Abstract
Banana fruit is a widely cultivated and economically important crop, and it is susceptible to mechanical damage. The effects of three different phospholipase D inhibitors (0.1% n-butanol, 0.05% 2-butanol, and 0.1% hexanal) on the cell membrane integrity and membrane lipid metabolism in wounding banana fruits during storage were investigated. The results indicated that wounded banana treated with phospholipase D inhibitors showed significant (p < 0.05) delay in the ripening and senescence process than the control group after a 9-day storage. Of the three PLD inhibitors, hexanal showed the best effect in maintaining firmness, suppressing the increase of total soluble solids (TSS) and respiration rate, and reducing malondialdehyde (MDA) content and cell membrane permeability of wounded banana fruits. Furthermore, hexanal more efficiently decreased the phospholipase D (PLD) and lipoxygenase (LOX) activities, lowered the contents of phosphatidic acid (PA) and diacylglycerol (DAG), and inhibited the reduction of phosphatidylcholine (PC) and phosphatidylinositol (PI) after 6 days of storage, compared to n-butanol or 2-butanol. These results demonstrate that application of hexanal treatment may be a reliable method to delay the senescence of harvested bananas subjected to mechanical wounding.
1. Introduction
The banana (Musa acuminata L.) is one of the most important fruit crops of the world that plays a very important role in the national economy of developing countries. It is a climacteric fruit that usually has a short postharvest shelf life under tropical conditions [1,2]. Banana fruit is easily affected by mechanical damage during postharvest handling, packaging, or transportation, which can result in a substantial decline in quality and reduce the commercial value of the fruits [3]. Deterioration of the membrane is an early and important feature of plant cells that undergo mechanical injury [4]. Many studies have confirmed that the integrity of cell membrane loss is related to lipid peroxidation or phospholipid degradation [5,6,7].
Phospholipase D (PLD), which is widely present in plant tissues, is a key enzyme that can catalyze the hydrolysis of phospholipids in cell membranes [8]. Mechanical stress stimulates PLD activity by promoting the combination of PLD and the cell membrane [9]. PLD has been shown to be activated in response to mechanical wounding in postharvest cabbage [10] and cucumber fruit [11]. PLD catalyzes the hydrolysis of the phosphodiester bond of the glycerolipid phosphatidylcholine (PC) to form phosphatidylic acid (PA) and choline. Hydrogen peroxide and free radicals are produced by the activities of a series of enzymes, and these are toxic to the cell membrane system and can lead to the deterioration of fruit quality [9]. In addition, PLD responds to mechanical stress through its hydrolysate; therefore, the inhibition of PLD activity in postharvest fruits can maintain the integrity of fruit cell membranes and reduce damage to fruits, thus improving fruit quality and delaying postharvest fruit aging.
Previous studies have found that PLD activity can be selectively inhibited by alcohols, such as n-butanol and 2-butanol, and aldehydes, such as hexanal [12,13,14]. Several technologies involving the application of PLD inhibitors to prolong the shelf life of postharvest fruit are currently being explored [14,15]. PLD inhibitors can inhibit the expression of corresponding functions or effects by blocking the production of secondary messengers, such as PA, to regulate the activity of PLD. The activities of PLD and LOX, the expression of related genes by n-Butanol treatment, were inhibited, and the change in membrane lipids content was delayed during ambient storage of pear and litchi fruits [16,17]. The 2-Butanol treatment markedly preserved the fruit quality of longan fruit, which improved its postharvest quality [18]. Earlier studies have also demonstrated that hexanal significantly improved preservative quality and prolonged the shelf life of sweet cherry [19], green-house tomato [20], guava [21], and bell peppers [22,23]. However, there is little information available regarding the effect of PLD inhibitor treatment on the membrane lipid metabolism of postharvest fruit in response to mechanical wounding stress.
In our previous study, we provided evidence that PLD is associated with banana fruit senescence and involved in the signaling pathways in response to mechanical wounding stress [24]. We also cloned the full-length cDNA of ‘Guijiao No. 6’ banana PLD genes (PLDγ, PLDα1, PLDα2, and PLDζ), registered in GenBank (accession no. MK516209, MK516210, MK516211, and MK516212), and studied the expression of banana PLD genes in response to Colletotrichum musae infection [25]. However, the report is lacking information about the effect of PLD inhibitors on membrane lipid metabolism of bananas in response to wounding stress. To gain deeper insights into the mechanism associated with the regulatory role of PLD in mechanical injury-induced senescence of fruit, the effects of treatment with n-butanol, 2-butanol, and hexanal on membrane lipid metabolism of wounded banana fruit were investigated, and the effects of PLD in biosynthetic pathways associated with fruit quality were also evaluated. The results may contribute some valuable information to maintain the quality of postharvest bananas during storage.
