The Artichoke “Bianco di Pertosa”: The Enhancement of Crop Residues through Environmentally Friendly Uses
Abstract
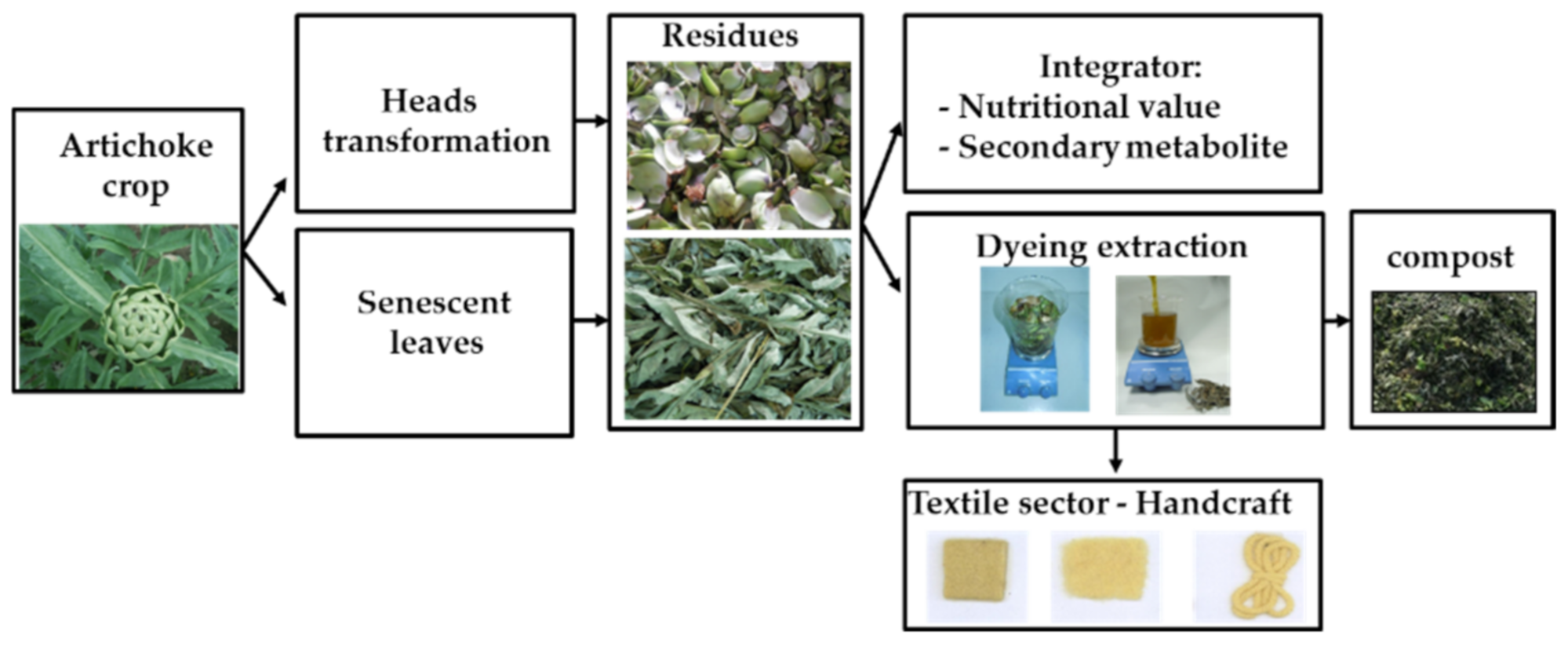
1. Introduction
2. Materials and Methods
2.1. Agronomic Measurements
2.2. Reagent and Chemicals
2.3. Determination of Nutritional Label
2.4. Determination of Minerals
2.5. Determination of Fatty Acids
2.6. Dyeing Process
- Dried bracts with a ratio between the material to be dyed and the plant material used for the preparation of the colour bath of 1:1, temperature of 70 °C for 1 h;
- Dried leaves using the ratio of 1:1, temperature of 70 °C for 1 h (Extraction Process 1);
- Dried leaves using the ratio of 1:1, temperature of 70 °C for 30 min, and temperature of 60 °C for the next 90 min (Extraction Process 2).
