssp. monococcum) Wheatgrass. J. Agri. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef] [PubMed]
- Sander, W.; Hogewoning, G.; Trouwborst, H.; Maljaars, H.; Poorter, W.; van Ieperen, J.H. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Abidi, F.; Girault, T.; Douillet, O.; Guillemain, G.; Sintes, G.; Laffaire, M.; Ahmed, H.B.; Smiti, S.; Huché-Thélier, L.; Leduc, N. Blue light effects on rose photosynthesis and photomorphogenesis. Plant Biol. 2013, 15, 67–74. [Google Scholar] [CrossRef]
- Macedo, A.F.; Leal-costa, M.V.; Tavares, E.S.; Salgueiro Lage, C.L.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Poorter, H.; Anten, N.P.R.; Marcelis, L.F.M. Physiological mechanisms in plant growth models: Do we need a supra-cellular systems biology approach? Plant Cell Environ. 2013, 36, 1673–1690. [Google Scholar] [CrossRef]
- Jilani, A.; Kar, S.; Bose, S.; Tripathy, B.C. Regulation of the carotenoid content and chloroplast development by levulinic acid. Physiol. Plant 1996, 96, 139–145. [Google Scholar] [CrossRef]
- Hoober, J.K.; Eggink, L.L. Assembly of light-harvesting complex II and biogenesis of thylakoid membranes in chloroplasts. Photosyn. Res. 1999, 61, 197–215. [Google Scholar] [CrossRef]
- Tanaka, A.; Ito, H.; Tanaka, R.; Tanaka, N.K.; Yoshida, K.; Okada, K. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Plant Biol. 1998, 95, 12719–12723. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, V.; Tripathy, B.C. Photoregulation of the greening process of wheat seedlings grown in red light. Plant Mol. Biol. 2005, 59, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red- and blue-light emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult. 2008, 92, 147–153. [Google Scholar] [CrossRef]
- Kurilcik, A.; Canova, M.R.; Dapkuniene, S.; Zilinskaite, S.; Kurilcik, G. In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Cent. Eur. J. Biol. 2008, 2, 161–167. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Raschke, K. Effect of light quality on stomatal opening in leaves of Xanthium strumarium L. J. Plant Physiol. 1981, 68, 1170–1174. [Google Scholar] [CrossRef]