2. Materials and Methods
2.1. Plant Material and Sample Treatment
Banana fruits (Musa acuminate L. cv. ‘Guijiao No. 6’) were harvested at the mature green stage (firmness 378.8 N, total soluble solids content 3.5, hue angle value 109.8) from a commercial orchard in Nanning, Guangxi province in July 2021 and immediately transported to the laboratory at the Guangxi Academy of Agricultural Sciences (Nanning city, Guangxi province of China). Banana fruits of similar size and maturity period, without infection or physical injury, were selected. Each banana was punched with four holes starting from the middle of the equator and punctured at 3-cm intervals using a stainless-steel puncher [24]. Each wound was 2.0-mm deep and 4.0-mm in diameter. A total of 720 fruits were randomly divided into four groups for the following postharvest treatment: (a) Banana fruits were dipped in 0.1% (v/v) n-butanol aqueous solution for 5 min; (b) banana fruits were dipped in 0.05% (v/v) 2-butanol aqueous solution for 5 min; (c) banana fruits were dipped for 5 min in 0.1% (v/v) hexanal dissolved in water containing Tween 20; (d) banana fruits were dipped in sterile distilled water for 5 min as the control. Three biological replicates were performed. Each replicate for each treatment contained 60 fruits. These concentrations were selected on the basis of the preliminary experiments, which showed that 0.1% n-butanol, 0.05% 2-butanol, and 0.1% hexanal delayed the senescence of mechanically wounded fruits.
All the fruits were placed in unsealed polyethylene bags (0.03-mm thick) and stored at 25 °C, with 90–95% relative humidity for 15 days. During the storage period, samples were collected at 0, 3, 6, 9, 12, and 15 days, lyophilized in liquid nitrogen, and then stored at −80 °C until further analysis.
2.2. Firmness, Total Soluble Solids and Respiration Rate
The firmness of the banana fruits was measured by a penetrometer (FT-327, Facchini, Alfonsine, Italy), equipped with a cylindrical aluminum plunger (diameter = 11 mm). The banana fruits were cut from the middle after the peels were removed. Five fruits from each treatment were randomly collected each time, and two points of each cut surface were measured. The results represented the mean of 10 individual measurements and were expressed in Newtons (N). The TSS content was measured by using a hand refractometer (Fisher Scientific, Ottawa, ON, Canada) according to the AOAC method.
The respiration rate of the banana fruits was measured after various storage times. Three fruits were sealed inside a 4.2 L glass container (one container per replicative) at 25 °C for 2 h. About 1 mL of headspace gas was withdrawn from the bottle and injected into a gas chromatograph (GC-9A, Shimadzu, Kyoto, Japan). Carbon dioxide concentration in the headspace was determined with a thermal conductivity detector (TCD) and a Poropak N column (Shimadzu, Kyoto, Japan). The respiration rate was expressed on a fresh weight (FW) basis [26]. Three biological replicates were performed.
2.3. Measurement of Cell Membrane Permeability and MDA Content
Membrane permeability was measured in accordance with the method of Song et al. [27], with a slight modification. Ten peel discs from three banana fruits for each replicate were used for the determination of cell membrane permeability. Three replicates were carried out. Electrolyte leakage was determined with a conductivity meter (METTLER TOLEDO, Zurich, Switzerland).
MDA content was measured according to the method reported by Sun et al. [17], with minor modifications. Peel powder (0.25 g) from three banana fruits was homogenized for 10 min in 5 mL of 0.1% (w/v) trichloroacetic acid and centrifuged for 15 min at 15,000× g. Next, one milliliter of the supernatant was mixed with 3 mL of 0.5% 2-thiobarbituric acid. The mixture was then heated for 20 min at 95 °C and cooled in an ice bath immediately, followed by centrifugation at 15,000× g for 15 min to get a clear solution. The supernatants were collected and the absorption spectra at OD450, OD532, and OD600 were recorded, and the MDA content was calculated as the following equation: MDA content (nmol g−1 FW) = [6.45 (OD532 − OD600) − 0.56 OD450] × 5 mL/0.25 g. Three biological replicates were performed.
2.4. Assays of PLD and LOX Activities
Activities of PLD and LOX were determined as previously described [25,28]. Peel tissue (1 g) from three banana fruits for each replicate was used for extraction and activity analysis. Results were expressed as U g−1 based on the mass of the protein. Three biological replicates were performed.