2.7. Statistical Analysis
3. Results and Discussion
3.1. Study Area and Product Description
3.2. The Agronomic Measurements
3.3. The Nutritional Label
3.4. The Dyeing Characterisation
4. Final Considerations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zayed, A.; Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. LWT—Food Sci. Technol. 2020, 132, 109883. [Google Scholar] [CrossRef]
- Acquadro, A.; Barchi, L.; Portis, E.; Mangino, G.; Valentino, D.; Mauromicale, G.; Lanteri, S. Genome reconstruction in Cynara cardunculus taxa gains access to chromosome-scale DNA variation. Sci. Rep. 2017, 7, 5617. [Google Scholar] [CrossRef] [PubMed]
- Curci, P.L.; De Paola, D.; Danzi, D.; Vendramin, G.G.; Sonnante, G. Complete chloroplast genome of the multifunctional crop globe artichoke and comparison with other Asteraceae. PLoS ONE 2015, 10, e0120589. [Google Scholar] [CrossRef] [PubMed]
- Pignone, D.; Sonnante, G. Wild artichokes of South Italy: Did the story begin here? Genet. Resour. Crop Evol. 2004, 51, 577–580. [Google Scholar] [CrossRef]
- FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 August 2022).
- ISTAT. Aggiornamento del 6° Censimento dell’agricoltura. 2022. Available online: http://dati.istat.it/ (accessed on 5 September 2022).
- Grabowska, A.; Caruso, G.; Mehrafarin, A.; Kalisz, A.; Gruszecki, R.; Kunicki, E.; Sękara, A. Application of modern agronomic and biotechnological strategies to valorise worldwide globe artichoke (Cynara cardunculus L.) potential—An analytical overview. Ital. J. Agron 2018, 13, 1252. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- De Faria Coelho-Ravagnani, C.; Corgosinho, F.C.; Sanches, F.L.F.Z.; Prado, C.M.M.; Laviano, A.; Mota, J.F. Dietary recommendations during the COVID-19 pandemic. Nutrients 2021, 13, 1752. [Google Scholar]
- Rodriguez-Leyva, D.; Pierce, G.N. The Impact of Nutrition on the COVID-19 Pandemic and the Impact of the COVID-19 Pandemic on Nutrition. Nutrients 2021, 13, 1752. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Beograd, Serbia, 2021. [Google Scholar]
- Fratianni, F.; Tucci, M.; De Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- European Commission. UE Circular Economy Action Plan. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 3 September 2022).
