Abstract
Due to the growing knowledge about the microorganism–plant relationship, medicinal plants have gained great attention in their bio fertilization programs using biostimulants based on microorganisms. Plectranthus amboinicus (Lour.) Spreng. is a perennial herb belonging to the family Lamiaceae and has therapeutic and nutritional properties attributed to its natural phytochemical compounds, which are highly valued in the pharmaceutical industry. A pot experiment was conducted to evaluate the efficiency of Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) yeast strains in concentrations of 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL−1 on the growth performance, productivity, and antioxidant activity of P. amboinicus plants. Yeast applications promoted growth attributes, nutritional value, and antioxidant activity in P. amboinicus leaves. Candida apicola exhibited the greatest root growth, herb weight, and essential oil production; it also stimulated carbohydrates, protein, and mineral content, as well as DPPH and FRAP activities. Whereas Rhodotorula muciligenese recorded the lowest values in this respect, among the concentrations used, the 1 × 107 CFU mL−1 concentration showed the highest values in this respect. These new findings showed that the foliar application of Candida apicola not only maximized the growth and productivity but also maximized the nutritional value and antioxidant activity of P. amboinicus.
1. Introduction
Despite the importance of chemical fertilizers in the agriculture sector and crop production, they cause air and groundwater pollution, as well as having a pivotal role in climate change [1]. Agroecosystem protection and plant development stimulation are critical issues, so it is necessary to alternate this traditional agricultural technique with a safer one. Huge efforts have been made to reduce reliance on such chemical fertilizers by developing biologically based biostimulants as an alternative. Using biostimulants is recommended as a unique approach to enhance the sustainability of agricultural systems and minimize chemical fertilizer usage [2]. Biological biostimulants are living microorganisms that can promote plant growth by several techniques, such as providing essential amino acids and vitamins, enhancing the nutrient supply and uptake, as well as improving root system development [3,4,5].
Yeasts are eukaryotic organisms that are classified as fungi, with about 1,500 species described [6]. Despite yeast populations being generally high in the rhizosphere and soil [7], reports about the role of yeasts as plant growth promoters are very limited [8,9]. Yeasts have exhibited environmental niche adaptations, including the capacity to colonize the rhizosphere alongside plant roots [7,8,10]. Many reports indicate that yeast strains can promote plant growth by a variety of mechanisms, such as phosphate solubilization [11], producing siderophore [12], phytohormones [13], and nitrogen and sulfur oxidation [7], as well as mycorrhizal-root colonization promotion [14]. Additionally, yeast can be used as a biocontrol agent [15]. Yeast stimulated active ingredient synthesis in Silybum marianum L. [16], increased productivity and essential oil quality [3], and improved root growth and nutrient levels [3,17]. Candida sp. promoted the plant growth of Phaseolus vulgaris plants grown under normal and drought stress conditions [9]. Furthermore, the application of Candida tropicalis significantly improved the growth and productivity of maize plants [18]. Ignatova [19] indicated that Rhodotorula mucilaginosa has high IAA-producing and plant growth-promoting potential.
Plectranthus amboinicus (Lour.) Spreng. (Indian borage), a perennial herb, is one of the Lamiaceae members and native to the tropics and warm areas of Africa, Asia, and Australia [20]. This plant has nutritional and curative traits due to its high content of active compounds that are extremely valuable in the pharmaceutical industry. Its leaves have the capability to produce an essential oil that has high quantities of carvacrol [21], thymol [22], β-caryophyllene, α-humulene, γ-terpinene, p-cymene, α-terpineol, and β-selinene [23,24], active ingredients, which exhibit various pharmacological properties [25,26], including antitumor, antioxidant, antimicrobial, anti-inflammatory, anti-epileptic, wound healing, larvicidal, and analgesic activities [23,27,28,29,30,31]. It is widely used in folk medicine for treating constipation, colds, headaches, asthma, coughs, and fever conditions. Moreover, P. amboinicus leaves are used as flavoring agents and are often eaten raw as a component in traditional food [32,33]. Due to the aroma of P. amboinicus leaves, it has horticultural properties, as it is cultivated in gardens and pots [34].
Biostimulants enhance plant growth and stimulate active ingredient synthesis, improving nutrient availability and nutrient uptake of many crop plants [35,36]. However, the plant species vary in their response to different biostimulants. To verify this hypothesis, four yeast strains of Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis as biological biostimulants were used as a foliar spray to evaluate their efficiency on the growth, productivity, and essential oil yield, as well as the antioxidant activities of Plectranthus amboinicus (Lour.) Spreng.