2.5. Measurement of the Contents of Cellular Membrane Phospholipids
Membrane lipids of PC, PI, and PA were extracted according to the modified methods of Lin et al. [29] and Kong et al. [30]. Briefly, 5 g of banana peel tissue were ground with CHCl3-MeOH (2:1, with 0.01% butylated hydroxytoluene) mixed solvents before centrifugation with 15,000× g for 15 min at 4 °C, after ultrasonic extraction. Acetone (1 mL) was added to the homogenate and oscillated thrice (2 min each time), followed by evaporation to dryness using nitrogen. CHCl3-MeOH mixed liquor (1 mL) was added to the extract, and samples were analyzed on the Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) with a Lichrospher silica column (250 × 4 mm, 5 μm). The contents of PC, PI, and PA were expressed as mmol kg−1, based on the FW of the banana pericarp. Peel tissue (1 g) was used to measure DAG content by adopting the double antibody sandwich method using an enzyme-linked immunosorbent assay kit (Shangle, Shanghai, China). The results were expressed as mg kg−1 fresh weight of banana peel tissue.
2.6. Statistical Analysis
The experiments were performed in triplicate (n = 3) and were arranged in a completely randomized design. The results were presented as the mean ± standard error of three replicates. Experimental data was analyzed by one-way analysis of variance (ANOVA) using the SPSS 24.0 software (SPSS Inc., Chicago, IL, USA). Mean differences were established by Fisher’s least significant difference test (p < 0.05).
3. Results
3.1. Firmness, Total Soluble Solids Content and Respiration Rate
Firmness values in all the banana fruits gradually decreased throughout the storage period (Figure 1A). n-Butanol-, 2-butanol-, and hexanal-treated samples exhibited significantly higher (p < 0.05) firmness than that of the control samples after 6 days. In particular, the banana with hexanal treatment had the highest firmness value (p < 0.05) of 203.88 N on day 15, followed by the n-butanol and 2-butanol treatments. As shown in Figure 1B, the TSS content of the banana pulp tissues increased in all the groups during storage. However, the TSS content of the PLD inhibitor-treated group was lower than that in the control group, especially after day 9 (p < 0.05). In particular, TSS content of the hexanal-treated sample was the lowest (p < 0.05) (5.88%) among all the groups on days 12 and 15.
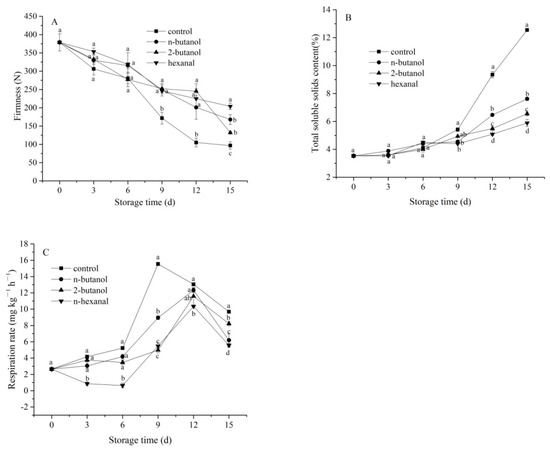
Figure 1.
Effect of phospholipase D inhibitors treatment on firmness (A), total soluble solids content (B), and respiration rate (C) of postharvest banana fruit in response to mechanical wounding stress. The vertical bars in each mean indicate the standard error (n = 3). Different letters among treatments at the same time point indicate significant differences (p < 0.05).
As shown in Figure 1C, the respiration rate of mechanically wounded banana fruits displayed a representative climacteric mode over the storage period. The respiration rate of the control group rapidly increased after day 6, reached a maximum (15.54 mg kg−1 h−1) on day 9, and finally decreased. Treatment with PLD inhibitors induced lower levels of respiration rate over the entire storage period, and respiration rate peaked on day 9 of storage, 3 days after the peak was observed in the control group. Moreover, the hexanal-treated samples had the lowest respiration rate (p < 0.05) among the three treatments on days 3, 6, 12, and 15. According to these results, respiration was strongly inhibited by hexanal treatment during the storage of banana fruit, indicating that hexanal could suppress and delay the occurrence of climacteric respiration peaks.
3.2. Cell membrane Permeability and MDA Content
Membrane permeability of fruit correlated negatively with membrane integrity and increased sharply after wounding [24]. Along with an increase in storage time, the control had the highest cell membrane permeability among all treatments (Figure 2A). The PLD inhibitors alleviated the increase in cell membrane permeability in the mechanically wounded banana fruits and significantly (p < 0.05) lowered the cell membrane permeability after 9 days. However, there was no significant difference (p > 0.05) between the three treated samples during the entire storage period. As shown in Figure 2B, the MDA content of the control banana fruit rapidly increased from 2.83 nmol g−1 to 12.11 nmol g−1 during the 15 days of storage. The MDA content of hexanal-treated samples was lowest (p < 0.05) from day 6, followed by the 2-butanol- and n-butanol-treated samples. This indicated that hexanal showed stronger effects than n-butanol and 2-butanol, with regard to the reduction of oxidative damage, maintenance of membrane integrity, and delay of tissue senescence during storage of banana fruits in response to mechanical wounding stress.
