Abstract
Anthyllis barba-jovis is a salt and drought tolerant evergreen shrub, native of the western-central Mediterranean coasts, with ornamental characteristics that make it worthy to be exploited for commercial use as an ornamental and landscape plant. Therefore, the present study aimed to determine germination as affected by seed-coat, temperature, photoperiod, and seed storage period, as a first approach to introduce the species into the floriculture industry. Seeds scarified or non-scarified, recently harvested or after storage at room temperature in the dark for 12, 24, or 36 months were placed for germination in vitro on Murashige and Skoog (MS) medium, under 16 h photoperiod (LD) or continuous darkness, at 5–35 °C, at 5 °C intervals. Seed pre-treatment by mechanical scarification with sandpaper highly promoted their germinability. Seeds germinated in all treatments at varying percentages. Photoperiod had no significant effect on germination. Cardinal temperatures for germination were defined at 35 °C and 5 °C (possibly even lower, particularly for up to 1-year-old seeds, which germinated at 30–58% at 5 °C when scarified). Temperatures from 15 to 25 °C were optimal for germination of recently harvested or 1-year-old seeds (82–98% when scarified), whereas older seeds germinated at higher percentages at 20 °C (65–97% when scarified), thus long storage affected both the range of optimal temperatures for germination and the germination percentage. Storage reduced germination mostly of non-scarified seeds. Three years after harvesting A. barba-jovis seeds germinated at high percentages (77%) at 20 °C and LD when scarified, while without scarification germination was less than 10% in all treatments.
1. Introduction
The plant communities of Mediterranean ecosystems being particularly rich and hosting approximately 25,000 species of vascular plants are a large pool of species that could have a very significant impact on urban and suburban landscaping [1]. A significant number of these species are drought and heat tolerant and show high ornamental potential that makes them suitable for xeriscaping. Furthermore, many of them are associated with the ancient Greek and Roman mythology and are therefore of high interest as components of botanic, historical, or other thematic gardens.
Anthyllis barba-jovis (f. Fabaceae, Jupiter’s Beard, Spur Valerian), native to the western-central Mediterranean Basin [2,3,4] is a perennial, silvery, evergreen, shrub, up to 1.5 m tall, found in different habitats, blooming from April to June bearing big inflorescences with pale yellow flowers [5]. The leaves are feathered, alternated, odd-pinnate, small (3–5 cm), with scattered hairs of silvery grey-green colors on the top surface and silky silvery white-grey on the underside [6]. A. barba-jovis is resistant to salty sea spray and it is native in rocky, calcareous cliffs, being a protected species in France and Croatia [7,8,9]. The species is included in a risk category in Italy although the areas inhabited by the species are not affected directly by anthropogenic modification [10].
A. barba-jovis has the potential to be introduced as an ornamental landscape plant in the floriculture industry, appropriate for urban and peri-urban green spaces, including archaeological sites [11], for xeriscaping, coastal planting, and degraded soil areas. Its tolerance to high salinity, wind, heat, and drought makes it even more suitable for areas threatened by soil erosion [2,12]. Apart from this, the species is of nutritional and medicinal interest; it is important as an alternative food for bees in Mediterranean coastal areas [13] and has been studied for possible use in the pharmaceutical industry, due to its antioxidants and flavanols content in the aerial parts and seeds [14].
Temperature and photoperiod are two basic, ecological factors of high importance for seed germination, affecting both the germination percentage and speed via seed imbibition and the regulation of germination processes [15]. Therefore, there is a range of cardinal temperatures within which germination can take place. There are data on the appropriate temperature requirements of agronomic crops, but there are many native plant species of ornamental or medicinal value for which there is little information on their ‘thermal’ profile, i.e., the lowest, optimum, and maximum temperatures for seed germination [15].
A. barba-jovis has hard seeds; a common problem with hard seeds is that they either fail to germinate or germinate at very low rates, even under favorable environmental conditions [16]. As is the case with most members of Fabaceae [17], A. barba-jovis has seeds with a physical dormancy due to their water-impermeable teguments, which was not reduced one year after harvesting [12]. Seed coat impermeability prevents water absorption and, thus, embryo germination [18,19]. Usually, it is faced by seed pre-treatment with mechanical or chemical scarification before providing the adequate environmental conditions for germination. Scarification is applied by immersion in concentrated sulfuric acid or hot water, or by using sandpaper or a sharp instrument to chip or pierce the seed coat, while the optimal pre-treatment differs between species and even between seed lots [20].
Storage period and conditions are of high importance, affecting the germination process [21]. Dormancy-break can occur during dry storage of non-deep dormant seeds with physiological dormancy, while for hard coat seeds it has been proved that seed longevity is associated with their hard-impermeable surface [21]. Long-lived seed-banks are of high importance for species that inhabit areas with irregular rainfall [22]. Moreover, plants have evolved germination-regulating mechanisms to enforce dormancy depending on environmental conditions and, as a result, behavior of seeds is often heterogenous [23]. Thus, seed age and storage conditions are critical factors in seed germination studies.