- Yu, M.; Yang, Y.; Chen, F.; Zhu, F.; Qu, J.; Zhang, S. Response of agricultural multifunctionality to farmland loss under rapidly urbanizing processes in Yangtze River Delta, China. Sci. Total Environ. 2019, 666, 1–11. [Google Scholar] [CrossRef]
- Todorova, S.; Ikova, J. Multifunctional Agriculture: Social and Ecological impacts on the organic farms in Bulgaria. Procedia Econ. 2014, 9, 310–320. [Google Scholar] [CrossRef]
- Castro, C.G.; Trevisan, A.H.; Pigosso, D.C.A.; Mascarenhas, J. The rebound effect of circular economy: Definitions, mechanisms, and a research agenda. J. Clean. Prod. 2022, 345, 131–136. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Sadagopan, M.; Rosado, L. Circular economy—From review of theories and practices to development of implementation tools. Resour. Conserv. Recycl. 2018, 135, 190–201. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lafiandra, D.; Morone-Fortunato, I. Composizione chimica e valore nutritivo del carciofo (Cynara scolymus L.). In Studi sul Carciofo, a Cura di V. Marzi e V. Lattanzio, Atti III Congresso Internazionale sul Carciofo, Cynar e CNR Editori; Industria Grafica Laterza: Bari, Italy, 1981. [Google Scholar]
- Barracosa, P.; Barracosa, M.; Pires, E. Cardoon as a sustainable crop for biomass and bioactive compounds production. Chem. Biodivers. 2019, 16, e1900498. [Google Scholar] [CrossRef]
- Ben Salem, M.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods for Human Nutrition 2015, 70, 441–453. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2061–2108. [Google Scholar] [CrossRef]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; De Falco, E.; Rastrelli, L. Chemical profile and cellular antioxidant activity of artichoke by-products. Food Funct. 2016, 7, 4841. [Google Scholar] [CrossRef]
- Chihoub, W.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Valorisation of the green waste parts from turnip, radish, and wild cardoon: Nutritional value, phenolic profile and bioactivity evaluation. Food Res. Int. 2019, 126, 108651. [Google Scholar] [CrossRef]
- Claus, T.; Maruyama, S.A.; Palombini, S.V.; Montanher, P.F.; Bonafé, E.G.; de Oliveira Santos Junior, O.; Matsushita, M.; Visentainer, J.V. Chemical characterization and use of artichoke parts for protection from oxidative stress in canola oil. LWT—Food Sci. Technol. 2015, 61, 346–351. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131. [Google Scholar] [CrossRef]
- Meneses, M.; Megìas, M.D.; Madrid, M.J.; Martìnez-Teruel, A.; Hernàndez, F. Ensiling capacity, chemical composition and multiresidue evaluation of fresh artichoke (Cynara scolymus, L.) by-product to be used in ruminant feeding. Options Méditerr. 2005, 67, 351–354. [Google Scholar]