2. Materials and Methods
2.1. Location and Materials Source
This pot investigation was conducted at the Experimental Farm of South El-Tahreer Station, Horticulture Research Institute, Agricultural Research Center, Giza, Egypt during the successive seasons of 2019–2020. Cuttings of Plectranthus amboinicus (Lour.) were obtained from the National Gene Bank, Giza, Egypt. Yeast strains of Rhodotorula muciligenese (JGBTA-S1), Candida sake (ATCC: 14478), Candida apicola (CBS 4078), and Candida kunwiensis (CS 9678) were obtained from the Microbiology Department, Ain Shams University, Egypt.
2.2. Yeast Culturing
The yeast strains were grown on yeast peptone dextrose (YPD) liquid medium, which contains 20 g L−1 peptone, 10 g L−1 yeast extract, and 20 g L−1 glucose [37]. Then the medium was autoclaved at 121 °C for 20 min. Each strain was inoculated separately with a loop full and incubated at 30 °C for 48 h on a rotary shaker at 150 rpm. The culture media were centrifuged at 6000 rpm for 10 min. Yeast cream mass was washed twice using sterile distilled water to eliminate any remaining culture medium, and then yeast cells were suspended in sterile distilled water. Finally, a hemocytometer slide and a light microscope were used for adjusting the yeast cell count to the concentrations of 1 × 104, 1 × 107, and 1 × 109 CFU mL−1.
2.3. Experimental Design and Layout
The cuttings were planted in pots (35 × 50 cm) filled with sandy loam soil on February 1st for both seasons. Each pot contained one stem cutting, and the pots were placed in a greenhouse. The plants were foliar sprayed monthly with yeast strains of Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) in concentrations of 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL−1. The first application was made 44 days after planting. This investigation was performed in a randomized complete design with three replicates; each replicate included five pots (a plant per pot). All the pots were fertigated monthly with 2 g L−1 of a water-soluble NPK fertilizer (10-20-10). The experimental soil was analyzed, and its physical and chemical properties were as follows: 88.6% sand, 9.1% clay, 2.3% silt, pH: 7.9, EC: 1.2 dS m−1, N: 0.01%, P: 0.4 ppm, K: 9.5 meq 100 g−1, Fe: 4.1 ppm, Mn: 1.3 ppm, Zn: 0.9 ppm.
2.4. Growth Parameters
The plants were collected for growth and biochemical analysis after 150 days from planting. All plants from each treatment were separately collected and the shoots were weighed fresh. The roots were separated, cleaned, and washed under running tap water to remove any soil, and air-dried for 2 h, then their fresh weights were recorded. The plant height, number of branches and leaves, root length and weight, root: shoot length ratio, and phenotypic plasticity traits were estimated. The phenotypic plasticity index (PPI) was estimated according to Valladares et al. [38] using the following formula:
2.5. Essential Oil Extraction
The essential oil was extracted from the fresh herb using the water distillation method of Viuda-Martos et al. [39] for 3 h using the Clevenger apparatus for oil percent and oil yield determination. The fresh herbs (100 g) from each treatment were distilled in triplicate and the oil contents are presented as the average value. The extracted oil was dried using anhydrous sodium sulfate. Oil percent was expressed using the following formula:
2.6. Biochemical Analysis
At the harvest stage, leaf samples were collected and submerged immediately in liquid nitrogen, then pulverized to a fine powder using a mortar and maintained at −80 °C for antioxidant determination. Other leaf samples were collected and oven-dried at 60 °C till the constant weight was recorded for biochemical analysis.
2.6.1. Total Carbohydrates, Ash, and Protein Content
Total carbohydrates (%) were measured in dried leaves by the colorimetric method as mentioned by Dubois et al. [40]. Briefly, 1 mL of sample was mixed with phenol solution 5% (1 mL) and 5.0 mL sulfuric acid, then the mixture was shaken thoroughly and maintained for 20 min in a water bath at 23–30 °C. The developed color was determined at 490 nm wavelength throughout the UVVIS spectrophotometer analysis. Glucose was used as a standard for calibration curve. The ash content (%) of the leaf samples was assessed at 550 °C for overnight using a muffle furnace AOAC [41]. The protein content (%) was estimated by multiplying N percent by the conversion factor (6.25) of nitrogen-protein [42].