In a previous study on germination of A. barba-jovis [12], it was found that the optimal temperature for germination is 20 °C, but there is no information on the effect of photoperiod and long storage on germination. Further studies on germination could enhance the use of appropriate practices in nurseries in order to facilitate the use of the species as an ornamental and landscape plant.
Therefore, the present study aimed to investigate the germinability of A. barba-jovis by determining the optimal and cardinal germination temperatures, the effect of photoperiod, and the effect of storage period on the germination of the species, with the ultimate goal being the introduction of the species to the floricultural industry.
2. Materials and Methods
2.1. Seed Material
Seeds were harvested at the stage of full maturity in late November–early December 2012, 2013, and 2014, from an A. barba-jovis plant at the Botanic Garden of Philodassiki Society at Mt. Hymettus Aesthetic Forest, Attica, Greece (lat 37°57′37.0″ Ν, long 23°47′53.4″ Ε). The seeds were left to dry spread out in trays on a laboratory bench for 15 d and then stored in glass vessels that were put into Styrofoam boxes in a cupboard (to provide darkness) under room conditions (RH 40–60%, room temperature 21 ± 2 °C) until used in germination experiments. The seed pericarp was separated manually before the use of seeds in experimental treatments.
2.2. Seed Viability
In order to determine seed viability, seeds were submitted to 2,3,5-triphenil tetrazolium chloride (TZ) staining (1.0%), at 20 °C, in D, for 24 h. A total of 100 seeds were used (25 seeds/ Magenta™ glass vessel, 4 vessels containing 25 mL of TZ-solution each) for each test. The embryo of viable seeds was colored red. Embryos that had less than ½ cotyledon colored-red or non-colored hypocotyl were considered non-viable [24]. The coloration of the embryo was observed using a portable QS.20200-P (Euromex Microscopen, Arnhem, The Netherlands) microscope.
A viability test was performed before the seeds of a particular lot were used in a germination experiment. Thus, seeds of the 2014 harvest were tested for viability immediately after their harvest, seeds of the 2013 harvest were tested after 12 months of storage, and seeds of the 2012 harvest were tested after 24 and 36 months of storage.
2.3. Mechanical Scarification
Mechanical scarification of seeds took place by rubbing them between two sheets of sandpaper (No 100), for 1 min.
2.4. In Vitro Germination
Seeds were surface-sterilized with 0.92% sodium hypochlorite (NaClO) containing 1–2 drops of 0.1% Tween 20 (polyxyethylenesorbitan monolaurate, MERCK), for 20 min under continuous stirring, followed by three rinses of 3 min each with sterile distilled water. Then, seeds were put for germination in 9 cm Petri dishes containing 20 mL of half-strength Murashige and Skoog (MS) medium [25] with 20 g L−1 sucrose solidified with 8 g L−1 agar. The pH of the medium was adjusted to 5.7–5.8 before autoclaving at 120 °C for 20 min.
Four experiments were carried out, i.e., with (a) recently harvested (15 days old) seeds of the 2014 harvest, (b) 12-month-old seeds of the 2013 harvest, (c) 24-month-old seeds of the 2012 harvest, and (d) 36-month-old seeds of the 2012 harvest. In each of these four experiments, scarified and non-scarified seeds were used to test their germination capacity under various temperature and photoperiod conditions. The response to temperature was assessed in the range 5–35 °C, in 5 °C intervals, under continuous darkness (D) or long days (LD), i.e., Petri dish cultures were incubated at 5, 10, 15, 20, 25, 30, and 35 °C, either in 24 h D or in the LD condition of 16 h cool white fluorescent light (37.5 μmol·m−2·s−1)/8 h dark.
Germination was recorded every second day for a period up to 45 d. Germination was defined as the appearance of a radicle at least 2 mm long according to the rules of the International Seed Testing Association [26], and T50 was defined as the time for 50% of the final percentage of germination.
2.5. Experimental Design and Statistical Analysis
A factorial experiment with three main factors was carried out for each storage period of seeds (i.e., four experiments). The factors were: seed scarification pre-treatment (mechanical scarification or not), temperature (5, 10, 15, 20, 25, 30, and 35 °C), and photoperiod (LD or D). The experiments followed the completely randomized design, with five replications of 20 seeds each (100 seeds per treatment).
The significance of the results was tested by one-, two-, and three-way analysis of variance. The standard errors (SE) of the treatment means were calculated and means were compared by Student’s t test at p ≤ 0.05 (JMP 11.0 software; SAS Institute Inc., Cary, NC, USA). Τo ensure the homogeneity of the variance, the data of germination percentage were arcsine transformed before the statistical analysis. Principal coordinate analysis (PCA) was also used to assist in visualizing the data.