- Farag, M.A.; Elsebai, M.F.; Khattab, A.R. Metabolome based classification of artichoke leaf: A prospect for phyto-equivalency of its different leaf origins and commercial preparations. J. Pharm. Biomed. Anal. 2018, 158, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aceituno, L.; García-Sarrió, M.J.; Alonso-Rodriguez, B.; Ramos, L.; Sanz, M.L. Extraction of bioactive carbohydrates from artichoke (Cynara scolymus L.) external bracts using microwave assisted extraction and pressurized liquid extraction. Food Chem. 2016, 196, 1156. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, M.; Romani Pizzichini, A.; Pizzichini, D.; Russo, C.; Pinelli, P. Process for Producing Refined Nutraceutic Extracts from Artichoke Waste and from other Plants of the Cynara Genus; Isr Ecoindustria s.r.l.: Latina, Italy, 2008. [Google Scholar]
- Coinu, R.; Carta, S.; Urgeghe, P.; Mulinacci, N.; Pinelli, P.; Franconi, F.; Romani, A. Dose-effect study on the antioxidant properties of leaves and outer bracts of extracts obtained from Violetto di Toscana artichoke. Food Chem. 2007, 101, 524–531. [Google Scholar] [CrossRef]
- De Falco, E.; Caroccia, R.; Russo, L.; Lombardi, G.; Roscigno, G.; Gaeta, A. Proceedings VIII Convegno Biodiversità; Arti Grafiche Favia—Modugno (BA): Lecce, Italy, 2010; pp. 167–169. [Google Scholar]
- Bechtold, T.; Mussak, R.; Mahmud-Ali, A.; Ganglberger, E.; Geisseler, S. Extraction of natural dyes for textile dyeing from coloured plant wastes released from the food and beverage industry. J. Sci. Food Agric. 2006, 86, 233. [Google Scholar] [CrossRef]
- Grifoni, D.; Roscigno, G.; De Falco, E.; Vece, A.; Camilli, F.; Sabatini, F.; Fibbi, L.; Zipoli, G. Evaluation of Dyeing and UV Protective Properties on Hemp Fabric of Aqueous Extracts from Vegetal Matrices of Different Origin. Fibers Polym. 2020, 21, 1750–1759. [Google Scholar] [CrossRef]
- De Falco, E. Tingere con il Carciofo Bianco di Pertosa; Collana MIdA Agricoltura; Editore MIDA—Musei Integrati dell’ambiente: Pertosa, Italy, 2012. [Google Scholar]
- Archontoulis, S.V.; Struik, P.C.; Vos, J.; Danalatos, N. Phenological growth stages of Cynara cardunculus: Codification and description according to the BBCH scale. Ann. Appl. Biol. 2010, 156, 253–270. [Google Scholar] [CrossRef]
- Magnifico, V.; Pepe, R.; Rosti, A.; Palumbo, A.D.; Santonicola, L.; Donato, R. Il Carciofo Bianco di Pertosa. Inf. Agrar. 2005, 7, 61–64. [Google Scholar]
- De Falco, E.; Zanti, R.; Senatore, A.; Vitti, A. Opportunities of spontaneous edible plants collected in southern Italy (Campania Region) as functional food. Ital. J. Agron. 2019, 14, 1540. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Jiang, B.; Tsao, R.; Li, Y.; Miao, M. Food Safety: Food Analysis Technologies/Techniques. In Encyclopedia of Agriculture and Food Systems; Academic Press: Cambridge, MA, USA, 2014; pp. 273–288. [Google Scholar]
- Regulation EU. No 1169/2011 of the European parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union. 2011, L304, 18–63. [Google Scholar]
- European Union. Regulation (EEC) 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union. 1991, L248, 36–47. [Google Scholar]
- European Union. Regulation (EU) 2015/1833 of 12 October 2015. Off. J. Eur. Union. 2015, L266, 35–44. [Google Scholar]
- De Falco, E.; Di Novella, N. Le Piante Tintorie del Cilento e Vallo di Diano; Fondazione MiDA: Pertosa, Italy, 2011. [Google Scholar]
