Effect of Grafting Compatibility on Fruit Yield and Quality of Cantaloupe in a Mediterranean-Type Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Environmental Variables
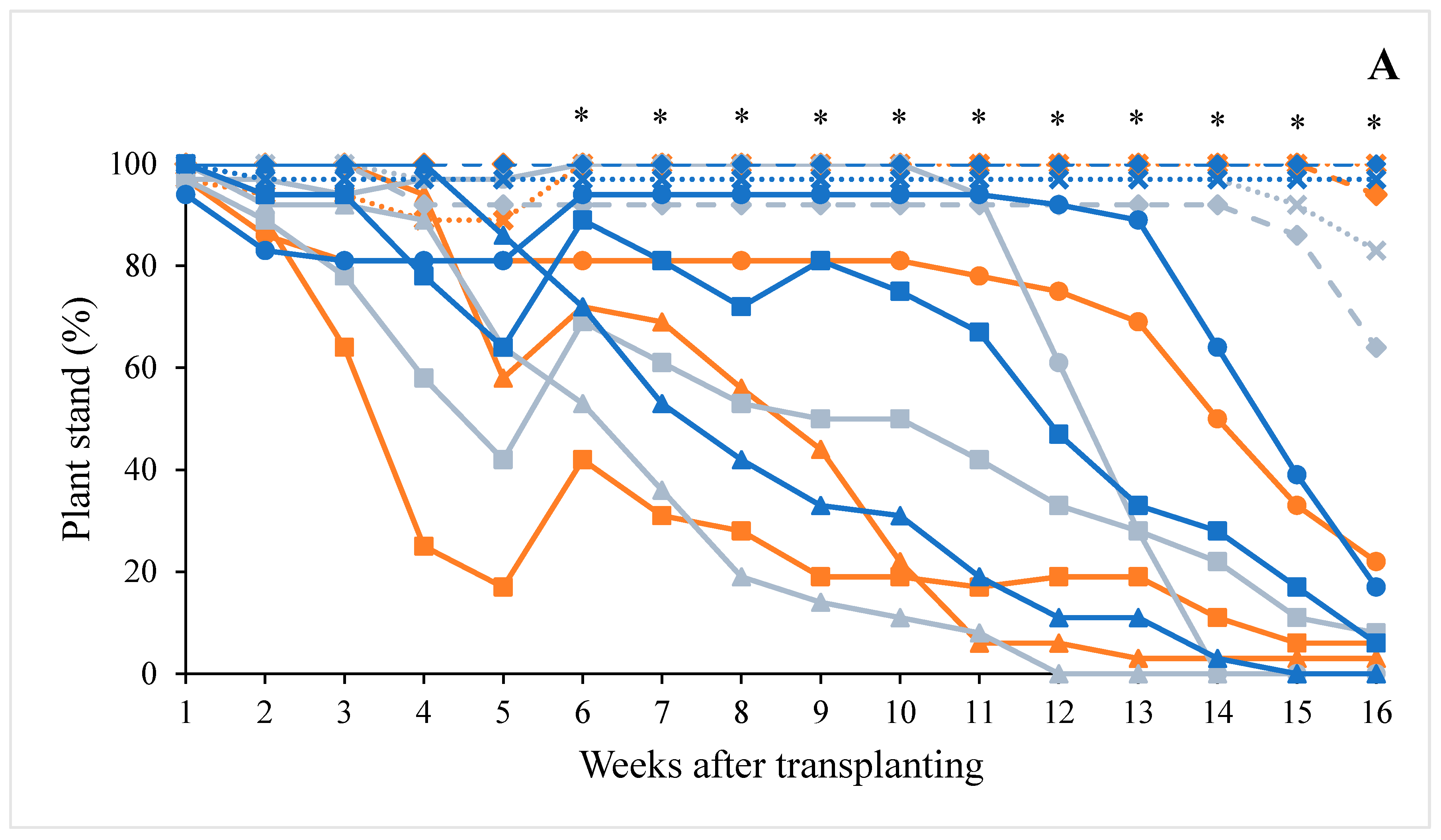
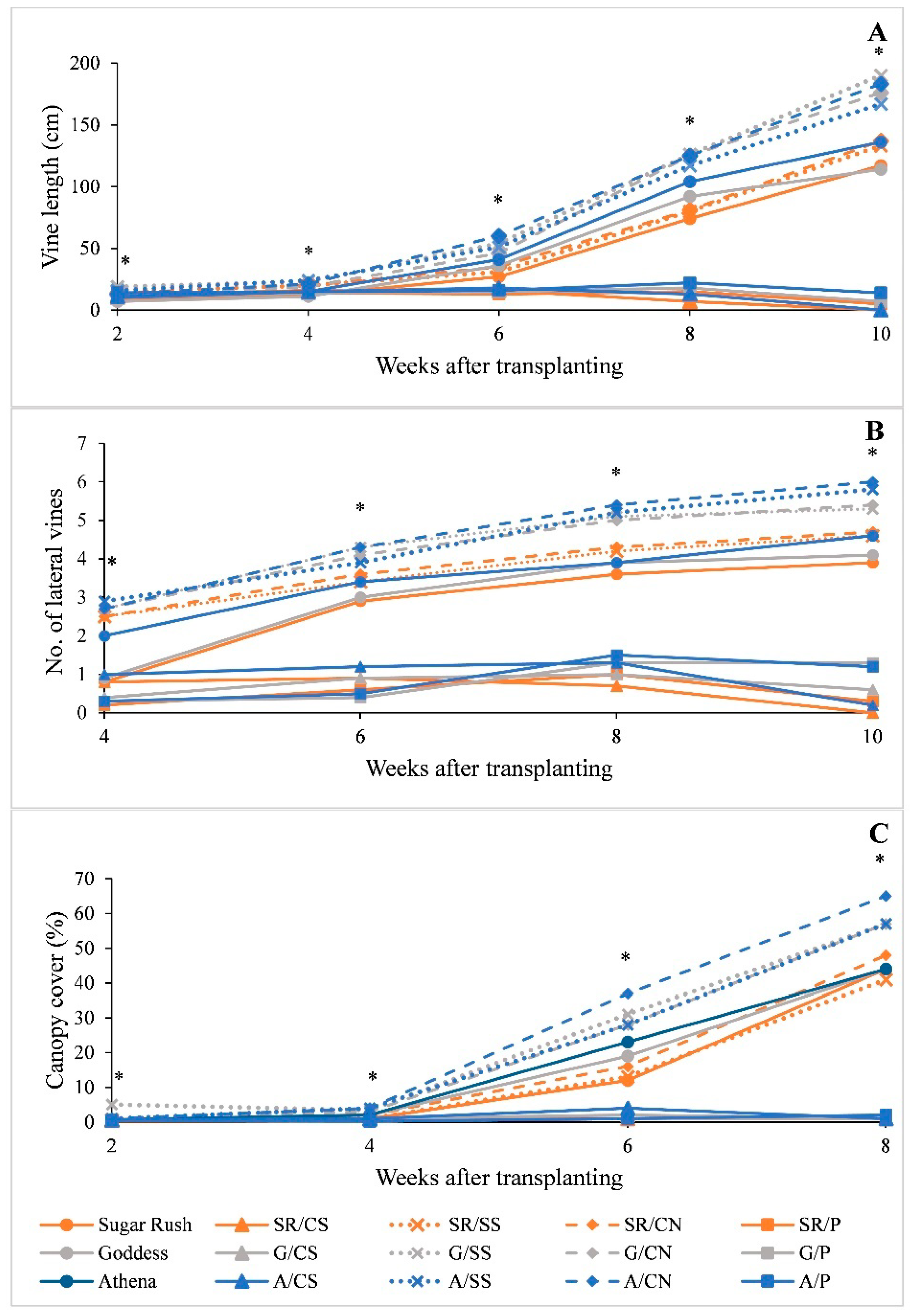