3. Results
3.1. Seed Viability
The embryos of viable A. barba-jovis seeds submitted to TZ test were stained red (Figure 1). Seeds when scarified (Figure 2) showed very high viability even after 24 months of storage at room temperature, while their viability was reduced by about 15% after 36 months of storage (Figure 3). Scarification allowed the dye to better penetrate the seed and determine viability, while in non-scarified seeds, viability was underestimated by about 35% compared to scarified seeds in all storage treatments (Figure 3).
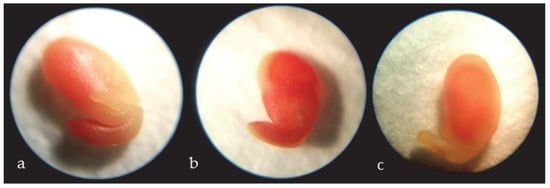
Figure 1.
Viable embryos A. barba-jovis, colored-red (cotyledon and radicle) (a,b) and non-viable embryo with ½ cotyledon colored-red (c).
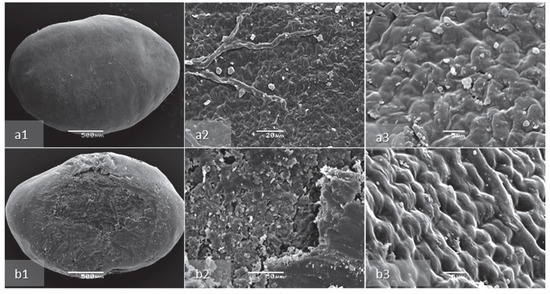
Figure 2.
Scanning electron microscopy image of non-scarified (a) and scarified by sandpaper (b) seed coat morphology of A. barba-jovis seeds, at low (1), medium (2), and high (3) magnification (×20, ×1000, ×2000, respectively). Non-scarified intact seed (a1); surface of exotesta of non-scarified seed (a2); surface of exotesta of non-scarified seed closer (a3); scarified intact seed (b1); exotesta after scarification and palisade layer (b2); surface of palisade area after the removal of exotesta (b3).
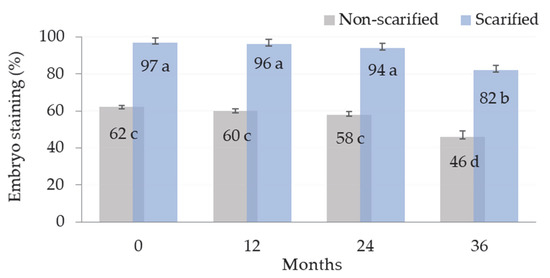
Figure 3.
Viability test results of A. barba-jovis seeds as affected by mechanical scarification (scarified or non-scarified seeds) and seed-storage period (0, 12, 24, or 36 months). F significant at p ≤ 0.001; mean (±SE) separation by Student’s t test at p ≤ 0.05; mean values followed by the same letter are not significantly different at p ≤ 0.05; n = 4, 25 seeds/vessel (total 100 seeds per treatment).
3.2. Seed Germination
Three-way ANOVA of germination percentages at each seed-storage period showed a significant interaction between the main experimental factors, i.e., scarification pre-treatment, temperature, photoperiod (three-way ANOVA results not presented). However, the data clearly showed that scarification promoted the percentage and speed of germination at all temperatures and storage periods, with the exception of germination at 35 °C (Figure 4, Figure 5 and Figure 6). Thus, we performed two-way ANOVA analyses of data separately for scarified and non-scarified seeds for each storage period.
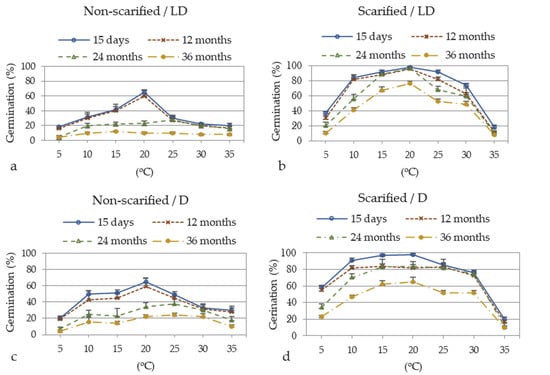
Figure 4.
Germination percentage of A. barba-jovis seeds as affected by scarification (non-scarified or scarified), incubation temperature (5, 10, 15, 20, 25, 30, or 35 °C), photoperiod (16 h light/8 h darkness: LD, continuous darkness: D) and seed-storage period (15 days and 12, 24, 36 months). Two-way ANOVA results for non-scarified seeds: 15-days storage period: Ftemperature × photoperiod NS, Ftemperature ***, Fphotoperiod **; 12-months storage period: Ftemperature×photoperiod NS, Ftemperature ***, Fphotoperiod *; 24-months storage period: Ftemperature×photoperiod NS, Ftemperature **, Fphotoperiod NS; 36-day storage period: Ftemperature×photoperiod *, Fone-way ANOVA ***. Two-way ANOVA results for scarified seeds: 15-day storage period: Ftemperature×photoperiod *, Fone-way ANOVA ***; 12-month storage period: Ftemperature×photoperiod *, Fone-way ANOVA ***; 24-month storage period: Ftemperature×photoperiod **, Fone-way ANOVA ***; 36-day storage period: Ftemperature×photoperiod **, Fone-way ANOVA ***; NS: non-significant at p ≤ 0.05; *, **, ***: significant at p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, respectively; n = 5, 20 seeds/Petri dish (total 100 seeds per treatment). SEs of the means are shown in each incubation temperature.
