Kiwifruit Adaptation to Rising Vapor Pressure Deficit Increases the Risk of Kiwifruit Decline Syndrome Occurrence
Abstract
1. Introduction
- Soil ridging, to improve roots aeration and avoid waterlogging;
- Addition of compost to soil, to mainly improve soil physical properties;
- Addition of commercial microbial consortia to soil, to improve soil biological fertility and promote plant response to stress;
- Addition of zeolite, to improve water turnover in soil;
- Addition of glycine-betaine, to protect leaves from desiccation.
2. Materials and Methods
2.1. Experimental Design and Trial Set Up
2.2. Materials
2.2.1. Compost
2.2.2. Microbial Consortium 1
2.2.3. Microbial Consortium 2
2.2.4. Zeolite
2.2.5. Glycine-Betaine
2.3. Evaluated Parameters
2.3.1. Leaf Gas Exchanges
2.3.2. Leaf Water Potential
2.3.3. Stem Growth
2.3.4. PSA and KIDS Symptoms
2.3.5. Microscopic Analysis of Roots Sections
2.3.6. Weather Data and Soil Water Potential
2.3.7. Processing of Data and Statistical Analysis
3. Results
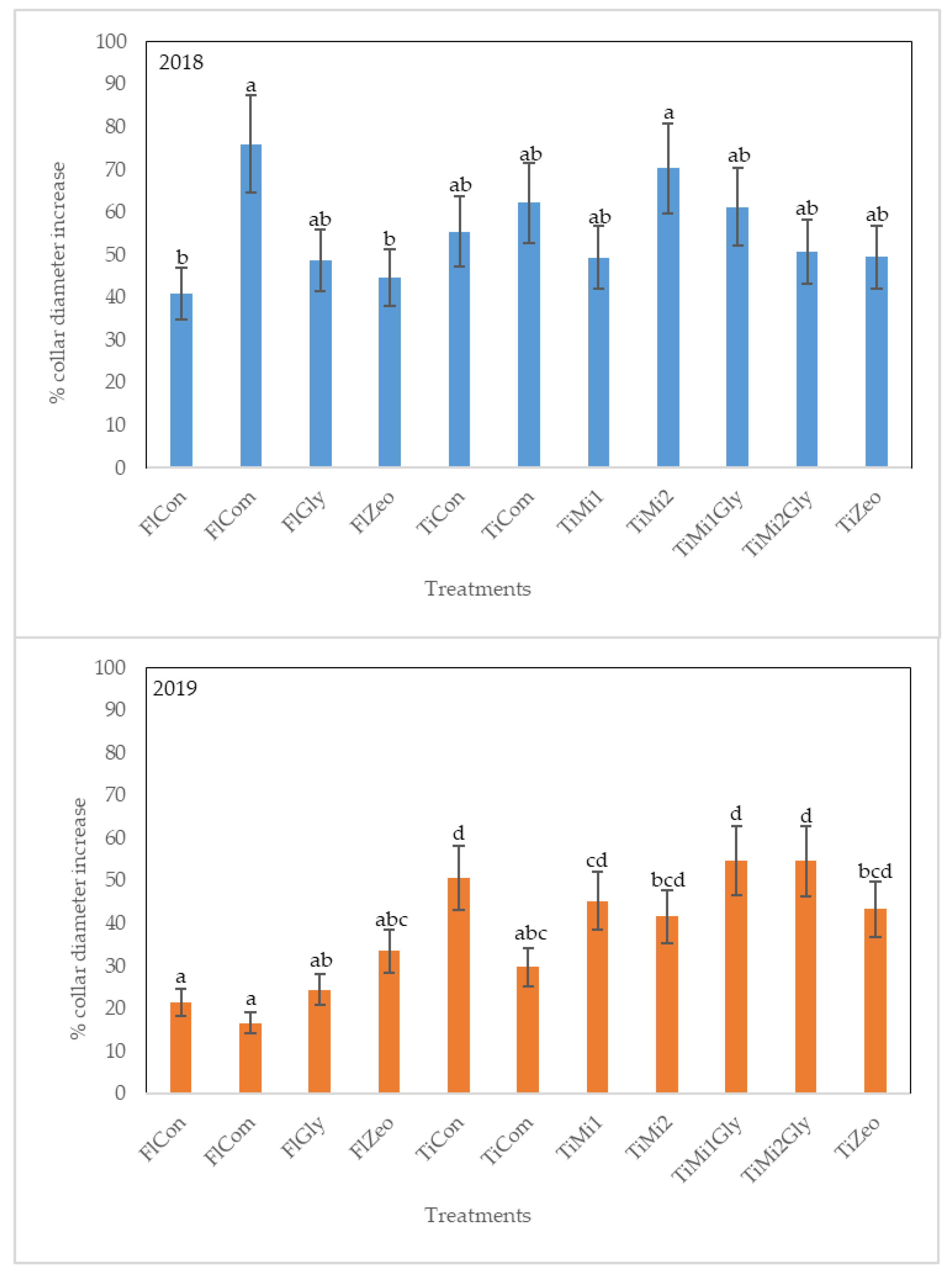
3.1. Stem Growth