2.2. Plant Growth Assessments
2.3. Fruit Harvest
2.4. Fruit Quality
2.5. Statistical Analysis
3. Results
3.1. Environmental Variables
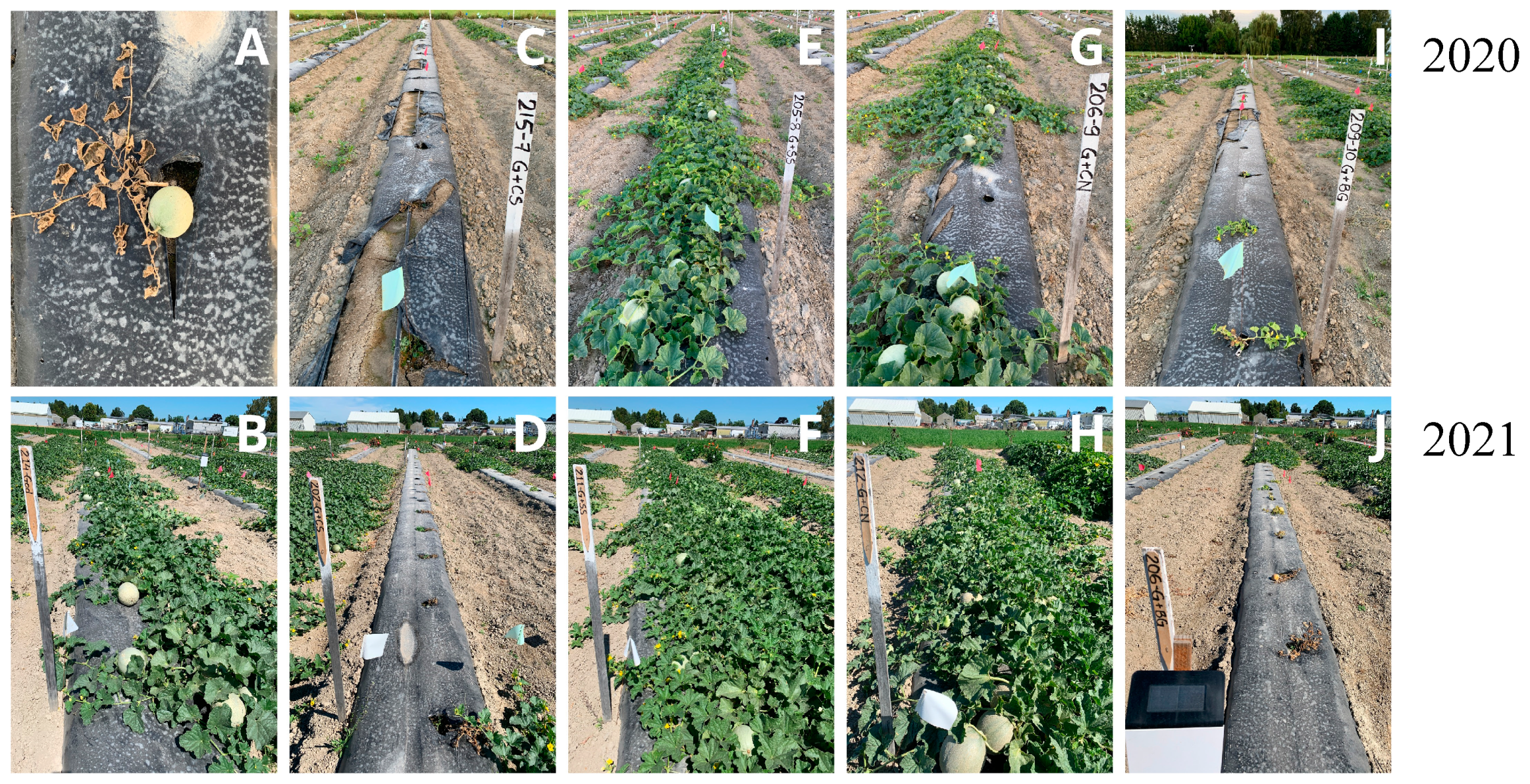
3.2. Plant Growth
3.3. Fruit Harvest
3.4. Fruit Quality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duncan, J.; Ewing, J. Specialty Melon Production for Small and Direct-Market Growers. 2015. Available online: https://attra.ncat.org/wp-content/uploads/2019/05/specialtymelon.pdf? (accessed on 10 October 2021).