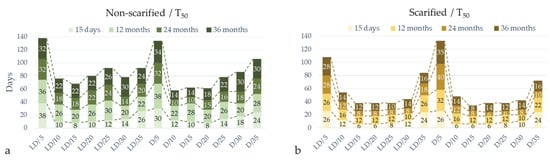
Figure 5.
Time for 50% germination (T50) of A. barba-jovis seeds as affected by scarification (non-scarified or scarified), incubation temperature (5, 10, 15, 20, 25, 30, or 35 °C), photoperiod (16 h light/8 h darkness: LD or continuous darkness: D) and seed-storage period (15 days or 12, 24, 36, months). Data (days) are marked on the bars.
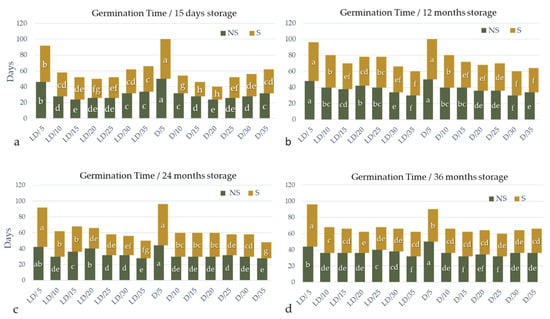
Figure 6.
Germination period of A. barba-jovis seeds as affected by scarification (non-scarified: NS or scarified: S), incubation temperature (5, 10, 15, 20, 25, 30, or 35 °C), photoperiod (16 h light/8 h darkness: LD or continuous darkness: D) and seed-storage period (15 days or 12, 24, 36, months). Two-way ANOVA results for non-scarified seeds: 15-day storage period: Ftemperature×photoperiod ***, Fone-way ANOVA ***; 12-month storage period: Ftemperature×photoperiod ***, Fone-way ANOVA ***; 24-month storage period: Ftemperature×photoperiod ***, Fone-way ANOVA ***; 36-day storage period: Ftemperature×photoperiod ***, Fone-way ANOVA ***. Two-way ANOVA results for scarified seeds: 15-day storage period: Ftemperature×photoperiod ***, Fone-way ANOVA ***; 12-month storage period: Ftemperature×photoperiod NS, Ftemperature ***, Fphotoperiod NS; 24-month storage period: Ftemperature×photoperiod NS, Ftemperature ***, Fphotoperiod NS; 36-day storage period: Ftemperature×photoperiod ***, Fone-way ANOVA ***; NS: non-significant at p ≤ 0.05; *, **, ***: significant at p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, respectively; n = 5, 20 seeds/Petri dish (total 100 seeds per treatment). Mean separation in non-scarified or scarified seeds by Student’s t, p ≤ 0.05; means followed by the same letter are not significantly different at p ≤ 0.05.
Seeds of A. barba-jovis germinated at a wide range of temperatures, 5–35 °C, with highest germination occurring in the 15–25 °C range, where scarified seeds germinated at 65–98% depending on the period of storage. Seeds harvested recently or stored for up to one year germinated at higher percentages, particularly in extreme temperatures and when non-scarified, compared to seeds stored for longer periods (Figure 4). Photoperiod had no significant effect on germination percentage, with the exception of non-scarified seeds harvested recently or stored for up to one year whose germination percentage was slightly promoted by D (significance of two-way ANOVA), particularly at suboptimal temperatures (Figure 4). Further, there was a significant interaction between photoperiod and temperature in germination percentages of scarified seeds and in most cases in germination time.
Recently harvested seeds when scarified germinated at 84–98% at 10–25 °C regardless of photoperiod, with T50 6–12 d and germination period 16–30 d (Figure 4, Figure 5 and Figure 6). At this temperature range, D induced faster completion of germination (16–26 d in D vs. 24–30 d in LD), and at 10 °C it induced higher germination percentage (91% in D vs. 84 in LD). Non-scarified seeds harvested recently reached 65% germination at 20 °C regardless of photoperiod, and their germination percentage was reduced even more at lower or higher temperatures. Germination percentage was reduced over 25 °C and below 10 °C regardless of photoperiod and scarification pre-treatment (Figure 4).