3.2. Bacterial Disease PSA and KiDS Symptoms
3.3. Leaf Water Potential
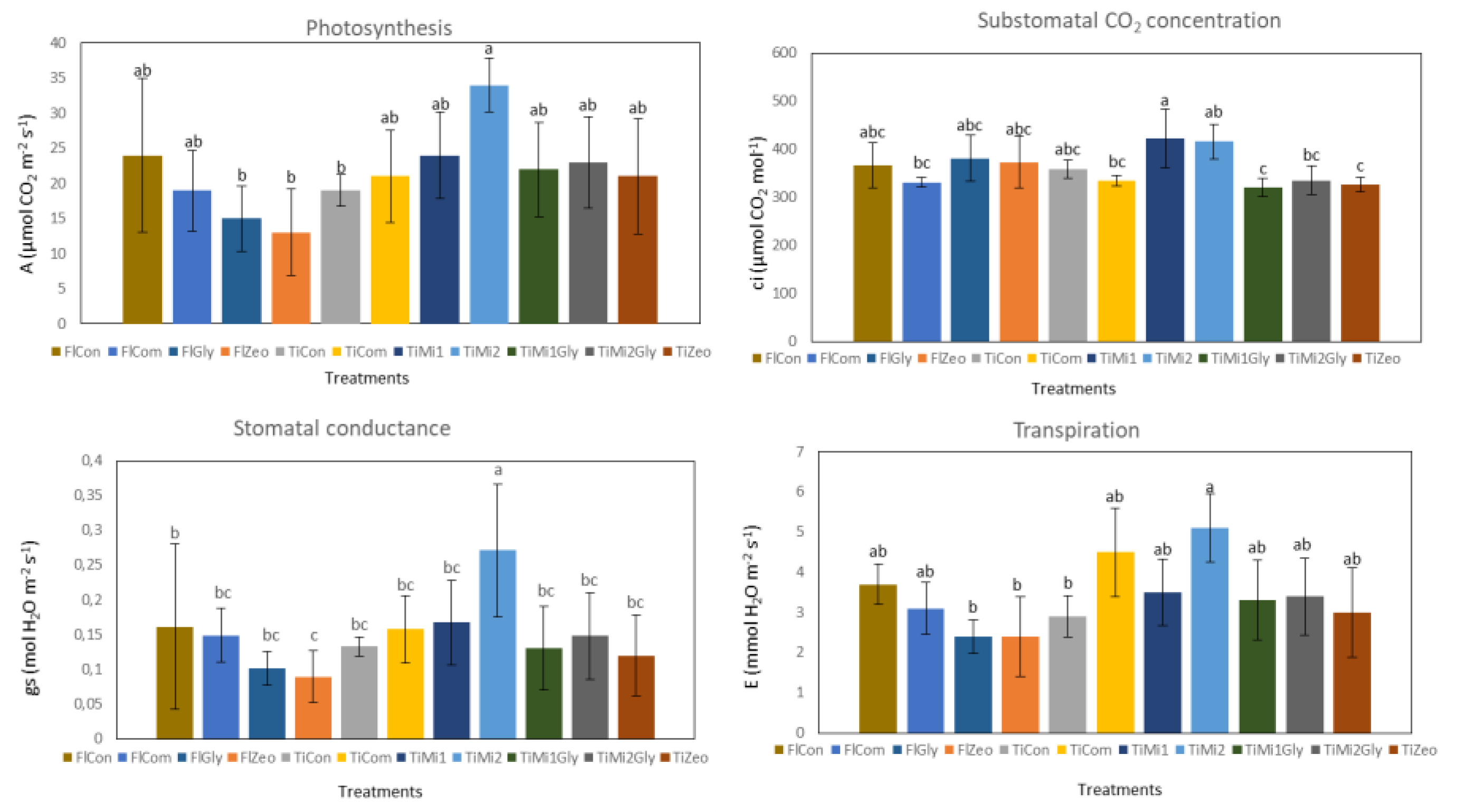
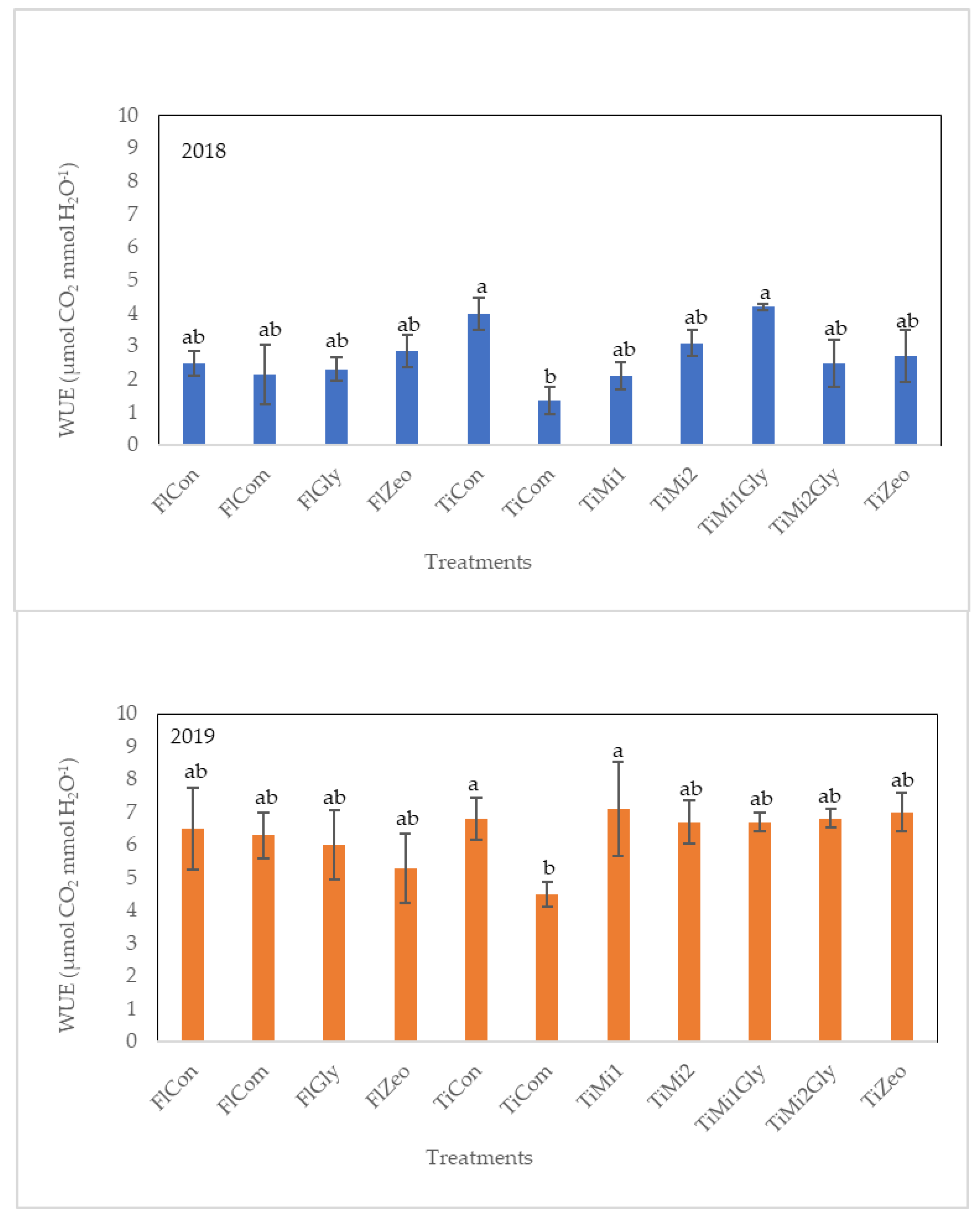
3.4. Leaf Gas Exchanges
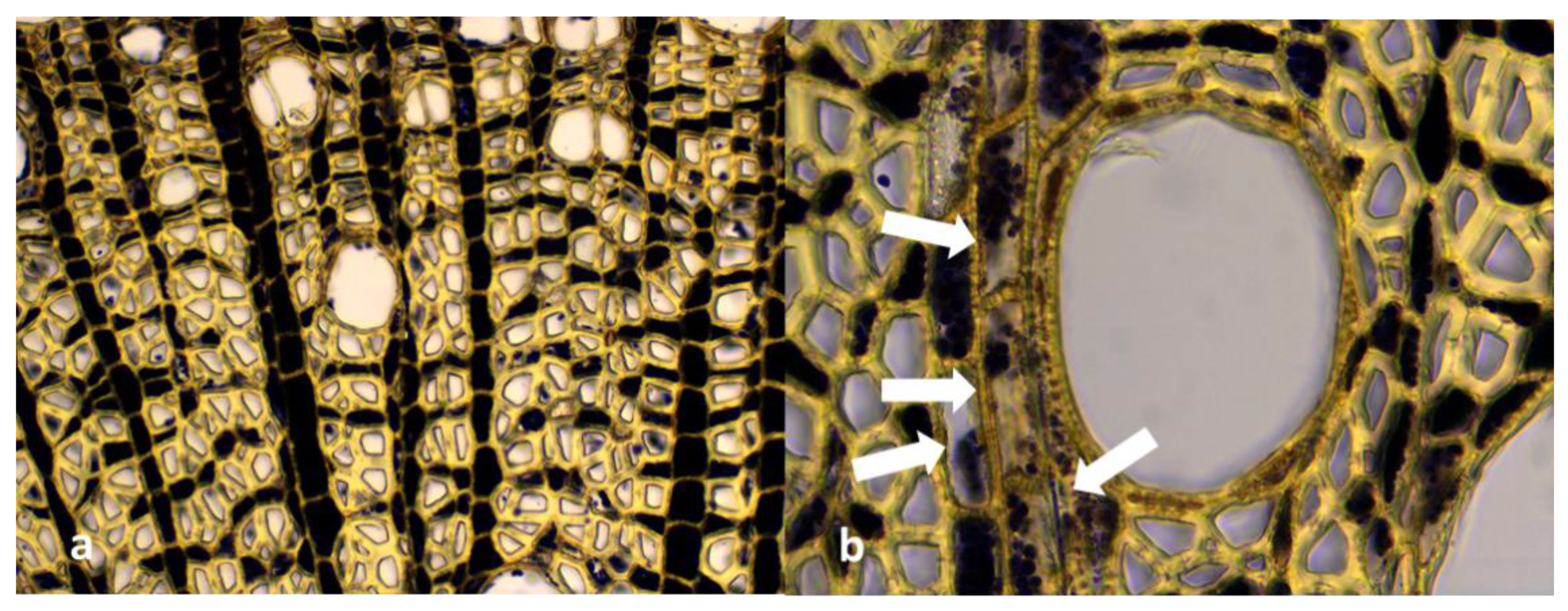
3.5. Microscopic Analysis of Roots Sections
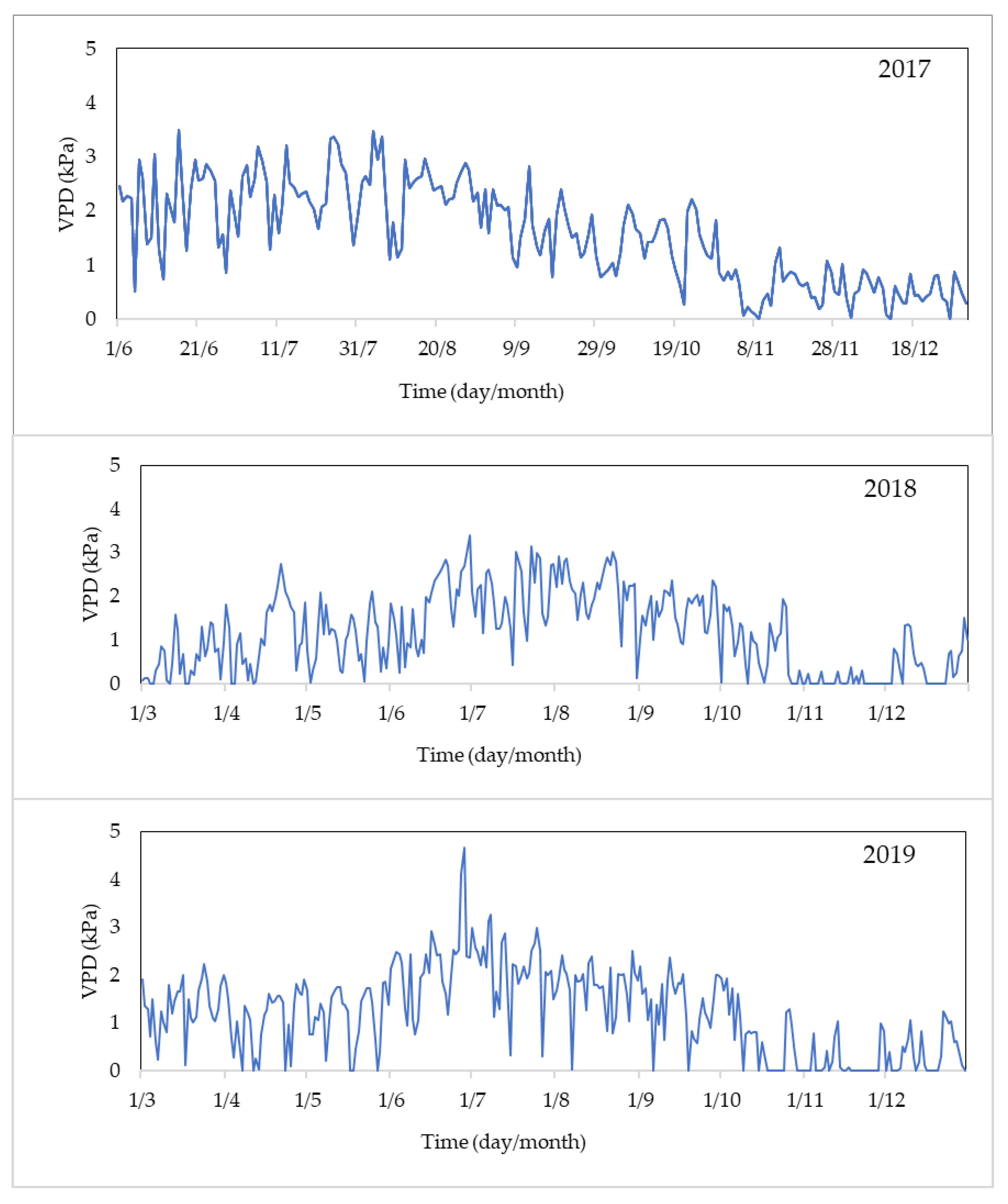
3.6. VPD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medda, S.; Fadda, A.; Mulas, M. Influence of Climate Change on Metabolism and Biological Characteristics in Perennial Woody Fruit Crops in the Mediterranean Environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendía, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; Available online: https://www.ipcc.ch/site/assets/uploads/2019/11/SRCCL-Full-Report-Compiled-191128.pdf (accessed on 15 July 2022).
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed]
- Pivovaroff, A.L.; Pasquini, S.C.; De Guzman, M.E.; Alstad, K.P.; Stemke, J.S.; Santiago, L.S. Multiple strategies for drought survival among woody plant species. Funct. Ecol. 2016, 30, 517–526. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Borrego, I.; Chan, A.M.; Adams, H.D.; Dickman, L.T.; Hudson, P.J.; McBranch, N.; Michaletz, S.T.; Pockman, W.T.; et al. Tree water dynamics in a drying and warming world. Plant Cell Environ. 2017, 40, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- von Arx, G.; Archer, S.R.; Hughes, M.K. Long-term functional plasticity in plant hydraulic architecture in response to supplemental moisture. Ann. Bot. 2012, 109, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- FAO. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 July 2022).