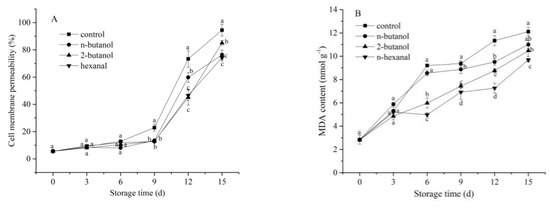
Figure 2.
Effect of phospholipase D inhibitors treatment on cell membrane permeability (A) and MDA content (B) of postharvest banana fruit in response to mechanical wounding stress. The vertical bars in each mean indicate the standard error (n = 3). Different letters among treatments at the same time point indicate significant differences (p < 0.05).
3.3. Activities of PLD and LOX
PLD is the initial enzyme involved in lipid degradation in the cell membrane. It is a key enzyme in phospholipid catalytic decomposition, which catalyzes the production of phospholipid degradation products, such as PA and choline [8]. The results of this study showed that PLD activity correlated with mechanical wounding. As shown in Figure 3A, PLD activity showed an overall increasing trend throughout the storage period. Compared with the control treatment, 2-butanol, n-butanol, and hexanal treatments significantly (p < 0.05) inhibited PLD activity by approximately 80.38%, 91.05%, and 66.16% on day 9, respectively. The PLD activity of the hexanal-treated sample was the lowest among the three treatments from day 6. A similar trend was observed for LOX activity (Figure 3A). LOX activity in the control group gradually increased from 24.23 U g−1 to 135.01 U g−1. Nevertheless, PLD inhibitor treatment significantly prevented the increase in LOX activity from day 6, compared with that observed in the control. Moreover, the hexanal-treated sample exhibited the lowest LOX activity (p < 0.05) among all treatments from day 9. These data indicate that the enhanced PLD and LOX activities could lead to phospholipid hydrolysis and lipid peroxidation of cell membranes.
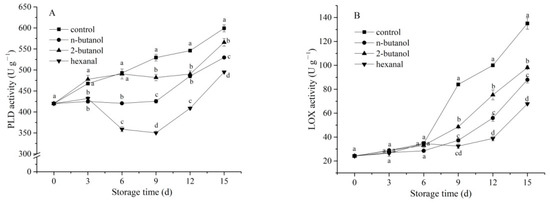
Figure 3.
Effect of phospholipase D inhibitors treatment on PLD (A) and LOX activity (B) of postharvest banana fruit in response to mechanical wounding stress. The vertical bars in each mean indicate the standard error (n = 3). Different letters among treatments at the same time point indicate significant differences (p < 0.05).
3.4. Changes in Membrane Phospholipids Component
Four phospholipids were detected in mechanically wounded banana fruits, including PA, PC, PI, and DAG. PA and DAG could be converted into each other through phosphorylation and dephosphorylation by PA phosphatase and DAG kinase [31]. As shown in Figure 4A,B, PA and DAG contents of all the groups significantly increased after day 6. Compared with those in the control, the three treated samples exhibited lower PA and DAG contents. In addition, the hexanal-treated sample possessed the lowest PA and DAG contents (p < 0.05) after day 9, followed by the 2-butanol- and n-butanol-treated samples. Figure 3C,D illustrates that the PC and PI contents in the banana fruits of the control group decreased rapidly during the 15 days of cold storage. Fruit samples treated with 2-butanol, n-butanol, and hexanal exhibited slower reduction as time progressed. Among the three treatments, the hexanal-treated sample possessed the highest PC and PI contents (p < 0.05) after day 9, followed by the 2-butanol- and n-butanol-treated samples. The results indicated that hexanal-treated mechanically wounded banana fruits had relatively lower PA and DAG contents and delayed PC and PI degradation during storage.
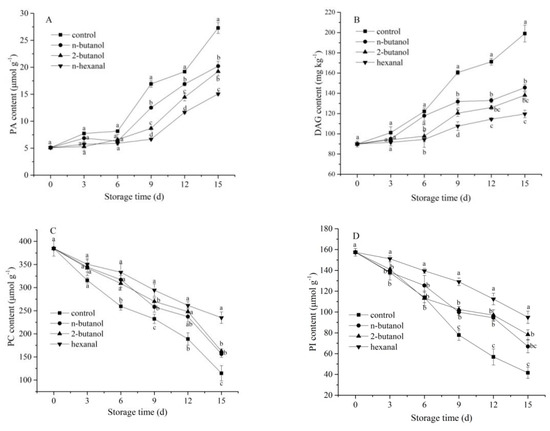
Figure 4.