2.6.2. Radical Scavenging (DPPH Assay)
The assay of DPPH (1,1-diphenyl-2-picrylhydrazyl) was performed following the procedures of Brand-William et al. [43] and Badhani et al. [44], with minor modifications. A 5 mL ethanol (80%) was mixed with 100 μM DPPH, then 1 mL of leaf sample extract was mixed with 3 mL of cation DPPH and maintained in the dark at room temperature for 20 min. The wavelength of 520 nm was used to measure the reduction in absorbance. The readings were presented in mg ascorbic acid equivalent (AAE) per 100 g−1 FW.
2.6.3. Ferric Reducing Antioxidant Potential (FRAP)
The assay of ferric reducing antioxidant potential (FRAP) was estimated calorimetrically as the procedure of Benzie and Strain [45] and Badhani et al. [44] with little modifications. The FRAP reagent consisted of 20 mM ferric chloride (10:1:1, v/v/v), 10 mM TPTZ (2,4,6-tri-2-pyridyl-1,3,5-triazin), and 300 mM acetate buffer (pH-3.6). A total of 3.0 mL of FRAP reagent was mixed with 0.1 mL of methanolic leaf extract and maintained for 8 min at 37 °C. A UV-VIS spectrophotometer was used for FRAP determination at the wavelength of 593 nm. Ascorbic acid was used as a blank sample, and the readings were expressed in mg ascorbic acid equivalent (AAE) per 100 g−1 FW.
2.6.4. Nutrients Estimation
Dried leaves were grinded for element analysis. A 0.5 g of leaf sample was digested using sulfuric and perchloric acids to measure the nutrient content [46]. The total N (%) was estimated using the modified micro Kjeldahl method described by Black et al. [47]. The P (mg 100 g−1) content was determined colorimetrically using stannous chloride phosphomolybdic-sulfuric acid, as described by Jackson [46], and the K (mg 100 g−1) content was determined using a flame-photometer, as described by Jackson and During [48].
2.7. Statistical Analysis