- Color, M.; Munsell, A.H. Munsell Book of Color; Glossy Edition; NoseLab Equipment s.r.l.: Nova Milanese, Italy, 1976. [Google Scholar]
- UNI EN ISO 105-B02:2014—Textiles. Tests for Colour Fastness Colour Fastness to Artificial Light: Xenon Arc Fading Lamp Test. Available online: https://www.en-standard.eu/bs-en-iso-105-b02-2014-textiles-tests-for-colour-fastness-colour-fastness-to-artificial-light-xenon-arc-fading-lamp-test/ (accessed on 15 May 2022).
- Bianco, V.V.; Calabrese, N.; Zalum Cardon, M. Letteratura, Pittura, Cultura In “Il Carciofo e il Cardo”; Art Servizi Editoriali: Bologna, Italy, 2009; pp. 32–43. [Google Scholar]
- Portis, E.; Mauromicale, G.; Barchi, L.; Mauro, R.; Lanteri, S. Population structure and genetic variation in autochthonous globe artichoke germplasm from Sicily Island. Plant Sci. 2005, 168, 1591–1598. [Google Scholar] [CrossRef]
- Martin-Gorriz, B.; Gallego-Elvira, B.; Martínez-Alvarez, V.; Maestre-Valero, J.F. Life cycle assessment of fruit and vegetable production in the Region of Murcia (south-east Spain) and evaluation of impact mitigation. J. Clean. Prod. 2020, 265, 121656. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids and antioxidants in edible wild plants. Biol. Res. 2004, 37, 263–277. [Google Scholar] [CrossRef]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 Fatty Acids and Risk for Cardiovascular Disease. Circulation 2009, 119, 902–907. [Google Scholar] [CrossRef]
- Angelini, R.; Calabrese, N.; Ponti, I. Il Carciofo e il Cardo; Bayer CropScience srl: Milano, Italy, 2009. [Google Scholar]
- Domínguez-Fernández, M.; Irigoyen, Á.; de los Angeles Vargas-Alvarez, M.; Ludwig, I.A.; De Peña, M.P.; Cid, C. Influence of culinary process on free and bound (poly)phenolic compounds and antioxidant capacity of artichokes. Int. J. Gastron. 2021, 25, 100389. [Google Scholar] [CrossRef]
- Di Venere, D.; Pieralice, M.; Linsalata, V.; Gatto, M.A.; Sergio, L.; Calabrese, N. Biochemical evaluation of artichoke cultivars propagated by seed. Acta Hortic. 2016, 1147, 89–94. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Globe artichoke leaves and floral stems as a source of bioactive compounds. Ind. Crops Prod. 2013, 44, 44–49. [Google Scholar] [CrossRef]
- Marzi, V.; Lattanzio, V. Studi sul carciofo. In Proceedings of the 3° Congresso Internazionale sul Carciofo, Bari, Italy, 27–30 November 1979. [Google Scholar]
- Bechtold, T.; Mahmud-Ali, A.; Mussak, R. Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K.W., Ed.; Woodhead Publishing: Sawston, UK, 2007; pp. 502–533. [Google Scholar]
- De Falco, E.; Roscigno, G. Valorizzazione delle risorse territoriali attraverso il recupero di residui agricoli e piante spontanee come coloranti naturali per la bioedilizia. In Proceedings of the XII Convegno Nazionale Biodiversità 2018, Teramo, Italy, 13–15 June 2018. [Google Scholar]

| Head | Field 1 (Pertosa) | Field 2 (Caggiano) | ||
|---|---|---|---|---|
| Primary | Secondary | Primary | Secondary | |
| Parameters 1 | ||||