Effect of phospholipase D inhibitors treatment on cell membrane PA content (A), DAG content (B), PC content (C), and PI content (D) of postharvest banana fruit in response to mechanical wounding stress. The vertical bars in each mean indicate the standard error (n = 3). Different letters among treatments at the same time point indicate significant differences (p < 0.05).
4. Discussion
PLD has been implicated in the response of plants to wounding. Previous work has conclusively demonstrated that the activation of PLD may also play a key role in mediating wound-induced lipid hydrolysis [8]. The regulation of PLD activity can significantly affect postharvest fruit quality. Different PLD inhibitors, such as primary alcohols, N-acylethanolamine, and lysophosphatidyl ethanolamine [32,33], have been used in PLD research. The regulation of PLD activity may have important impacts on the maintenance of quality of postharvest fruit. Several PLD inhibitors, such as 2-butanol, n-butanol, and hexanal, can preserve the physiological quality of postharvest fruits, thereby prolonging their shelf life [19,21,22,34]. Particular features of banana ripening and senescence are the decrease in fruit firmness and changes in sugar contents, which are regulated by respiration. The present study showed that PLD inhibition with 2-butanol, n-butanol, and hexanal maintained firmness inhibited the increase in TSS and reduced the respiration rate, especially after day 9 (p < 0.05). Among the three treatments, the hexanal-treated sample presented the highest firmness, as well as the lowest TSS content and respiration rate on day 15, suggesting that hexanal maintained the best quality of banana fruit by delaying organic matter consumption and ethylene-induced senescence. Thus, the data indicated that hexanal treatment suppressed fruit softening and the occurrence of climacteric respiration peaks, and was effective in delaying ripening and preserving the postharvest qualities of banana fruits. Previous studies have conclusively indicated the beneficial effects of hexanal on several fruits and vegetables, such as tomato [15,20], apple [35], mango [36], and strawberry [37]. These studies demonstrated that hexanal improved the shelf life and visual attributes, without impaired fruit color or compromised flavor characteristics, which effectively enhanced the shelf life and quality [38,39].
Cell membrane permeability and MDA are indicators of lipid peroxidation and can reflect lipid peroxidation extent induced by oxidative stress [40]. A gradual increase in cell membrane permeability and MDA accumulation of banana fruit was observed during storage, suggesting a gradual loss of cell membrane integrity (Figure 2). Exposure of fruits to hexanal resulted in lower cell membrane permeability and MDA content than that with fruits in the 2-butanol, n-butanol, or control treatment groups. Hexanal showed stronger effects than n-butanol and 2-butanol, with regard to the reduction of oxidative damage, maintenance of membrane integrity, and delay of tissue senescence during storage of banana fruits in response to mechanical wounding stress. Our results are in agreement with those of previous studies [30,39], which found that hexanal treatment at optimal concentrations could reduce cell membrane permeability and MDA content, thereby reducing the damage to the membrane structure caused by lipid peroxidation. In addition, hexanal does not accumulate in the plant tissues, as it is converted to hexanol, which can be further metabolized through the respiratory cycle [20]. Therefore, being a GRAS (generally regarded as safe) component applied in the food industry, hexanal could be explored in postharvest technologies for different commodities.
PLD and LOX have been suggested as crucial participants in phospholipid degradation and membrane lipid peroxidation [39]. It has been proposed that they initiate lipolytic cascades during membrane degradation. In addition, the resulting phospholipid deterioration products may be further catalyzed by LOX to produce activated oxygen and lipid peroxides, which lead to cellular compartmentalization and membrane damage [40]. The present study showed that the activities of PLD and LOX of the banana fruits treated with PLD inhibitors were significantly lower than those in the control groups after day 6 (Figure 3). Of the three treatments, hexanal induced the lowest PLD and LOX activities (p < 0.05) after 9 days. Thus, the inhibition of postharvest senescence in the banana fruits by hexanal might have been mediated by lower activity of the membrane lipolytic enzymes and suppressed breakdown of cell membrane structures. Similarly, Padmanabhan et al. [23] found that the enhanced storability of harvested bell peppers exposed to hexanal vapor might be related to the maintenance of cell membrane structure through the inhibition of PLD and LOX. The results indicate that membrane lipid metabolism is involved in the storage of banana fruits exposed to mechanical wounding stress.