- Zeiger, E.; Zhu, J. Role of zeaxanthin in blue light photoreception and the modulation of light—CO2 interactions in guard cells. J. Exp. Bot. 1998, 49, 433–442. [Google Scholar] [CrossRef]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Karlsson, P.E.; Assmann, S.M. Rapid and specific modulation of stomatal conductance by blue- light in ivy (Hedera helix): An approach to assess the stomatal limitation of carbon assimilation. J. Plant Physiol. 1990, 94, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.M.; Mackowiak, C.L.; Sager, J.C. Soybean Stem Growth under High-Pressure Sodium with Supplemental Blue Lighting. Agron. J. 1991, 83, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of Seven Diverse Species to Blue and Green Light: Interactions with Photon Flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Shi, L.; Shen, P.; Li, Y. Leaf color formation mechanisms in Alternanthera bettzickiana elucidated by metabolite and transcriptome analyses. Planta 2022, 255, 59. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Kim, H.J.; Myriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar]
- Barker, A.V.; Maynard, D.N. Nutritional factors affecting nitrate accumulation in spinach. Commun. Soil Sci. Plant Anal. 1971, 2, 471–478. [Google Scholar] [CrossRef]
- Stagnari, F.; Di Bitetto, V.; Pisante, M. Effects of N fertilizers and rates on yield, safety and nutrients in processing spinach genotypes. Sci. Hortic. 2007, 114, 225–233. [Google Scholar] [CrossRef]
- Abubaker, S.M.; Abu-Zahra, T.R.; Alzubi, Y.A.; Tahboub, A.B. Nitrate accumulation in spinach (Spinacia oleracea L.) tissues under different fertilization regimes. J. Food Agric. Environ. 2010, 8, 778–780. [Google Scholar]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A Review of Environment Effects on Nitrate Accumulation in Leafy Vegetables Grown in Controlled Environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; El-Nakhel, C.; Rippa, M.; Mormile, P.; Corrado, G.; Rouphael, Y.; Mori, M. Assessment of Yield and Nitrate Content of Wall Rocket Grown under Diffuse-Light-or Clear-Plastic Films and Subjected to Different Nitrogen Fertilization Levels and Biostimulant Application. Horticulturae 2022, 8, 138. [Google Scholar] [CrossRef]
- European Community. Commission Regulation (EU) No 1258/2011 of 2 December 2011. Off. J. Eur. Union 2011, L320, 15–17. [Google Scholar]
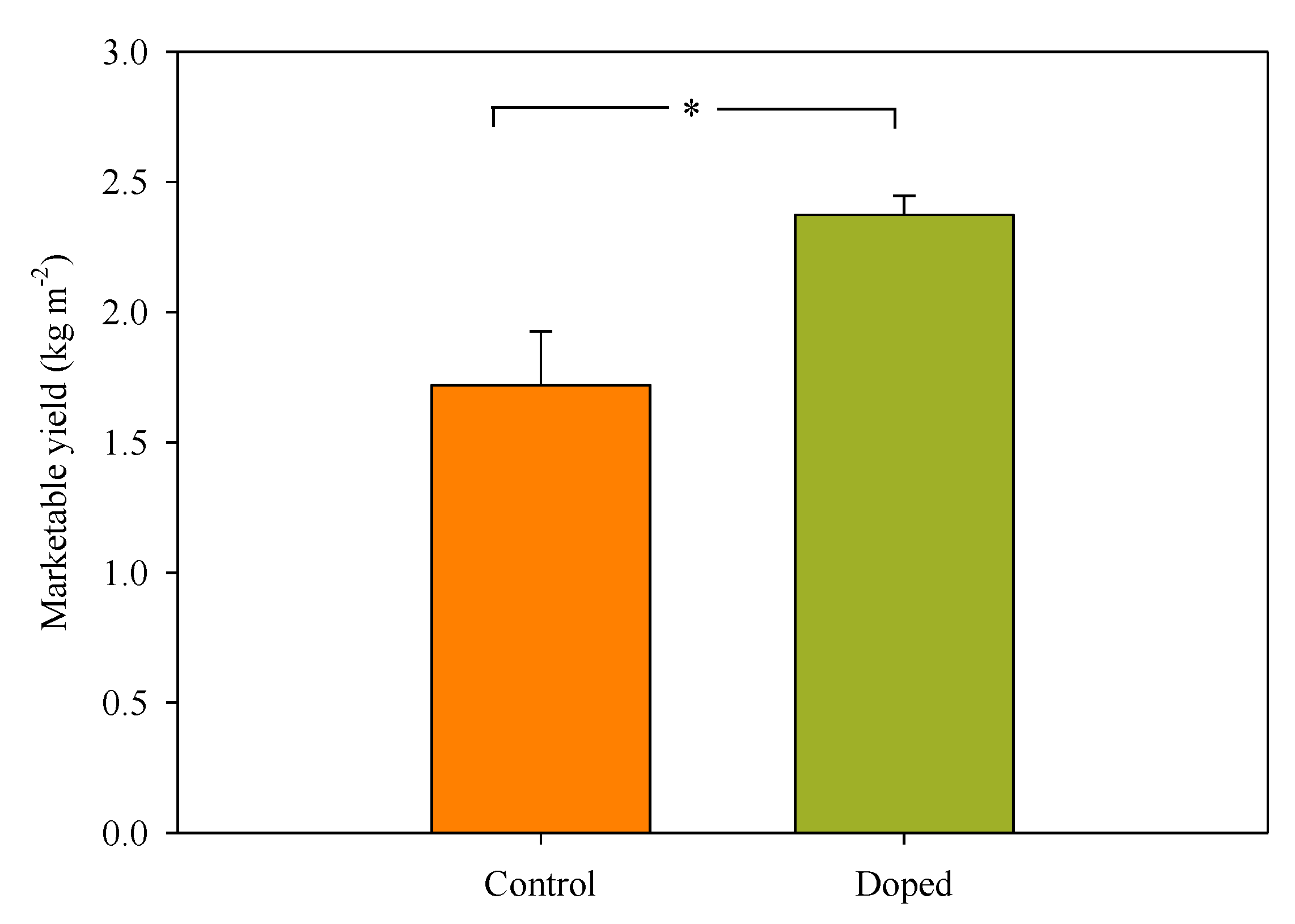
| Treatment | Head | Leaf | Stem | |||
|---|---|---|---|---|---|---|
| fw | Number | dm | fw | Height | Diameter | |
| g Head−1 | n | % | g | cm | cm | |