- Scortichini, M.; Marcelletti, S.; Ferrante, P.; Petriccione, M.; Firrao, G. Pseudomonas syringae pv. actinidiae: A re-emerging, multi-faceted, pandemic pathogen. Mol. Plant Pathol. 2012, 13, 631–640. [Google Scholar] [CrossRef]
- Haye, T.; Weber, D.C. Special issue on the brown marmorated stink bug, Halyomorpha halys: An emerging pest of global concern. J. Pest Sci. 2017, 90, 987–988. [Google Scholar] [CrossRef]
- Bardi, L.; Nari, L.; Morone, C.; Faga, M.G.; Malusà, E. Possible Role of High Temperature and Soil Biological Fertility on Kiwifruit Early Decline Syndrome. Front. Agron. 2020, 2, 13. [Google Scholar] [CrossRef]
- Reid, J.B.; Tate, K.G.; Brown, N.S.; Cheah, L.H. Effects of flooding and alluvium deposition on kiwifruit (Actinidia deliciosa): 1. Early vine decline. J. Crop Hortic. Sci. 1991, 19, 247–257. [Google Scholar] [CrossRef]
- Tosi, L.; Giacopini, A.; Tacconi, G. La moria del kiwi, situazione e prospettive. Inform. Agrar. 2015, 744, 67–70. [Google Scholar]
- Tacconi, G.; Tosi, L.; Giacopini, A.; Bertaccini, A.; Mazzucchi, U.; Favaron, F. Vine decline in kiwifruit: Climate change and effect on waterlogging and Phythopthora in North Italy. In Proceedings of the 8th International Symposium on Kiwifruit, Dujiangyan, China, 18 September 2014. [Google Scholar]
- Tacconi, G.; Paltrinieri, S.; Mejia, J.F.; Fuentealba, S.P.; Bertaccini, A.; Tosi, L.; Giacopini, A.; Mazzucchi, U.; Favaron, F.; Sella, L.; et al. Vine decline in kiwifruit: Climate change and effect on waterlogging and Phytophthora in North Italy. Acta Hortic. 2015, 1096, 93–97. [Google Scholar] [CrossRef]
- Bardi, L. Early Kiwifruit Decline: A Soil-Borne Disease Syndrome or a Climate Change Effect on Plant–Soil Relations? Front. Agron. 2020, 2, 3. [Google Scholar] [CrossRef]
- Smith, G.S.; Judd, M.J.; Miller, S.A.; Buwalda, J.G. Recovery of kiwifruit vines from transient waterlogging of the root system. New Phytol. 1990, 115, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Savé, R.; Serrano, L. Some physiological and growth responses of kiwi fruit (Actinidia chinensis) to flooding. Physiol. Plant 1986, 66, 75–78. [Google Scholar] [CrossRef]
- Smith, G.S.; Buwalda, J.G.; Green, T.G.A.; Clark, C.J. Effect of oxygen supply and temperature at the root on the physiology of kiwifruit vines. New Phytol. 1989, 113, 431–437. [Google Scholar] [CrossRef]
- Reid, J.B.; Tate, K.G.; Brown, N.S. Effects of flooding and alluvium deposition on kiwifruit (Actinidia deliciosa). N. Zeal. J. Crop Hortic. Sci. 1992, 20, 283–288. [Google Scholar] [CrossRef]
- Tacconi, G.; Giacopini, A.; Vittone, G.; Nari, L.; Spadaro, D.; Savian, F.; Ermacora, P.; Saro, F.; Morone, C.; Bardi, L.; et al. Il punto sulla moria del kiwi a 8 anni dalla sua comparsa. Inform. Agrar. 2019, 21, 34–36. [Google Scholar]
- Ferguson, A.R. Kiwifruit: A botanical review. Horticult. 1984, 6, 1–64. [Google Scholar] [CrossRef]
- Lemon, C.W.; Considine, J.A. Anatomy and histochemistry of the root system of the kiwifruit vine, Actinidia deliciosa var. deliciosa. Ann. Bot. 1993, 71, 117–129. [Google Scholar] [CrossRef]
- Condon, J.M. Aspects of kiwifruit stem structure in relation to transport. Acta Hortic. 1991, 297, 419–426. [Google Scholar] [CrossRef]
- Smith, G.S.; Clark, C.J.; Boldingh, H.L. Seasonal Accumulation of Starch by Components of the Kiwifruit Vine. Ann. Bot. 1992, 70, 19–25. [Google Scholar] [CrossRef]