Lipids make up 70% of the botanic cell membrane [28]. PC and PI are the primary structural phospholipid components of botanic cells. PLD can initiate the oxylipin pathway and decomposition of phospholipids through catalyzing the breakdown of PC into the hydrolysis products of PA and choline [34]. As presented in this study, the wounded banana fruits treated with PLD inhibitors exhibited lower PLD activity (Figure 3A) and lower levels of PA and DAG (Figure 4A,B) but higher PC and PI contents (Figure 4C,D) than those in the control fruit. Therefore, the degradation of PC and PI and the increase in PA and DAG might be related to higher cell membrane permeability, with impaired membrane integrity subsequently leading to postharvest senescence. Moreover, compared with 2-butanol or n-butanol, hexanal more effectively delayed the degradation of phospholipid components, including PC and PI, and produced less degradation products, such as PA and DAG. The results agreed with those recently reported by Cheema et al. [22], which showed that hexanal could effectively maintain integrity of the membrane structure in sweet bell peppers during storage and consequently retard senescence.
5. Conclusions
Hexanal, a PLD inhibitor, significantly inhibited membrane permeability and MDA accumulation, maintained membrane integrity, and thus delayed the mechanical injury-induced senescence of the fruit. Additionally, hexanal treatment delayed the degradation of phospholipid components, including PC and PI, and produced fewer PA and DAG degradation products. Furthermore, the activities of PLD and LOX were effectively inhibited by hexanal. The current study demonstrated that membrane lipid metabolism plays a key role in the senescence of mechanically wounded banana fruits, and hexanal treatment can alleviate developmental disorders by regulating membrane lipid metabolism. However, the molecular mechanisms and genes involved in membrane lipid metabolism during storage needs to be ascertained in future research.
Author Contributions
Conceptualization, L.L. and J.S. (Jian Sun); methodology, P.Y., X.D., L.L., X.H. and C.L.; formal analysis, J.S. (Jinfeng Sheng), M.X., J.T. and Y.T.; investigation, P.Y., L.L., D.L., Z.L., F.H. and J.L.; resources, J.S. (Jian Sun); data curation, J.S. (Jinfeng Sheng), M.X., M.H., Z.S. and T.G.; writing—original draft, L.L. and P.Y.; writing—review and editing, L.L. and X.D.; funding acquisition, J.S. (Jian Sun) and X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Guangxi Natural Science Foundation (2021GXNSFGA196001), National Natural Science Foundation of China (31860579, 32160732), earmarked fund for CARS-31, and the Foundation of Fundamental Research Project from the Guangxi Academy of Agricultural Sciences (2021YT111, 2021YT112).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Z.; Pu, H.; Shan, S.; Zhang, P.; Li, J.; Song, H.; Xu, X. Melatonin enhanced chilling tolerance and alleviated peel browning of banana fruit under low temperature storage. Postharvest Biol. Technol. 2021, 179, 111571. [Google Scholar] [CrossRef]
- Pongprasert, N.; Srilaong, V. A novel technique using 1-MCP microbubbles for delaying postharvest ripening of banana fruit. Postharvest Biol. Technol. 2014, 95, 42–45. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Shan, Y.; Zhou, Y.; Liang, L.; Qu, H.; Jiang, Y.; Chen, J. Determination of H+ and Ca2+ fluxes in cold-stored banana fruit using non-invasive micro-test technology. Postharvest Biol. Technol. 2019, 153, 169–175. [Google Scholar] [CrossRef]
- Peng, Y.; Mao, L.C. Salicylic acid, ethephon, and methyl jasmonate induce the expression of phospholipase D in mechanically-wounded cucumber. J. Hortic. Sci. Biotechnol. 2011, 86, 235–240. [Google Scholar] [CrossRef]
- Zien, C.A.; Wang, C.; Wang, X.; Welti, R. In vivo substrates and the contribution of the common phospholipase D, PLDα, to wound-induced metabolism of lipids in Arabidopsis. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2001, 1530, 236–248. [Google Scholar] [CrossRef]
- Bourtsala, A.; Farmaki, T.; Galanopoulou, D. Phospholipases Dα and δ are involved in local and systemic wound responses of cotton (G. hirsutum). Biochem. Biophys. Rep. 2017, 9, 133–139. [Google Scholar] [CrossRef]