| Control | 215.0 | 30.5 | 5.91 | 18.16 | 4.38 | 2.10 |
| Doped | 296.7 | 28.5 | 4.94 | 13.50 | 4.05 | 1.95 |
| * | ns | * | ns | * | ns | |
| Treatment | L* | a* | b* |
|---|---|---|---|
| Control | 41.6 | −4.64 | 15.27 |
| Doped | 44.7 | −8.08 | 22.57 |
| ** | ** | ** |
| Treatment | Chlorophyll a | Chlorophyll b | Total Chlorophylls | Carotenoids | Nitrates |
|---|---|---|---|---|---|
| mg g−1 fw | mg g−1 fw | mg g−1 fw | µg g−1 fw | mg kg−1 fw | |
| Control | 0.756 | 0.313 | 1.069 | 394.0 | 3065.4 |
| Doped | 0.894 | 0.411 | 1.304 | 394.7 | 3492.1 |
| ns | ns | ns | ns | * |
| Treatment | ABTS | HAA | Total Phenols | TAA |
|---|---|---|---|---|
| mM Trolox 100g−1 dw | mM AA 100g−1 dw | mg Gallic Acid g−1 dw | mg g−1 fw | |
| Control | 23.18 | 8.50 | 3.19 | 26.99 |
| Doped | 21.51 | 8.49 | 2.48 | 24.69 |
| ns | ns | * | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mola, I.; Conti, S.; Bartak, M.; Cozzolino, E.; Ottaiano, L.; Giordano, D.; Melchionna, G.; Mormile, P.; Rippa, M.; Beltrame, L.; et al. Greenhouse Photoluminescent PMMA Panels Improve the Agronomical and Physiological Performances of Lettuce (Lactuca sativa L.). Horticulturae 2022, 8, 913. https://doi.org/10.3390/horticulturae8100913
Di Mola I, Conti S, Bartak M, Cozzolino E, Ottaiano L, Giordano D, Melchionna G, Mormile P, Rippa M, Beltrame L, et al. Greenhouse Photoluminescent PMMA Panels Improve the Agronomical and Physiological Performances of Lettuce (Lactuca sativa L.). Horticulturae. 2022; 8(10):913. https://doi.org/10.3390/horticulturae8100913
Chicago/Turabian StyleDi Mola, Ida, Stefano Conti, Milos Bartak, Eugenio Cozzolino, Lucia Ottaiano, Davide Giordano, Giuseppe Melchionna, Pasquale Mormile, Massimo Rippa, Luca Beltrame, and et al. 2022. "Greenhouse Photoluminescent PMMA Panels Improve the Agronomical and Physiological Performances of Lettuce (Lactuca sativa L.)" Horticulturae 8, no. 10: 913. https://doi.org/10.3390/horticulturae8100913
APA StyleDi Mola, I., Conti, S., Bartak, M., Cozzolino, E., Ottaiano, L., Giordano, D., Melchionna, G., Mormile, P., Rippa, M., Beltrame, L., El-Nakhel, C., Corrado, G., Rouphael, Y., & Mori, M. (2022). Greenhouse Photoluminescent PMMA Panels Improve the Agronomical and Physiological Performances of Lettuce (Lactuca sativa L.). Horticulturae, 8(10), 913. https://doi.org/10.3390/horticulturae8100913