One-year-old seeds when scarified germinated at 82–96% at 10–25 °C regardless of photoperiod, with T50 12–16 d and a germination period 32–40 d (Figure 4, Figure 5, Figure 6 and Figure 7), i.e., germination was delayed compared to seeds that were recently harvested, while non-scarified seeds only reached 30–60% germination percentage, which was completed in a similar time period to recently harvested seeds, although non-scarified seeds showed delayed T50 (22–28 d). Germination percentage was reduced at temperatures lower than 10 °C and higher than 25 °C Figure 4, Figure 5 and Figure 6).
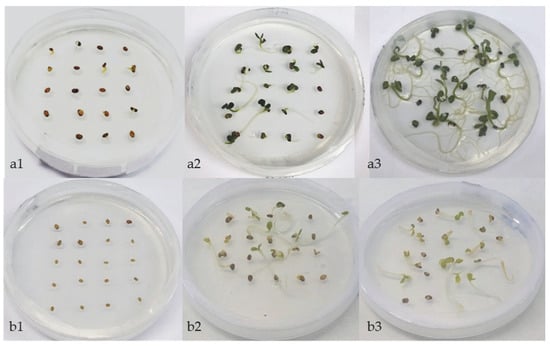
Figure 7.
Scarified seeds of A. barba-jovis, 12 months old, in Petri dishes, containing half strength MS, at 20 °C, under 16 h light/8 h darkness: LD (a) or continuous darkness: D (b), after 4 (1), 18 (2), and 28 (3) days of incubation.
Two-year-old seeds when scarified germinated at 89–97% at 15 and 20 °C in LD, while in D germination percentages were reduced to 83–84%. The germination of non-scarified seeds was reduced much more, reaching 22–38% at most favorable temperatures, i.e., 15–25 °C (Figure 4).
The highest germination percentage for three-year-old seeds when scarified was 77%, at 20 °C, in LD (Figure 4b). In D the highest germination rate of scarified seeds was 62–65% at 15–20 °C (Figure 4d). Non-scarified seeds germinated at very low percentages, the germination percentage being in the range of 22–24% at the most favorable temperatures (20–30 °C) in D (Figure 4a,c).
PCA analysis (Figure 8) transformed the original data of germination and different parameters in a set of uncorrelated new variables (principal components including eigenvalues > 1). PCA produced three components, in declining order of importance and explained 79.18% of the total variability among the germination percentages of scarified and non-scarified seeds. The first PC component (PC1) accounted for 40.91% of the total variation and was defined by T50 and GT. The second PCA component (PC2) explained another 21.59% of the total variation and was defined by the seed age, the temperature, and the germination percentage. The third PCA component (PC3) includes only photoperiod explaining 16.67% of total variation (Table 1) confirming the two-way ANOVA.
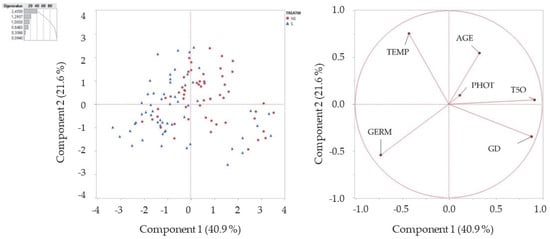
Figure 8.
Principal components calculation (PCA) of variation on germination data.

Table 1.
Results of PCA of variation on germination data.
4. Discussion
The optimal temperatures for germination of Mediterranean species overall are in the range of 15–20 °C [27,28,29] and for Anthyllis spp. 20 °C was found most favorable for germination [12,30]. In the present study it was found that the range of optimal temperatures for germination of A. barba-jovis seeds recently harvested or stored for up to one year was quite wide, i.e., 10–25 °C. This partly verifies the results of Morbidoni et al. [12], according to which scarified 1-year-old seeds of A. barba-jovis (stored at room temperature as in our work and placed for germination in the dark) germinated up to 84% at 20 °C, but germination percentage was significantly reduced at lower and higher temperatures. In our work, reduction in germination percentage under dark at 15 °C and 25 °C compared to 20 °C was found only after three years of storage at room temperatures.
At very high or very low temperatures (35 °C or 5 °C) germination was significantly reduced, especially at 35 °C; however, germination occurred at quite high percentages (63–91% respectively, depending on photoperiod conditions for up to 2-year-old scarified seeds) even at 30 °C or 10 °C, contrary to Morbidoni et al.’s [12] results where germination was much lower (5–55%, respectively) at the same thermal conditions, probably due to genotypic variation between different clones of the species. Germination of A. barba-jovis took place even at 5 °C, reaching as high as 55–58% under dark condition for scarified seeds recently harvested or stored for up to one year. Thus, although 35 °C could be defined as cardinal for germination of A. barba-jovis seeds, 5 °C may not be, as germination was significant at this temperature and lower temperatures were not tested.