- Lechthaler, S.; Kiorapostolou, N.; Pitacco, A.; Anfodillo, T.; Petit, G. The total path length hydraulic resistance according to known anatomical patterns: What is the shape of the root-to-leaf tension gradient along the plant longitudinal axis? J. Theor. Biol. 2020, 502, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys. In U.S. Department of Agriculture Handbook 436, 2nd ed.; Natural Resources Conservation Service (USDA): Washington DC, USA, 1999. [Google Scholar]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap Pressure in Vascular Plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Deloire, A.; Pellegrino, A.; Rogiers, S. A few words on grapevine leaf water potential. IVES Tech. Rev. VineWine 2020. [Google Scholar] [CrossRef]
- Eades, H.W. Iodine as an indicator of sapwood and heartwood. For. Chron. 1937, 13, 470–477. [Google Scholar] [CrossRef]
- Calderón-Orellana, A.; Silva, D.I.; Bastías, R.M.; Bambach, N.; Aburto, F. Late-season plastic covering delays the occurrence of severe water stress and improves intrinsic water use efficiency and fruit quality in kiwifruit vines. Agric. Water Manag. 2021, 249, 106795. [Google Scholar] [CrossRef]
- Zheng, S.; Cuia, N.; Gong, D.; Wang, Y.; Hu, X.; Fenga, Y.; Zhang, Y. Relationship between stable carbon isotope discrimination and water use efficiency under deficit drip irrigation of kiwifruit in the humid areas of South China. Agric. Water Manag. 2020, 240, 106300. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental Factors Influence Plant Vascular System and Water Regulation. Plants 2019, 8, 65. [Google Scholar] [CrossRef]
- Fonti, P.; von Arx, G.; Garcıa-Gonzalez, I.; Eilmann, B.; Sass-Klaassen, U.; Gartner, H.; Eckstein, D. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef]
- Salleo, S.; Trifilò, P.; Lo Gullo, M.A. Phloem as a possible major determinant of rapid cavitation reversal in stems of Laurus nobilis (laurel). Funct. Plant Biol. 2006, 33, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Salleo, S.; Jansen, S. More than just a vulnerable pipeline: Xylem physiology in the light of ion-mediated regulation of plant water transport. J. Exp. Bot. 2011, 62, 4701–4718. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Zeppel, M.J.; Anderegg, W.R.; Bloemen, J.; De Kauwe, M.G.; Hudson, P.; Ruehr, N.K.; Powell, T.L.; von Arx, G.; Nardini, A. Xylem embolism refilling and resilience against drought-induced mortality in woody plants: Processes and trade-offs. Ecol. Res. 2018, 33, 839–855. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, H.; Batelaan, O.; McVicar, T.R.; Long, T.; Piao, S.; Liang, V.; Liu, B.; Jin, Z.; Simmons, C.T. Contrasting responses of water use efficiency to drought across global terrestrial ecosystems. Sci. Rep. 2016, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- McAneney, K.J.; Judd, M.J. Observations on kiwifruit (Actinidia chinensis Planch.) root exploration, root pressure, hydraulic conductivity, and water uptake. New Zealand J. Agric. Res. 2012, 26, 507–510. [Google Scholar] [CrossRef]
- Spinelli, F.; Donati, I.; Vanneste, J.L.; Costa, M.; Costa, G. Real time monitoring of the interactions between Pseudomonas syringae pv. actinidiae and Actinidia species. Acta Hortic. 2011, 913, 461–465. [Google Scholar]