- USDA ERS-Fruit and Tree Nuts Yearbook Tables. Available online: https://www.ers.usda.gov/data-products/fruit-and-tree-nuts-data/fruit-and-tree-nuts-yearbook-tables/#Melons (accessed on 4 April 2022).
- Maynard, D.N.; Hochmuth, G.J. Knott’s Handbook for Vegetable Growers, 5th ed.; J. Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hartz, T.; Cantwell, M.; Mickler, J.; Mueller, S.; Stoddard, S.; Turini, T. Cantaloupe Production in California. 2008. Available online: https://anrcatalog.ucanr.edu/pdf/7218.pdf (accessed on 25 February 2021).
- Nair, A.; Krzton-Presson, J. Commercial Melon Production. 2016. Available online: https://store.extension.iastate.edu/product/Commercial-Melon-Production (accessed on 12 September 2021).
- Gordon, R. Researchers Find That Grafting Increases Melon Yields. 2015. Available online: https://www.growingproduce.com/vegetables/melon-research-produces-higher-yields-with-grafting/ (accessed on 22 April 2022).
- Fita, A.; Picó, B.; Nuez, F. Melon Roots under Stress: Melon Vine Decline. Plant Stress 2007, 1, 93–104. [Google Scholar]
- Martyn, R.D. Late-season vine declines of melons: Pathological, cultural or both? Acta Hortic. 2007, 731, 345–356. [Google Scholar] [CrossRef]
- Kubota, C.; McClure, M.A.; Kokalis-Burelle, N.; Bausher, M.G.; Rosskopf, E.N. Vegetable Grafting: History, Use, and Current Technology Status in North America. HortScience 2008, 43, 1664–1669. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Yang, L.; Yan, W.; He, Z. Study on cold tolerance of different rootstocks of melon seedlings. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 052032. [Google Scholar] [CrossRef]
- Ohletz, J.L.; Loy, J.B. Grafting melons increases yield, extends the harvest season, and prevents sudden wilt in new England. HortTechnology 2021, 31, 101–114. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Colla, G.; Suárez, C.M.C.; Cardarelli, M.; Rouphael, Y. Improving nitrogen use efficiency in melon by grafting. HortScience 2010, 45, 559–565. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Fiorillo, A.; Rouphael, Y.; Rea, E. Enhancing nitrogen use efficiency in cucurbitaceae crops by grafting. Acta Hortic. 2012, 952, 863–869. [Google Scholar] [CrossRef]
- Crinò, P.; Bianco, C.L.; Rouphael, Y.; Colla, G.; Saccardo, F.; Paratore, A. Evaluation of rootstock resistance to Fusarium wilt and gummy stem blight and effect on yield and quality of a grafted ‘Inodorus’ melon. HortScience 2007, 42, 521–525. [Google Scholar] [CrossRef]
- Guan, W.; Zhao, X.; Huber, D.J. Grafting with an interspecific hybrid squash rootstock accelerated fruit development and impaired fruit quality of Galia melon. HortScience 2015, 50, 1833–1836. [Google Scholar] [CrossRef]
- Kolayli, S.; Kara, M.; Tezcan, F.; Erim, F.B.; Sahin, H.; Ulusoy, E.; Aliyazicioglu, R. Comparative study of chemical and biochemical properties of different melon cultivars: Standard, hybrid, and grafted melons. J. Agric. Food Chem. 2010, 58, 9764–9769. [Google Scholar] [CrossRef] [PubMed]
- Nisini, P.T.; Colla, G.; Granati, E.; Temperini, O.; Crinò, P.; Saccardo, F. Rootstock resistance to Fusarium wilt and effect on fruit yield and quality of two muskmelon cultivars. Sci. Hortic. 2002, 93, 281–288. [Google Scholar] [CrossRef]
- Andrews, P.K.; Marquez, C.S. Graft Incompatibility. In Horticultural Reviews; John Wiley & Sons: New York, NY, USA, 1993; Volume 15, pp. 183–232. [Google Scholar]