There are a number of works reporting similar optimum temperature requirements for other members of Mediterranean vegetation, i.e., 15 °C for Dianthus fruticosus and Globularia alypum [31,32], 15–20 °C for Coridothymus capitatus, Origanum vulgare subsp. hirtum, and Satureja thymbra [27], 20–25 °C for Sideritis athoa [33] and Sideritis syriaca L. ssp. syriaca [34], 15–25 for Teucrium capitatum [35], 10–30 for Clinopedium nepeta and C. creticum [36,37]. However, none of the above species showed such wide thermal range for high germination percentages as A. barba-jovis. Thus, the wide thermal range, from 5 °C to 35 °C, for effective germination, and the ability to germinate rapidly, indicate the high potential of A. barba-jovis to establish in a number of countries, as an ornamental plant or a plant for landscape restoration.
Mechanical scarification by sandpaper accelerated germination and improved the overall germination percentage both under LD and D. Recently harvested seeds and seeds after one year of storage germinated at 60–65% without scarification pre-treatment at the optimal germination temperature of 20 °C. Scarification increased germination percentages two- to three-fold in all treatments, except when seeds were put for germination at 35 °C, where scarification was slightly inhibitory, possibly because scarified seeds or substrate were drying at this high temperature. The promotion of germination by scarification confirmed their physical dormancy, in agreement with previous studies on the species [12] and as is usually the case in most members of the Fabaceae family [17].
Scarifying with sandpaper was the most effective method in comparison to chemical scarification in many species [38,39,40,41,42]. Scarification with concentrated sulfuric acid as an alternative to mechanical scarification, although in many cases has been proven a very effective method resulting in high germination percentages, could be risky for the environment or the seeds [41,43]. Mechanical scarification by hand, using sandpaper or a blade, is suggested for testing the effect of dormancy break on germination in the laboratory [41], enhancing germination in several hard-seeded species, facilitating water entry and exchange of gas. It activates enzymatic hydrolysis, and the consequence is the promotion of seed germination [44]. The method is easy and, moreover, it has a low environmental footprint [45].
Seeds showed high vitality, even when stored at room temperature. Storage reduced seed germination, particularly when incubated for germination under D and when excided at two years, but even 3-year-old seeds germinated at 77% with T50 = 12 and completed their germination in 26 d, when put for germination at 20 °C and LD. Seed longevity is an adaptive aspect of high importance to ensure survival of the species in nature [46].
Anthropogenic pressure is strong in many areas of the planet and biodiversity is endangered. Landscape designers and ornamental horticulture could use a series of new, commercially attractive, agronomic, and horticultural plants of high ornamental value. Furthermore, members of Fabaceae are adapted to arid and semi-arid environments and are of high capacity to grow in poor soil being basal species for Mediterranean ecosystems [47]. Seed germination is a critical stage for undomesticated species and plants grown from seeds have high quality; therefore, ecophysiology studies could lead to the production of a high number of plants enjoying a prolonged lifespan [48,49].
5. Conclusions
The present study revealed that A. barba-jovis seeds, either recently harvested or after being stored at room temperature for three years, germinated at very high percentages when scarified by sandpaper regardless photoperiod.
For recently harvested or 1-year-old seeds, the optimal temperatures for germination were from 15 °C to 25 °C (82–98% germination for scarified seeds, 27–65% for non-scarified), while older seeds germinated at higher percentages (65–97% when scarified, 10–23% when non-scarified) at 20 °C; thus, long storage affected both the range of optimal temperatures for germination and the percentage of germination.
Therefore, this work determined the range of temperatures that induce the maximum germination percentages of A. barba-jovis seeds in relation to storage period, as well as the time required to achieve germination in relation to temperature and storage period. This knowledge is particularly useful for the promotion of the species for commercial exploitation in the floricultural industry.
Author Contributions
Conceptualization, M.P., G.V., and M.T.; methodology, M.P., K.B., G.V., and M.T.; validation, M.P., G.V., K.B., and M.T.; investigation, G.V. and M.T.; resources, M.P.; data curation, G.V. and M.T.; writing—original draft preparation, M.P., K.B., and G.V.; writing—review and editing, M.P. and K.B.; visualization, G.V. and M.T.; supervision, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Education and Lifelong Learning, NRSF 2007-2013. (Project code: MIS 380237, Project: Thalis-ARCHEOSCAPE).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Arianoutsou, M. Landscape changes in Mediterranean Ecosystems of Greece: Implications for Fire and Biodiversity issues. J. Mediterr. Ecol. 2001, 2, 165–178. [Google Scholar]
- Biondi, E.; Vagge, I.; Mossa, L. On the phytosociological importance of Anthyllis barba-jovis L. Coll. Phytosoc. 1997, 27, 95–104. [Google Scholar]
- Paradis, G. Observations sur l’espèce rare et protégée Anthyllis barba-jovis L. (Fabaceae) en Corse: Description de ses stations et phytosociologie. J. Bot. Soc. Bot. 1997, 4, 33–44. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1992; Volume 1. [Google Scholar]
- Motta, F. Nel Mondo Della Natura; Enciclopedia di Scienze Naturali: Milano, Italy, 1992; Volume 1. [Google Scholar]
- Tutin, G.T.; Heywood, V.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb., D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1972; Volume 4. [Google Scholar]
- Trinajstic, I. Anthyllis barba-jovis L. Crvena Knjiga Biljnih Vrsta Republike Hrvatske, Ministarstvo Graditeljstva i Zastite Okolisa; Zavod za Zastitu Prirode: Zagreb, Croatia, 1994; pp. 21–23. [Google Scholar]
- Danton, P.; Baffray, M. Inventaire des Plantes Protégées en France; Nathan & A.F.C.E.V.: Paris, France; Mulhouse, France, 1995; p. 294. [Google Scholar]
- Ballesteros, D.; Meloni, F.; Bacchetta, G. (Eds) Manual for the Propagation of Selected Mediterranean Native Plant Species. Ecoplantmed ENPI CBC-MED 2015, 127. Available online: http://www.ecoplantmed.eu/en/publications/propagation_manual (accessed on 28 September 2022).