In this study, a randomized complete design was used as an experimental layout. The experimental design included two factors: (1) four yeast strains (Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis) and (2) four concentrations (0, 1 × 104, 1 × 107, and 1 × 109 CFU mL−1). The experiment was repeated twice in two different seasons with three replicates for each treatment. In order to compare the significant differences between treatments, the analysis of variance (ANOVA) was made according to Snedecor and Cochran [49]. Tukey’s test was used for post-hoc analysis (p < 0.05).
3. Results
3.1. Vegetative Growth
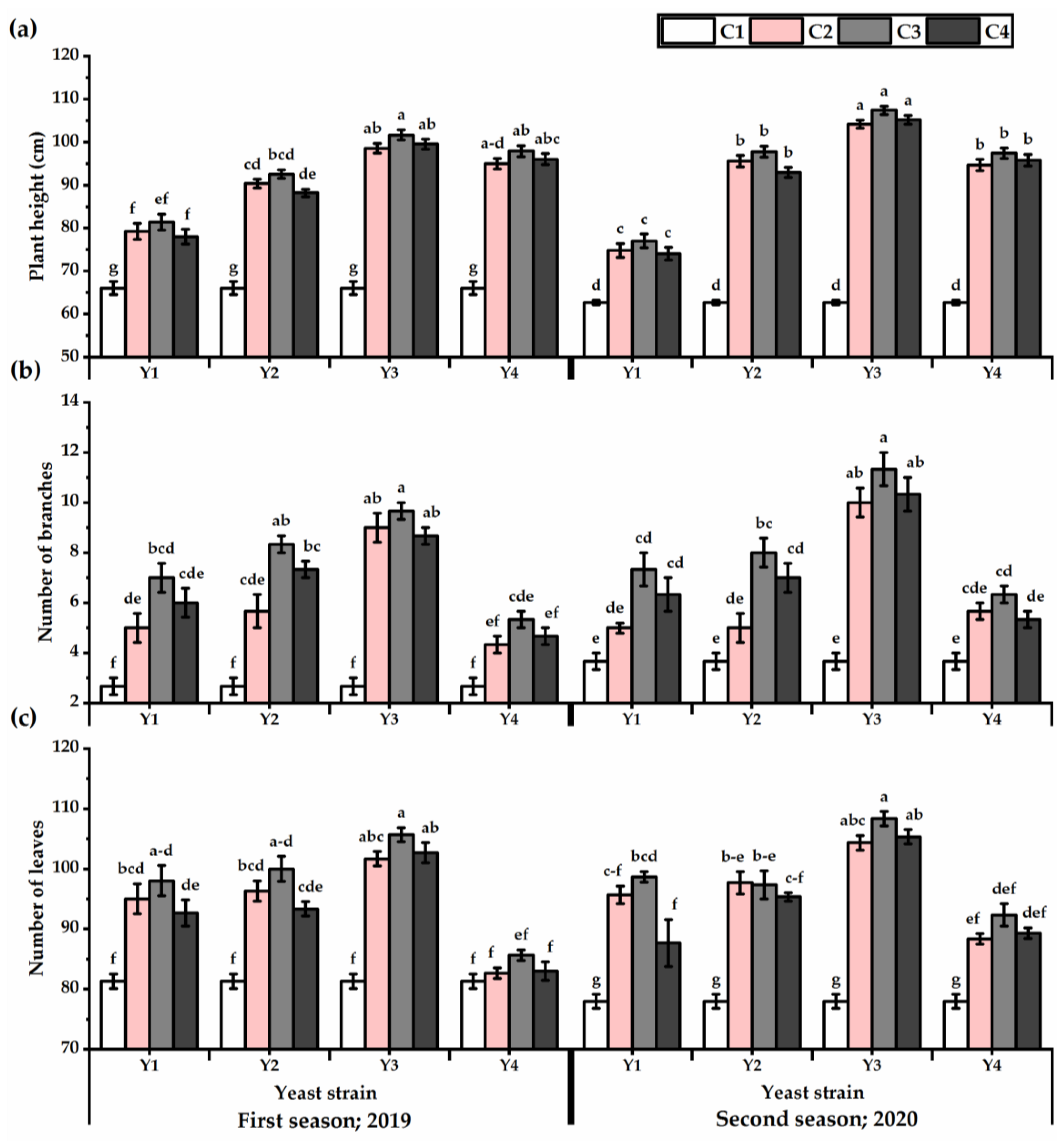
Foliar application with yeast strains significantly improved the growth performance of P. amboinicus plants (Figure 1). The Y3 treatment significantly maximized the plant height, number of branches, and leaves values, as compared to other yeast strains for both seasons (Table S1). Increasing the concentration led to an increase in growth attributes to reach the maximum growth when the plants were foliar sprayed with C3 application. The tallest plants were obtained by Y3×C3 treatment, which gave 101.6 cm for the first season despite there being no significant differences between Y3 × C3 and Y3 × C2, Y3 × C4, Y4 × C2, Y4 × C3, and Y4 × C4 treatments. Furthermore, Y3 × C3 treatment recorded the highest plant height value (107.4 cm for the second seasons), albeit the differences between Y3 × C2 and Y3 × C4 treatments were non-significant. The Y1 × C4 application resulted in the smallest increase in plant height, with 15% higher than control plants.
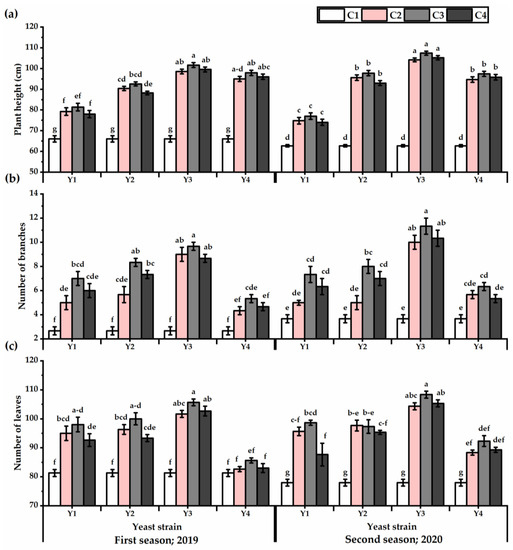
Figure 1.
Plant height (cm) (a), number of branches (b), and number of leaves (c) of Plectranthus amboinicus (Lour.) Spreng. plants subjected to Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) yeast strains foliar application at 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL–1 concentrations during 2019 and 2020 seasons. Data are mean value ± SE. Means with different letters for each season significantly differed, using Tukey’s test at p ≤ 0.05 level, n = 15.