- Lee, J.M. Cultivation of grafted vegetables I. Current status, grafting methods, and benefits. HortScience 1994, 29, 235–239. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Sakata, Y.; López-Galarza, S.; Maroto, J.V.; Lee, S.-G.; Huh, Y.C.; Sun, Z.; Miguel, A.; King, S.R.; et al. Cucurbit Grafting. Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Edelstein, M.; Burger, Y.; Horev, C.; Porat, A.; Meir, A.; Cohen, R. Assessing the effect of genetic and anatomic variation of cucurbita rootstocks on vigour, survival and yield of grafted melons. J. Hortic. Sci. Biotechnol. 2004, 79, 370–374. [Google Scholar] [CrossRef]
- Camalle, M.D.; Sikron, N.; Zurgil, U.; Khadka, J.; Pivonia, S.; Pěnčík, A.; Novák, O.; Fait, A.; Tel-Zur, N. Does scion–rootstock compatibility modulate photoassimilate and hormone trafficking through the graft junction in melon–pumpkin graft combinations? Plant Sci. 2021, 306, 110852. [Google Scholar] [CrossRef]
- Espen, L.; Cocucci, M.; Sacchi, G.A. Differentiation and functional connection of vascular elements in compatible and incompatible pear/quince internode micrografts. Tree Physiol. 2005, 25, 1419–1425. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Taji, A.; Backhouse, D.; Oda, M. Anatomy and physiology of graft incompatibility in solanaceous plants. J. Hortic. Sci. Biotechnol. 2008, 83, 581–588. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, S.; Shu, S.; Xu, Y.; Sun, J. Isolation and expression pattern analysis of CmRNF5 and CmNPH3L potentially involved in graft compatibility in cucumber/pumpkin graft combinations. Sci. Hortic. 2018, 227, 92–101. [Google Scholar] [CrossRef]
- Schöning, U.; Kollmann, R. Phloem translocation in regenerating in vitro heterografts of different compatibility. J. Exp. Bot. 1997, 48, 289–295. [Google Scholar] [CrossRef]
- Xiong, M.; Liu, C.; Guo, L.; Wang, J.; Wu, X.; Li, L.; Bie, Z.; Huang, Y. Compatibility evaluation and anatomical observation of melon grafted onto eight cucurbitaceae species. Front. Plant Sci. 2021, 12, 762889. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Zhao, X.; Hassell, R.; Thies, J. Defense mechanisms involved in disease resistance of grafted vegetables. HortScience 2012, 47, 164–170. [Google Scholar] [CrossRef]
- Cantaloup Grades and Standards. Agricultural Marketing Service. Available online: https://www.ams.usda.gov/grades-standards/cantaloup-grades-and-standards (accessed on 30 December 2021).
- Davis, A.R.; Perkins-Veazie, P.; Hassell, R.; Levi, A.; King, S.R.; Zhang, X. Grafting Effects on Vegetable Quality. HortScience 2008, 43, 1670–1672. [Google Scholar] [CrossRef]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of Grafting on Product Quality of Fruit Vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Guan, W.; Zhao, X.; Huber, D.J.; Sims, C.A. Instrumental and sensory analyses of quality attributes of grafted specialty melons. J. Sci. Food Agric. 2015, 95, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Dabirian, S.; Inglis, D.; Miles, C.A. Grafting watermelon and using plastic mulch to control verticillium wilt caused by Verticillium Dahliae in Washington. HortScience 2017, 52, 349–356. [Google Scholar] [CrossRef]
- Devi, P.; Perkins-Veazie, P.; Miles, C.A. Rootstock and plastic mulch effect on watermelon flowering and fruit maturity in a Verticillium Dahliae–infested field. HortScience 2020, 55, 1438–1445. [Google Scholar] [CrossRef]
- Wimer, J.; Inglis, D.; Miles, C. Evaluating grafted watermelon for verticillium wilt severity, yield, and fruit quality in Washington state. HortScience 2015, 50, 1332–1337. [Google Scholar] [CrossRef]
- The Washington Agricultural Weather Network. Available online: http://weather.wsu.edu/ (accessed on 4 November 2021).
- Soil Survey: Skagit County Washington. 1960. Available online: https://www.nrcs.usda.gov/Internet/FSE_MANUSCRIPTS/washington/skagitWA1960/skagitWA1960.pdf (accessed on 3 September 2021).