- Wang, C.; Zien, C.A.; Afitlhile, M.; Welti, R.; Hildebrand, D.F.; Wang, X. Involvement of Phospholipase D in Wound-Induced Accumulation of Jasmonic Acid in Arabidopsis. Plant Cell 2000, 12, 2237–2246. [Google Scholar] [CrossRef]
- Wang, X. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 2005, 139, 566–573. [Google Scholar] [CrossRef]
- Whitaker, B.D. Membrane lipid metabolism and oxidative stress involved in postharvest ripening, senescence, and storage disorders of fruits. Acta Hortic. 2012, 945, 269–282. [Google Scholar] [CrossRef]
- Thammawong, M.; Umehara, H.; Kaneta, T.; Nakamura, N.; Ito, Y.; Shiina, T.; Yoshida, M.; Soga, A.; Nakano, K. Changes in Gene Expression of Harvested Cabbage in Response to Mechanical Wound Stress. J. Acta Hortic. 2013, 1005, 117–123. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Qian, C.L.; Chen, J.C.; Peng, Y.; Mao, L.C. Responses of Phospholipase D and Lipoxygenase to Mechanical Wounding in Postharvest Cucumber Fruits. J. Zhejiang Univ. Sci. 2010, 11, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Paliyath, G.; Pinhero, R.G.; Yada, R.Y.; Murr, D.P. Effect of processing conditions on phospholipase D activity of corn kernel subcellular fractions. Agric. Food Chem. 1999, 47, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.; Paliyath, G. Microarray analysis of ripening-regulated gene expression and its modulation by 1-MCP and hexanal. Plant Physiol. Biochem. 2011, 49, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Paliyath, G.; Padmanabhan, P. Preharvest and Postharvest Technologies Based on Hexanal. In Postharvest Biology and Nanotechnology; Paliyath, G., Subramanian, J., Lim, L.-T., Subramanian, K.S., Handa, A.K., Mattoo, A.K., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2019; pp. 89–101. [Google Scholar]
- Pak Dek, M.S.; Padmanabhan, P.; Subramanian, J.; Paliyath, G. Inhibition of tomato fruit ripening by 1-MCP, wortmannin and hexanal is associated with a decrease in transcript levels of phospholipase D and other ripening related genes. Postharvest Biol. Technol. 2018, 140, 50–59. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Wei, B.; Cheng, S.; Ji, S. Membrane lipid metabolism in relation to core browning during ambient storage of ‘Nanguo’ pears. Postharvest Biol. Technol. 2020, 169, 111288. [Google Scholar] [CrossRef]
- Sun, J.; You, X.R.; Li, L.; Peng, H.X.; Su, W.Q.; Li, C.B.; He, Q.G.; Liao, F. Effects of a phospholipase D inhibitor on postharvest enzymatic browning and oxidative stress of litchi fruit. Postharvest Biol. Technol. 2011, 62, 288–294. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Sun, J.; Li, C.; Sheng, J.; Zheng, F.; Liao, F.; He, X.; Liu, G.; Ling, D.; et al. Effects of 2-butanol on quality and physiological characteristics of longan fruit stored at ambient temperature. Postharvest Biol. Technol. 2015, 101, 96–102. [Google Scholar] [CrossRef]
- Sharma, M.; Jacob, J.K.; Subramanian, J.; Paliyath, G. Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 239–247. [Google Scholar] [CrossRef]
- Cheema, A.; Padmanabhan, P.; Subramanian, J.; Blom, T.; Paliyath, G. Improving quality of greenhouse tomato (Solanum lycopersicum L.) by pre- and postharvest applications of hexanal-containing formulations. Postharvest Biol. Technol. 2014, 95, 13–19. [Google Scholar] [CrossRef]
- Gill, K.S.; Dhaliwal, H.S.; Mahajan, B.V.C.; Paliyath, G.; Boora, R.S. Enhancing postharvest shelf life and quality of guava (Psidium guajava L.) cv. Allahabad Safeda by pre-harvest application of hexanal containing aqueous formulation. Postharvest Biol. Technol. 2016, 112, 224–232. [Google Scholar] [CrossRef]
- Cheema, A.; Padmanabhan, P.; Amer, A.; Parry, M.J.; Lim, L.-T.; Subramanian, J.; Paliyath, G. Postharvest hexanal vapor treatment delays ripening and enhances shelf life of greenhouse grown sweet bell pepper (Capsicum annum L.). Postharvest Biol. Technol. 2018, 136, 80–89. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Cheema, A.S.; Todd, J.F.; Lim, L.-T.; Paliyath, G. Ripening responses, fruit quality and phospholipase D gene expression in bell peppers exposed to hexanal vapor. Postharvest Biol. Technol. 2020, 170, 111317. [Google Scholar] [CrossRef]