Yeast strains exhibited efficiency in stimulating more branches and leaves, as revealed by the highest values when Y3 treatments were applied (7.5 and 8.8 for the first and second seasons, respectively, for number of branches and 83.2 and 87 for the first and second seasons, respectively, for leaves number). The C3 concentration significantly showed the tallest plants, which carry the greatest number of branches and leaves. In this regard, the Y4-treated plants had lower values (4.3 and 5.3 for the first and second seasons, respectively). On the other hand, untreated plants significantly gave the lowest growth performance. The interaction of Y3 × C3 significantly gave the highest growth.
3.2. Root Traits
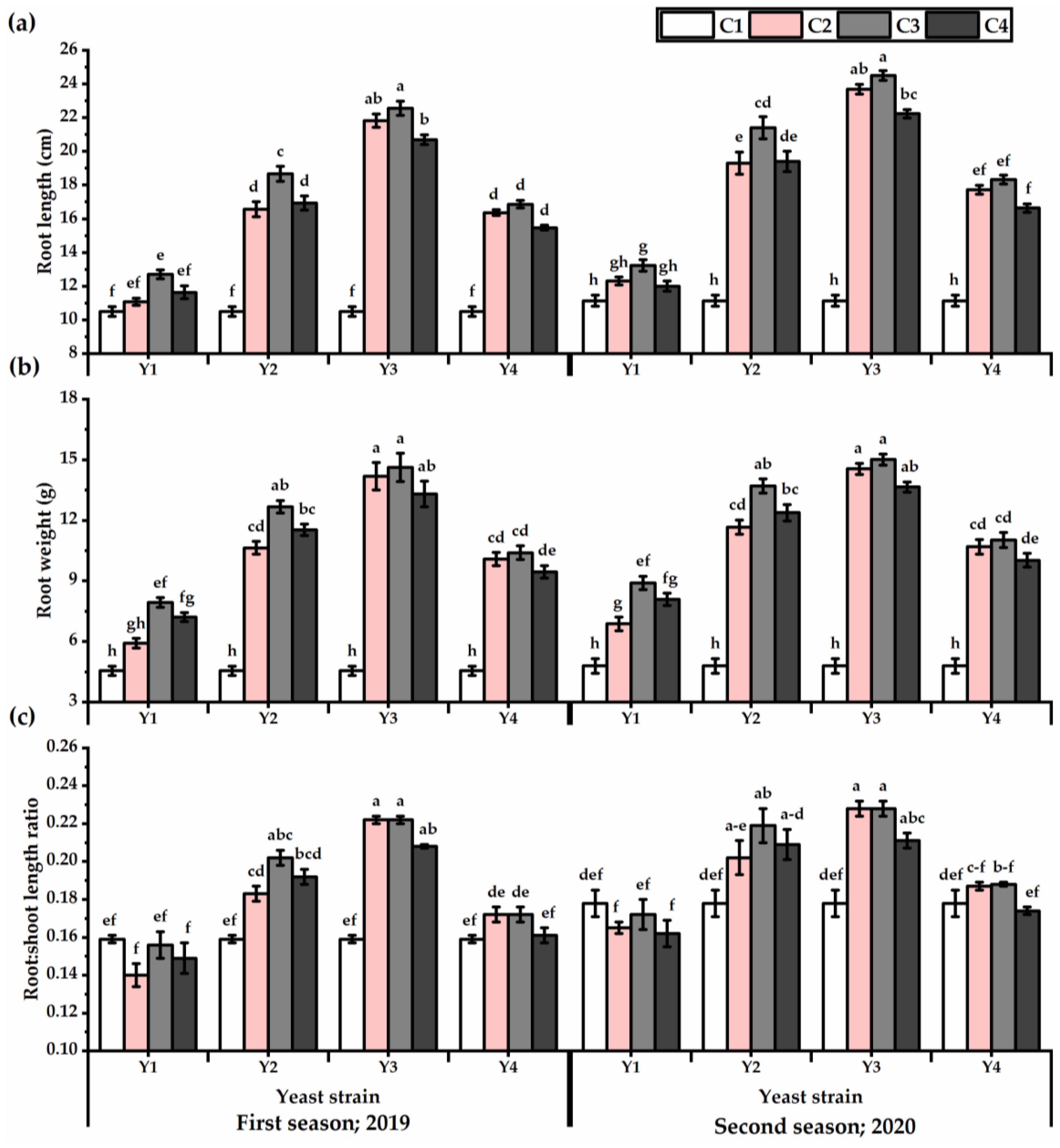
The results presented in Figure 2 reveal the root weight and length values of P. amboinicus plants, as affected by different yeast strains’ foliar application. The root traits showed significant differences within yeast strains applied, as both root weight and length values showed the maximum values as affected by Y3 application relative to other yeast strains (Table S2). Regarding the concentration, C3-plants significantly showed the highest root growth. On the other hand, C1-plants presented the lowest values in this respect. The Y3 × C2 and Y3 × C3 treatments outperformed other treatments in this respect, as they gave the highest root weight and length values. The lowest root weight (4.6 and 4.8 g for the first and second seasons, respectively) and length values (10.5 and 12.3 cm for the first and second seasons, respectively) were obtained from the untreated plants. The highest root: shoot length ratio were noticed by P. amboinicus plants subjected to Y3 at C2 and C3 concentrations and Y2 × C3 treatment for both seasons.