- Zhao, X.; Guan, W. Rootstock Selections and Important Considerations in Melon and Watermelon Grafting. 2018. Available online: http://www.vegetablegrafting.org/wp/wp-content/uploads/2018/04/Rootstock3-30-18.pdf (accessed on 20 May 2020).
- Miles, C.; Hesnault, L.; Johnson, S.; Kreider, P.; Dabirian, S. Vegetable Grafting: Watermelon. 2016. Available online: https://s3.wp.wsu.edu/uploads/sites/2709/2021/05/FS100E-1.pdf (accessed on 23 September 2021).
- Patrignani, A.; Ochsner, T.E. Canopeo: A powerful new tool for measuring fractional green canopy cover. J. Agron. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- OECD Fruit and Vegetable Scheme. Guidelines on Objective Tests to Determine Quality of Fruit and Vegetables, Dry and Dried Produce. 2018. Available online: https://www.oecd.org/agriculture/fruit-vegetables/publications/guidelines-on-objective-tests.pdf (accessed on 5 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 12 December 2021).
- Novel Watermelon Rootstock Knocks out Disease and Pests. Available online: https://www.ars.usda.gov/news-events/news/research-news/2019/novel-watermelon-rootstock-knocks-out-disease-and-pests/ (accessed on 3 February 2022).
- King, S.R.; Davis, A.R.; Zhang, X.; Crosby, K. Genetics, breeding and selection of rootstocks for solanaceae and cucurbitaceae. Sci. Hortic. 2010, 127, 106–111. [Google Scholar] [CrossRef]
- Aloni, B.; Karni, L.; Deventurero, G.; Levin, Z.; Cohen, R.; Katzir, N.; Lotan-Pompan, M.; Edelstein, M.; Aktas, H.; Turhan, E.; et al. Physiological and biochemical changes at the rootstock-scion interface in graft combinations between cucurbita rootstocks and a melon scion. J. Hortic. Sci. Biotechnol. 2008, 83, 777–783. [Google Scholar] [CrossRef]
- Beltrán, R.; Vicent, A.; Garcia-Jiménez, J.; Armengol, J. Quantification of Monosporascus cannonballus ascospores in muskmelon fields in eastern Spain. J. Phytopathol. 2007, 155, 248–250. [Google Scholar] [CrossRef]
- Cohen, R.; Pivonia, S.; Burger, Y.; Edelstein, M.; Gamliel, A.; Katan, J. Toward integrated management of Monosporascus wilt of melons in Israel. Plant Dis. 2000, 84, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Martyn, R.D.; Miller, M.E. Monosporascus root rot and vine decline: An emerging disease of melons worldwide. Plant Dis. 1996, 80, 716. [Google Scholar] [CrossRef]
- Stanghellini, M.E.; Kim, D.H.; Rasmussen, S.L. Ascospores of Monosporascus cannonballus: Germination and distribution in cultivated and desert soils in Arizona. Phytopathology 1996, 86, 509. [Google Scholar] [CrossRef]
- Devi, P.; Perkins-Veazie, P.; Miles, C. Impact of grafting on watermelon fruit maturity and quality. Horticulturae 2020, 6, 97. [Google Scholar] [CrossRef]
- Soteriou, G.A.; Kyriacou, M.C.; Siomos, A.S.; Gerasopoulos, D. Evolution of watermelon fruit physicochemical and phytochemical composition during ripening as affected by grafting. Food Chem. 2014, 165, 282–289. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Soteriou, G.A.; Rouphael, Y.; Siomos, A.S.; Gerasopoulos, D. Configuration of watermelon fruit quality in response to rootstock-mediated harvest maturity and postharvest storage. J. Sci. Food Agric. 2016, 96, 2400–2409. [Google Scholar] [CrossRef]