- Conti., F.; Manzi, A.; Pedrotti, F. Liste Rosse Regionali delle Piante d’Italia; WWF-S.B.I.; Università di Camerino: Camerino, Italy, 1997. [Google Scholar]
- Papafotiou, M.; Bertsouklis, K.F.; Martini, A.N.; Vlachou, G.; Akoumianaki-Ioannidou, A.; Kanellou, E.; Kartsonas, E.D. Evaluation of the establishment of native Mediterranean plant species suggested for landscape enhancement in archaeological sites of Greece. Acta Hortic. 2017, 1189, 177–180. [Google Scholar] [CrossRef]
- Morbidoni, M.; Estrelles, E.; Soriano, P.; Martínez-Solís, I.; Biondi, E. Effects of environmental factors on seed germination of Anthyllis barba-jovis L. Plant Biosyst. 2008, 142, 275–286. [Google Scholar] [CrossRef]
- Benelli, G.; Benvenuti, S.; Scaramozzino, P.L.; Canale, A. Food for honeybees? Pollinators and seed set of Anthyllis barba-jovis L. (Fabaceae) in arid coastal areas of the Mediterranean basin. Saudi J. Biol. Sci. 2017, 24, 1056–1060. [Google Scholar] [CrossRef]
- Pistelli, L.; Noccioli, C.; Bertoli, A.; Scapecchi, G.; Potenza, D. Chemical composition and volatile constituents of Anthyllis barba-jovis. Nat. Prod. Res. 2007, 21, 418–425. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Some Ecophysiological Aspects of Germination. In Seeds-Physiology of Development and Germination, 2nd ed.; Bewley, J.D., Black, M., Eds.; Plenum Press: New York, NY, USA, 1994; pp. 273–292. [Google Scholar]
- Tomer, R.P.; Kumari, P. Hard seed studies in black gram (Vigna mungo L.). Seed Sci. Technol. 1991, 19, 51–56. [Google Scholar]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Spec. Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Van Staden, J.; Manning, J.C.; Kelly, K.M. Legume seeds-The structure: Function equation. In Advances in Legume Research; Stirton, C.H.L., Zarucchi, J.L., Eds.; Monographs in Systematic Botany from the Missouri Botanical Garden no. 29.; Missouri Botanical Garden: St. Louis, MO, USA, 1989; pp. 417–450. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; p. 37. [Google Scholar]
- Wang, Y.R.; Hanson, J.; Mariam, Y.W. Effect of sulfuric acid pretreatment on breaking hard seed dormancy in diverse accessions of five wild Vigna species. Seed Sci. Technol. 2007, 35, 550–559. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Breaking Seed Dormancy during Dry Storage: A Useful Tool or Major Problem for Successful Restoration via Direct Seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Gutterman, Y. Strategies of seed dispersal and germination in plants inhabiting deserts. Bot. Rev. 1994, 60, 373–425. [Google Scholar] [CrossRef]
- Ellner, S. ESS germination fractions in randomly varying environments. I. Logistic-type models. Theor. Popul. Biol. 1985, 28, 50–79. [Google Scholar] [CrossRef]
- Moore, R.P.E. Handbook on Tetrazolium Testing, 1st ed.; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 1985; p. 99. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- International Seed Testing Association. International rules for seed testing. Seed Sci. Technol. 1999, 27, 333. [Google Scholar]
- Thanos, C.A.; Kadis, C.C.; Skarou, F. Ecophysiology of germination in the aromatic plants thyme, savory and oregano (Labiatae). Seed Sci. Res. 1995, 5, 161–170. [Google Scholar] [CrossRef]
- Kadis, C.; Georghiou, K. Seed dispersal and germination behavior of three threatened endemic labiates of Cyprus. Plant Spec. Biol. 2010, 25, 77–84. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Papafotiou, M. Seed germination of Arbutus unedo, A. andrachne and their natural hybrid A. andrachnoides in relation to temperature and period of storage. HortScience 2013, 48, 347–351. [Google Scholar] [CrossRef]
- Ibanez, A.N.; Passera, C.B. Factors affecting the germination of albaida (Anthyllis cytisoides L.), a forage legume of the Mediterranean coast. J. Arid Environ. 1997, 35, 225–231. [Google Scholar] [CrossRef]