| Height (cm) | 7.9 ±0.5 ab | 7.2 ± 0.4 b | 8.2 ± 0.8 a | 7.7 ± 0.6 ab |
| Diameter (cm) | 7.8 ± 1.3 a | 5.8 ± 0.6 b | 7.2 ± 0.6 a | 6.0 ± 0.7 b |
| Fresh total weight (g) | 142.8 ± 44.2 a | 80.3 ± 12.3 c | 119.4 ± 7.3 ab | 94.4 ± 13.5 bc |
| Fresh clean head weight (g) | 59.2 ± 20.4 a | 32.6 ± 4.5 b | 41.8 ± 7.4 b | 28.7 ± 1.9 b |
| Fresh discarded bracts (g) | 83.6 ± 26.0 a | 47.6 ± 8.2 b | 81.8 ± 14.5 a | 65.3 ± 12.4 ab |
| Discarded bracts/total weight (%) | 58.5 ± 4.0 b | 59.3 ± 2.3 b | 68.5 ± 5.1 a | 69.2 ± 3.7 a |
| Bracts discarded (n.) | 31.8 ± 8.5 a | 19.9 ± 2.8 b | 29.0 ± 3.9 a | 25.3 ± 3.3 ab |
| Bracts water content (%) | 86.4 ± 0.4 a | 86.0 ± 0.7 a | 84.4 ± 0.6 ab | 82.8 ± 0.9 b |
| Residual dry weight (g) | 11.4 ± 3.5 a | 6.7 ± 2.1 b | 12.7 ± 2.3 a | 11.2 ± 2.1 a |
| Diameter of Head (cm) | Bracts Discarded (n.) | Fresh Total Weight of Head (g) | Fresh Clean Head (g) | Discarded Bracts (g) | |
|---|---|---|---|---|---|
| Height of head (cm) | 0.63 | 0.54 | 0.66 | 0.44 | 0.74 |
| Diameter of head (cm) | - | 0.82 | 0.90 | 0.83 | 0.83 |
| Bracts discarded (n.) | - | - | 0.86 | 0.67 | 0.88 |
| Fresh total weight of head (g) | - | - | - | 0.89 | 0.92 |
| Fresh clean head (g) | - | - | - | - | 0.69 |
| Field 1 (Pertosa) | Field 2 (Caggiano) | |
|---|---|---|
| Leaves per stem (n.) | 16.7 ± 4.0 | 13.1 ± 2.4 |
| Fresh total weight (g) | 207.1 ± 59.7 | 150.6 ± 63.7 |
| Unitary fresh weight (g) | 12.4 ± 2.1 | 11.5 ± 2.1 |
| Water content (%) | 53.8 ± 1.2 | 65.7 ± 0.8 |
| Total dry weight (g) | 93.5 ± 27.6 | 56.3 ± 21.8 |
| Unitary dry weight (g) | 5.6 ± 1.0 | 4.3 ± 0.7 |
| Technical Data 1 | Quantity |
|---|---|
| Planting density (plants ha−1) | 10,000 |
| Stems per plant (n) | 4 |
| Primary heads per plant (n) | 5 |
| Secondary heads per plant (n) | 10 |
| Leaves per stem (n) 2 | 14.9 |
| Residues 2 | |
| Fresh biomass | |
| Bracts from primary heads (tons ha−1) | 4.1 |
| Bracts from secondary heads (tons ha−1) | 5.6 |
| Leaves in natural drying phase (tons ha−1) | 7.1 |
| Dried biomass | |
| Bracts from primary heads (tons ha−1) | 0.6 |
| Bracts from secondary heads (tons ha−1) | 0.8 |
| Leaves in natural drying phase (tons ha−1) | 2.8 |
| Composition 1 | Bracts | Leaves |
|---|---|---|
| Protein (g/100 g) | 16.6 ± 0.2 a | 7.6 ± 0.1 b |
| Fat (g/100 g) | 1.6 ± 0.1 b | 5.3 ± 0.1 a |
| Carbohydrates (g/100 g) | 44.9 ± 0.1 a | 42.7 ± 0.1 a |
| of which sugars (g/100 g) | 3.3 ± 0.1 a | 3.5 ± 0.1 a |
| Fibre (g/100 g) | 17.5 ± 0.1 a | 15.8 ± 0.1 a |
| Ashes (g/100 g) | 9.8 ± 0.1 b | 18.9 ± 0.1 a |
| Water content (%) | 9.7 ± 0.3 a | 9.8 ± 0.1 a |
| Energy (Kcal/100 g) | 356.8 ± 0.1 a | 330.6 ± 0.1 a |
| (Kj/100 g) | 1493.7 ± 0.1 a | 1383.8 ± 0.1 a |
| Bracts | Leaves | ||
| Unsaturated fatty acids (%) 1 | |||
| Palmitoleic | C16:1 | 0.3 ± 0.001 | 0.3 ± 0.005 |
| Margaroleic | C17:1 | tr | 0.2 ± 0.001 |
| Oleic | C18:1 | 5.2 ± 0.005 | 20.0 ± 0.005 |
| Linoleic | C18:2 | 18.8 ± 0.010 | 15.4 ± 0.015 |
| A-Linolenic/β-Linolenic | C18:3 | 10.5 ± 0.010 | 11.9 ± 0.025 |