- Sánchez, E.; Pollock, R.; Elkner, T.; Butzler, T.; Di Gioia, F. Fruit yield and physicochemical quality evaluation of hybrid and grafted field-grown muskmelon in Pennsylvania. Horticulturae 2021, 7, 69. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Massa, D.; Salerno, A.; Rea, E. Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 146–152. [Google Scholar] [CrossRef]

| Parameter | Assessment Period | Assessment Criteria |
|---|---|---|
| Plant stand | Weekly from one week after transplanting (WAT) until 16 WAT (2020) or 13 WAT (2021) | Total number of live plants per plot |
| Vine length | Every two weeks from 2 WAT until 10 WAT | Measured from the base of the crown to the tip of the longest vine from center six plants of each plot |
| Number of lateral vines | Every two weeks from 4 WAT until 10 WAT | Total number of vines growing from the main vine for six plants in the center of each plot |
| Percent canopy cover | Every two weeks from 2 WAT until 8 WAT | Measured for the center six plants of each plot. After hand weeding, digital photographs were taken at a 65-cm height centered above the plant in each plot. Images were analyzed using Canopeo application (ver. 2.0; Canopeo, Stillwater, OK) developed by the Soil Physics Research Group at Oklahoma State University [41]. |
| Environmental Variables z | 2020 y | 2021 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| June | July | August | September | October | Av./Total | May | June | July | August | September | Av./Total | |
| Average daily air temperature (°C) | 14.6 | 16.4 | 16.6 | 15.7 | 14.0 | 15.5 | 12.8 | 17.1 | 17.1 | 17.0 | 13.3 | 15.5 |
| Average daily min air temperature (°C) | 10.3 | 11.0 | 10.3 | 10.0 | 10.1 | 10.3 | 7.4 | 11.0 | 11.2 | 11.5 | 4.7 | 9.2 |
| Average daily max air temperature (°C) | 19.4 | 21.8 | 23.0 | 21.7 | 18.4 | 20.9 | 18.0 | 23.1 | 24.1 | 23.5 | 21.8 | 22.1 |
| Total air thermal accumulation | 134 | 333 | 540 | 715 | 736 | 2458 | 32 | 244 | 481 | 712 | 719 | 2188 |
| Total solar radiation (MJ·m−2) | 565 | 685 | 636 | 374 | 36 | 2296 | 225 | 718 | 734 | 515 | 42 | 2234 |
| Average relative humidity (%) | 79 | 77 | 77 | 81 | 93 | 81.4 | 77 | 73 | 75 | 77 | 75 | 75.4 |
| Total rainfall (mm) | 78.7 | 21.0 | 16.3 | 27.2 | 0.75 | 144 | 11.4 | 23.1 | 0 | 24.4 | 0 | 58.9 |
| Average soil temperature (°C) x | 19.3 | 21.5 | 20.9 | 17.7 | 15.9 | 19.1 | 17.8 | 21.9 | 22.5 | 20.5 | 17.3 | 20 |
| Total soil thermal accumulation | 249 | 608 | 948 | 1182 | 1212 | 4199 | 47 | 402 | 794 | 1123 | 1150 | 3516 |
| Average volumetric water content (cm3·cm−3) x | 0.28 | 0.32 | 0.32 | 0.32 | 0.34 | 0.3 | 0.21 | 0.28 | 0.27 | 0.28 | 0.31 | 0.3 |
| Plant Stand | Vine Length | No. of Lateral Vines | Canopy Cover | |||
|---|---|---|---|---|---|---|
| Treatment | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Year | 0.91 | <0.0001 | <0.0001 | <0.0001 | ||
| Week | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Treatment × Year | <0.0001 | 0.08 | 0.23 | 0.52 | ||
| Year × Week | <0.0001 | 0.0006 | 0.04 | <0.0001 | ||
| Treatment × Week | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Treatment × Year × Week | 0.01 | <0.0001 | <0.0001 | <0.0001 | ||