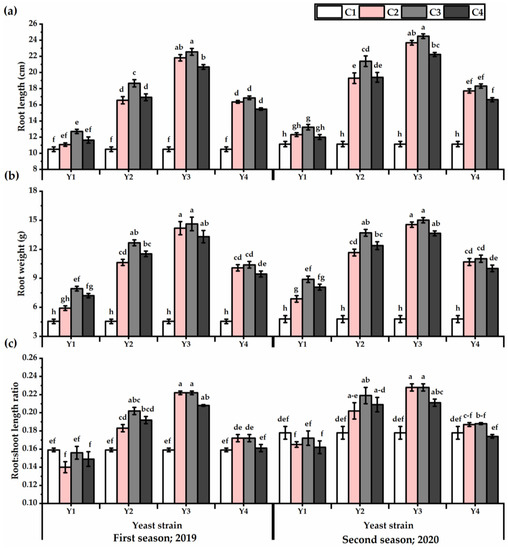
Figure 2.
Root length (cm) (a), root weight (g) (b), and root:shoot length ratio (c) of Plectranthus amboinicus (Lour.) Spreng. plants subjected to Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) yeast strains foliar application at 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL–1 concentrations during 2019 and 2020 seasons. Data are mean value ± SE. Means with different letters for each season significantly differed, using Tukey’s test at p ≤ 0.05 level, n = 15.
3.3. Yield Attributes and Phenotypic Plasticity
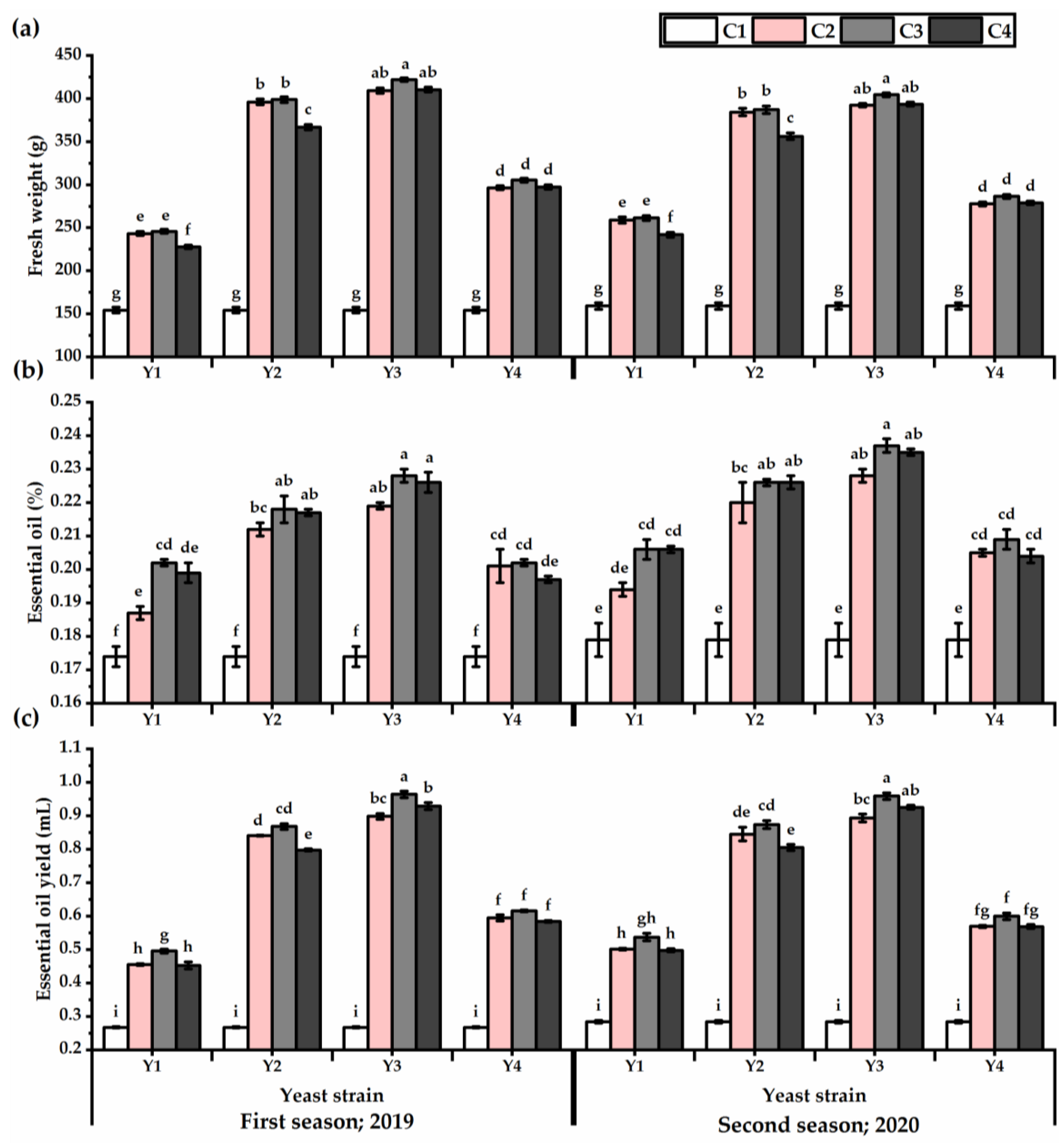
The herb fresh weight values exhibited in Figure 3 showed an improvement as affected by yeast treatments (Table S3), and Y3 outperformed the other strains in this respect (349 and 337.3 g for the first and second seasons, respectively). Treated plants with Y3 × C3 exhibited the heaviest weights, as recorded 342.9 and 334.7 g for the first and second seasons, respectively, while untreated plants presented the lowest values in this respect. Essential oil percent and yield