- Li, L.; He, X.; Sun, J.; Li, C.; Ling, D.; Sheng, J.; Zheng, F.; Liu, G.; Li, J.; Tang, Y.; et al. Responses of Phospholipase D and Antioxidant System to Mechanical Wounding in Postharvest Banana Fruits. J. Food Qual. 2017, 2017, 8347306. [Google Scholar] [CrossRef]
- Yi, P.; Li, L.; Sun, J.; He, X.; Li, C.; Sheng, J.; Xin, M.; Ling, D.; Li, Z.; Tang, Y.; et al. Characterization and Expression of Phospholipase D Putatively Involved in Colletotrichum musae Disease Development of Postharvest Banana Fruit. Horticulturae 2022, 8, 312. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Sun, J.; Xin, M.; Yi, P.; He, X.; Sheng, J.; Zhou, Z.; Ling, D.; Zheng, F.; et al. Synergistic effects of ultraviolet light irradiation and high-oxygen modified atmosphere packaging on physiological quality, microbial growth and lignification metabolism of fresh-cut carrots. Postharvest Biol. Technol. 2021, 173, 111365. [Google Scholar] [CrossRef]
- Song, L.L.; Liu, H.; You, Y.L.; Sun, J.; Yi, C.; Li, Y.B.; Jiang, Y.M.; Wu, J.S. Quality deterioration of cut carnation flowers involves in antioxidant systems and energy status. Sci. Hortic. 2014, 170, 45–52. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Lin, H.; Lin, M.; Lin, Y.; Wang, H.; Hung, Y.C. Salicylic acid treatment suppresses Phomopsis longanae Chi-induced disease development of postharvest longan fruit by modulating membrane lipid metabolism. Postharvest Biol. Technol. 2020, 164, 111168. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, M.; Lin, H.; Lin, M.; Hung, Y.; Lin, Y.; Chen, Y.; Wang, H.; Ritenourd, M.A. Phomopsis longanae-induced pericarp browning and disease development of longan fruit can be alleviated or aggravated by regulation of ATP-mediated membrane lipid metabolism. Food Chem. 2018, 269, 644–651. [Google Scholar] [CrossRef]
- Kong, X.; Ge, W.; Wei, B.; Zhou, Q.; Zhou, X.; Zhao, Y.; Ji, S. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Motes, C.M.; Pechter, P.; Yoo, C.M.; Wang, Y.S.; Chapman, K.D.; Blancaflor, E.B. Differential effects of two phospholipase D inhibitors 1-butanol and Nacylethanolamine, on in vivo cytoskeletal organization and Arabidopsis seedling growth. Protoplasma 2005, 226, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.T.; Logan, K.O.; Miller, A.C.; Kropf, D.L. Phospholipase D signaling regulates microtubule organization in the fucoid alga Silvetia compressa. Plant Cell Physiol. 2007, 48, 1764–1774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheng, L.; Zhou, X.; Liu, Z.Y.; Wang, J.W.; Zhou, Q.; Wang, L.; Zhang, Q.; Ji, S.J. Changed activities of enzymes crucial to membrane lipid metabolism accompany pericarp browning in ‘Nanguo’ pears during refrigeration and subsequent shelf life at room temperature. Postharvest Biol. Technol. 2016, 117, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.; Qi, Y.; Li, R.; Yang, Y.; Yan, D.; Liu, X.; Ren, X. Postharvest applications of n-butanol increase greasiness in apple skins by altering wax composition via effects on gene expression. Postharvest Biol. Technol. 2019, 155, 111–119. [Google Scholar] [CrossRef]
- Jincy, M.; Djanaguiraman, M.; Jeyakumar, P.; Subramanian, K.S.; Jayasankar, S.; Paliyath, G. Inhibition of phospholipase D enzyme activity through hexanal leads to delayed mango (Mangifera indica L.) fruit ripening through changes in oxidants and antioxidant enzymes activity. Sci. Hortic. 2017, 218, 316–325. [Google Scholar] [CrossRef]
- Misran, A.; Padmanabhan, P.; Sullivan, J.A.; Khanizadeh, S.; Paliyath, G. Composition of phenolics and volatiles in strawberry cultivars and influence of preharvest hexanal treatment on their profiles. Can. J. Plant Sci. 2015, 95, 115–126. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Jiang, Y.L.; Qu, H.X.; Duan, X.W.; Luo, Y.B.; Jiang, W.B. Energy aspects in ripening and senescence of harvested horticultural crops. Stewart Postharvest Rev. 2007, 3, 1–5. [Google Scholar]
- Lin, Y.F.; Lin, H.T.; Lin, Y.X.; Zhang, S.; Chen, Y.H.; Jiang, X.J. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Mao, L.; Pang, H.; Wang, G.; Zhu, C. Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biol. Technol. 2007, 44, 42–47. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).