| Eicosenoic | C20:1 | 1.3 ± 0.010 | 1.4 ± 0.005 |
| Total 2 | 36.1 ± 0.030 b | 49.2 ± 0.010 a | |
| Saturated fatty acids (%) 1 | Bracts | Leaves | |
| Myristic | C14:1 | 0.4 ± 0.005 | 0.3 ± 0.005 |
| Palmitic | C 16:1 | 11.6 ± 0.001 | 17.7 ± 0.005 |
| Margaric | C17:1 | 0.2 ± 0.005 | 0.2 ± 0.005 |
| Stearic | C 18:1 | 1.9 ± 0.005 | 4.2 ± 0.010 |
| Arachidic | C 20:1 | 5.7 ± 0.010 | 3.4 ± 0.010 |
| Behenic | C 22:1 | 1.1 ± 0.010 | 1.5 ± 0.005 |
| Lignoceric | C 24:1 | 1.1 ± 0.001 | 3.5 ± 0.001 |
| Total 2 | 22.0 ± 0.020 d | 30.6 ± 0.040 c | |
| Composition 1 | Bracts | Leaves | DRI (mg/d) |
|---|---|---|---|
| Sodium (mg 100 g−1) | 101.6 ± 0.1 fgh | 309.3 ± 0.1 d | 2000 |
| Potassium (mg 100 g−1) | 452.6 ± 0.2 c | 4029.3 ± 50.9 a | 2000 |
| Magnesium (mg 100 g−1) | 56.1 ± 0.1 fgh | 27.5 ± 1.4 h | 375 |
| Calcium (mg 100 g−1) | 154.2 ± 0.1 efg | 1309.8 ± 55.5 b | 800 |
| Iron (µg 100 g−1) | 268.2 ± 0.2 de | 44.6 ± 0.4 gh | 14 |
| Manganese (µg kg−1) | 64.9 ± 0.1 fgh | 14.7 ± 0.4 h | 2 |
| Copper (µg 100 g−1) | 161.2 ± 0.1 efg | 5.6 ± 0.4 i | 1 |
| Zinc (µg kg−1) | 173.5 ± 0.1 ef | 20.7 ± 0.9 h | 10 |
| Main Wavelengths of the Visible UV Spectrum (nm) | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bracts | 255 | 260 | 265 | 280 | 285 | 290 | 305 | 310 | 315 | 330 | 335 | 340 | 345 | 350 | 355 | 370 | 680 | 695 | |||||||||||
| Leaves (1) | 235 | 255 | 320 | 325 | 330 | 345 | 350 | ||||||||||||||||||||||
| Leaves (2) | 305 | 310 | 315 | 320 | 325 | 330 | 335 | 340 | 345 | 350 | 355 | 360 | 370 | 375 | 380 | 385 | 390 | 395 | 400 | 405 | |||||||||
| Munsell System | ||||
|---|---|---|---|---|
| Shades | Value | Chroma | Solidity | |
| Merino Wool Baby (Standard) | ||||
| Bract extraction | 7.5Y | 7 | 4 | 4 |
| Leaves (1) | 7.5Y | 8.5 | 8 | 4 |
| Leaves (2) | 10Y | 9 | 8 | 4 |
| Wool from native Italian sheep breeds | ||||
| Wool yarn (2) (Alto Tammaro, BN) | 5Y | 8.5 | 8 | 4 |
| Carded wool (2) (Alto Tammaro, BN) | 5Y | 8.5 | 8 | 4 |
| Felt (2) (Biella) | 7.5Y | 8.5 | 8 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Falco, E.; Senatore, A.; Roscigno, G.; Pergola, M. The Artichoke “Bianco di Pertosa”: The Enhancement of Crop Residues through Environmentally Friendly Uses. Horticulturae 2022, 8, 900. https://doi.org/10.3390/horticulturae8100900
De Falco E, Senatore A, Roscigno G, Pergola M. The Artichoke “Bianco di Pertosa”: The Enhancement of Crop Residues through Environmentally Friendly Uses. Horticulturae. 2022; 8(10):900. https://doi.org/10.3390/horticulturae8100900
Chicago/Turabian StyleDe Falco, Enrica, Antonello Senatore, Graziana Roscigno, and Maria Pergola. 2022. "The Artichoke “Bianco di Pertosa”: The Enhancement of Crop Residues through Environmentally Friendly Uses" Horticulturae 8, no. 10: 900. https://doi.org/10.3390/horticulturae8100900
APA StyleDe Falco, E., Senatore, A., Roscigno, G., & Pergola, M. (2022). The Artichoke “Bianco di Pertosa”: The Enhancement of Crop Residues through Environmentally Friendly Uses. Horticulturae, 8(10), 900. https://doi.org/10.3390/horticulturae8100900