| Days to first harvest | Yield/ha | Total fruit no per plant | Fruit wt | |||
| Treatment | <0.0001 | <0.0001 | 0.003 | <0.0001 | ||
| Year | <0.0001 | <0.0001 | <0.0001 | 0.01 | ||
| Treatment × Year | 0.52 | 0.40 | 0.74 | 0.001 | ||
| Fruit length | Fruit diameter | TSS | Firmness | TA | pH | |
| Treatment | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.002 | 0.002 |
| Year | 0.02 | 0.53 | 0.02 | <0.0001 | <0.0001 | <0.0001 |
| Treatment × Year | 0.36 | 0.08 | 0.02 | 0.002 | 0.17 | 0.38 |
| Treatment z | Days to First Harvest | Yield (t·ha−1) | Tot. Fruit Number per Plant | Weight per Fruit (kg) | |
|---|---|---|---|---|---|
| 2020 | 2021 | ||||
| Sugar Rush | 97 abc y | 11.4 d | 3.9 bc | 0.6 d | 0.9 d |
| SR/SS | 105 c | 18.8 cd | 4.5 abc | 1.1 bc | 1.1 cd |
| SR/CN | 102 bc | 19.4 cd | 4.5 abc | 1.0 bc | 1.1 cd |
| Goddess | 90 a | 15.9 d | 3.0 c | 1.5 ab | 1.5 b |
| G/SS | 92 ab | 33.6 ab | 4.9 abc | 1.8 a | 1.8 a |
| G/CN | 89 a | 27.9 bc | 4.3 abc | 1.8 a | 1.8 a |
| Athena | 100 abc | 18.8 cd | 4.3 abc | 0.9 cd | 1.4 bc |
| A/SS | 107 c | 31.7 ab | 5.0 ab | 1.8 a | 1.6 ab |
| A/CN | 103 bc | 40.2 a | 5.9 a | 2.0 a | 1.8 a |
| p-value | <0.0001 | <0.0001 | 0.004 | <0.0001 | <0.0001 |
| Treatment z | Fruit Length (cm) | Fruit Diameter (cm) | Fruit Firmness (N) | TSS (%) | TA (g·L−1) | pH | ||
|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | |||||
| Sugar Rush | 12 e y | 11 c | 56.6 a | 47.3 a | 13.1 abc | 15.7 a | 0.56 ab | 7.0 a |
| SR/SS | 14 d | 13 b | 49.0 b | 48.4 a | 14.7 a | 15.0 ab | 0.60 ab | 6.8 ab |
| SR/CN | 13 d | 13 b | 48.2 b | 46.8 a | 14.8 a | 15.1 a | 0.51 b | 7.0 a |
| Goddess | 15 bc | 14 ab | 41.5 c | 31.4 b | 11.9 bc | 11.6 c | 0.64 ab | 6.6 ab |
| G/SS | 16 a | 15 a | 42.3 c | 33.3 b | 12.5 abc | 12.4 c | 0.55 ab | 6.7 ab |
| G/CN | 16 ab | 15 a | 43.6 c | 32.4 b | 12.4 abc | 12.7 c | 0.54 ab | 6.8 ab |
| Athena | 14 cd | 13 b | 44.5 c | 30.9 b | 10.6 c | 12.5 c | 0.65 ab | 6.5 b |
| A/SS | 16 a | 15 a | 49.7 c | 39.0 ab | 13.9 ab | 13.2 bc | 0.77 a | 6.4 b |
| A/CN | 16 a | 15 a | 43.5 c | 37.9 ab | 12.6 abc | 13.1 bc | 0.77 a | 6.5 b |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0004 | <0.0001 | 0.002 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, S.; Mattupalli, C.; Miles, C. Effect of Grafting Compatibility on Fruit Yield and Quality of Cantaloupe in a Mediterranean-Type Climate. Horticulturae 2022, 8, 888. https://doi.org/10.3390/horticulturae8100888
Shrestha S, Mattupalli C, Miles C. Effect of Grafting Compatibility on Fruit Yield and Quality of Cantaloupe in a Mediterranean-Type Climate. Horticulturae. 2022; 8(10):888. https://doi.org/10.3390/horticulturae8100888
Chicago/Turabian StyleShrestha, Srijana, Chakradhar Mattupalli, and Carol Miles. 2022. "Effect of Grafting Compatibility on Fruit Yield and Quality of Cantaloupe in a Mediterranean-Type Climate" Horticulturae 8, no. 10: 888. https://doi.org/10.3390/horticulturae8100888
APA StyleShrestha, S., Mattupalli, C., & Miles, C. (2022). Effect of Grafting Compatibility on Fruit Yield and Quality of Cantaloupe in a Mediterranean-Type Climate. Horticulturae, 8(10), 888. https://doi.org/10.3390/horticulturae8100888