- Papafotiou, M.; Stragas, J. Seed germination and in vitro propagation of Dianthus fruticosus. L. Acta Hortic. 2009, 813, 481–484. [Google Scholar] [CrossRef]
- Bertsouklis, K.F.; Papafotiou, M. Studies on propagation of Globularia alypum L. Acta Hortic. 2010, 885, 73–77. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kalantzis, A. Seed germination and in vitro propagation of Sideritis athoa. Acta Hortic. 2009, 813, 471–476. [Google Scholar] [CrossRef]
- Thanos, C.A.; Doussi, M.A. Ecophysiology of seed germination in endemic Labiates of Crete. Israel J. Plant Sci. 1995, 43, 227–237. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N. In Vitro Seed and Clonal Propagation of the Mediterranean Aromatic and Medicinal Plant Teucrium capitatum. HortScience 2016, 51, 403–411. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, Μ.; Bertsouklis, K. Studies on seed germination and micropropagation of Clinopodium nepeta a medicinal and aromatic plant. HortScience 2019, 54, 1558–1564. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, Μ.; Bertsouklis, K. Seed germination, micropropagation from adult and juvenile origin explants and address of hyperhydricity of the Cretan endemic herb Calamintha cretica. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 1504–1518. [Google Scholar] [CrossRef]
- Travlos, I.S.; Economou, G.; Karamanos, A.I. Germination and emergence of the hard seed coated Tylosema esculentum (Burch) A. Schreib in response to different pre-sowing seed treatments. J. Arid Environ. 2007, 68, 501–507. [Google Scholar] [CrossRef]
- Talei, D.; Valdiani, A.; Abdullah, M.; Hassan, S.A. A rapid and effective method for dormancy breakage and germination of King of Bitters (Andrographis paniculata Nees.) seeds. Maydica 2012, 57, 98–105. [Google Scholar]
- Rodrigues-Junior, A.G.; Baskin, C.C.; Baskin, J.M.; Garcia, Q.S. Sensitivity cycling in physically dormant seeds of the Neotropical tree Senna multijuga (Fabaceae). Plant Biol. J. 2008, 20, 698–706. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, J.; Baskin, C.; Benakashani, F. A meta-analysis of the effects of treatments used to break dormancy in seeds of the megagenus Astragalus (Fabaceae). Seed Sci. Res. 2020, 30, 224–233. [Google Scholar] [CrossRef]
- Khuat, Q.V.; Kalashnikova, E.A.; Kirakosyan, R.N.; Nguyen, H.T.; Baranova, E.N.; Khaliluev, M.R. Improvement of In Vitro Seed Germination and Micropropagation of Amomum tsao-ko (Zingiberaceae Lindl.). Horticulturae 2022, 8, 640. [Google Scholar] [CrossRef]
- Bonner, F.T.; Franklin, T.; Karrfalt, R.P.; Robert, P. (Eds.) The Woody Plant Seed Manual. In Agricultural Handbook; No 727; U.S. Department of Agriculture, Food Service: Washington, DC, USA, 2008; p. 1223. [Google Scholar]
- Missanjo, E.; Maya, C.; Kapira, D.; Banda, H.; Kamanga-Thole, G. Effect of seed size and pretreatment methods on germination of Albizia lebbeck. Int. Sch Res. Not. 2013, 2013, 969026. [Google Scholar] [CrossRef]
- Bouteiller, X.P.; Porté, A.J.; Mariette, S.; Monty, A. Using automated sanding to homogeneously break seed dormancy in black locust (Robinia pseudoacacia L., Fabaceae). Seed Sci. Res. 2017, 27, 243–250. [Google Scholar] [CrossRef]
- Guàrdia, R.; Gallart, F.; Ninot, J.M. Soil seed bank and seedling dynamics in badlands of the Upper Llobregat basin (Pyrenees). Catena 2000, 40, 189–202. [Google Scholar] [CrossRef]
- Acherkouk, M.; Aberkani, K.; Sabre, I.A.; Maatougui, A.; Amhamdi, H.; Halou, B. Effect of seeds pretreatment and storage on improvement of the germination and emergence of Anthyllis cytisoides L. Afr. J. Agric. Res. 2017, 12, 2642–2650. [Google Scholar] [CrossRef]
- Giusti, L.; Grau, A. Inhibidores de la germinación en Atriplex cordobensis Gand. et Stucker (Chenopodiaceae). Lilloa 1983, 36, 143–149. [Google Scholar]
- Adams, C.R.; Early, M.P. Principles of Horticulture, 4th ed.; Elsevier Butterworth-Heinemann Publication: Burlington, MA, USA, 2004; p. 70. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).