- Roos, I.M.M.; Hattingh, M.J. Systemic invasion of plum leaves and shoots by Pseudomonas syringae pv. syringae introduced into petioles. Phytopathology 1987, 77, 1253–1257. [Google Scholar]
- De Pascali, M.; Vergine, M.; Sabella, E.; Aprile, A.; Nutricati, E.; Nicolì, F.; Buja, I.; Negro, C.; Miceli, A.; Rampino, P.; et al. Molecular Effects of Xylella fastidiosa and Drought Combined Stress in Olive Trees. Plants 2019, 8, 437. [Google Scholar] [CrossRef]
- Sabella, E.; Aprile, A.; Genga, A.; Siciliano, T.; Nutricati, E.; Nicolì, F.; Vergine, M.; Negro, C.; De Bellis, L.; Luvisi, A. Xylem cavitation susceptibility and refilling mechanisms in olive trees infected by Xylella fastidiosa. Sci. Rep. 2019, 9, 9602. [Google Scholar] [CrossRef]
- Sabella, E.; Moretti, S.; Gärtner, H.; Luvisi, A.; De Bellis, L.; Vergine, M.; Saurer, M.; Cherubini, P. Increase in ring width, vessel number and δ18O in olive trees infected with Xylella fastidiosa. Tree Physiol. 2020, 44, 1583–1594. [Google Scholar] [CrossRef]
- Petit, G.; Bleve, G.; Gallo, A.; Mita, G.; Montanaro, G.; Nuzzo, V.; Zambonini, D.; Pitacco, A. Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europaea: Revisiting a tale of plant–pathogen interaction. Plants 2021, 13, 2–4. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Balzano, A.; Wheeler, E.A.; Baas, P. Tyloses and gums: A review of structure, function and occurrence of vessel occlusions. IAWA J. 2016, 37, 186–205. [Google Scholar] [CrossRef]
- Bardi, L.; Malusà, E. Drought and nutritional stresses in plant: Alleviating role of rhizospheric microorganisms. In Abiotic Stress: New Research; Haryana, N., Punj, S., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 1–57. [Google Scholar]




| Treatment | Bacterial Disease PSA Symptoms (% Affected Plants) | KiDS Symptoms (% Affected Plants) |
|---|---|---|
| FlCon | 16.7 | 100 |
| FlCom | 42.1 | 100 |
| FlGly | 20.0 | 100 |
| FlZeo | 9.5 | 100 |
| TiCon | 15.0 | 91 |
| TiCom | 55.9 | 66.7 |
| TiMi1 | 20.0 | 91.7 |
| TiMi2 | 18.4 | 100 |
| TiMi1Gly | 16.7 | 90.9 |
| TiMi2Gly | 18.2 | 90 |
| TiZeo | 19.0 | 91.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardi, L.; Nari, L.; Morone, C.; Solomita, M.; Mandalà, C.; Faga, M.G.; Migliori, C.A. Kiwifruit Adaptation to Rising Vapor Pressure Deficit Increases the Risk of Kiwifruit Decline Syndrome Occurrence. Horticulturae 2022, 8, 906. https://doi.org/10.3390/horticulturae8100906
Bardi L, Nari L, Morone C, Solomita M, Mandalà C, Faga MG, Migliori CA. Kiwifruit Adaptation to Rising Vapor Pressure Deficit Increases the Risk of Kiwifruit Decline Syndrome Occurrence. Horticulturae. 2022; 8(10):906. https://doi.org/10.3390/horticulturae8100906
Chicago/Turabian StyleBardi, Laura, Luca Nari, Chiara Morone, Mauro Solomita, Claudio Mandalà, Maria Giulia Faga, and Carmela Anna Migliori. 2022. "Kiwifruit Adaptation to Rising Vapor Pressure Deficit Increases the Risk of Kiwifruit Decline Syndrome Occurrence" Horticulturae 8, no. 10: 906. https://doi.org/10.3390/horticulturae8100906
APA StyleBardi, L., Nari, L., Morone, C., Solomita, M., Mandalà, C., Faga, M. G., & Migliori, C. A. (2022). Kiwifruit Adaptation to Rising Vapor Pressure Deficit Increases the Risk of Kiwifruit Decline Syndrome Occurrence. Horticulturae, 8(10), 906. https://doi.org/10.3390/horticulturae8100906