traits showed an increase, as influenced by yeast applications, and Y3 exhibited the highest values in this respect. Plants subjected to foliar application with Y3 × C3 presented an improvement in the essential oil yield of 72.2 and 70% relative to untreated plants for the first and second seasons, respectively. The Y1 × C4 treatment significantly showed the lowest essential oil yield after untreated plants, despite achieving an increase in the essential oil yield of about 41 and 43% for the first and second seasons, respectively, relative to untreated plants.
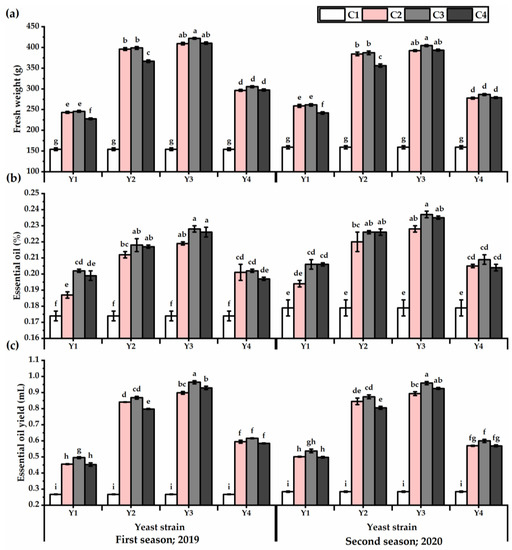
Figure 3.
Fresh weight (g) (a), essential oil (%) (b), and essential oil yield (mL) (c) of Plectranthus amboinicus (Lour.) Spreng. plants subjected to Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) yeast strains foliar application at 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL–1 concentrations during 2019 and 2020 seasons. Data are mean value ± SE. Means with different letters for each season significantly differed, using Tukey’s test at p ≤ 0.05 level, n = 15.
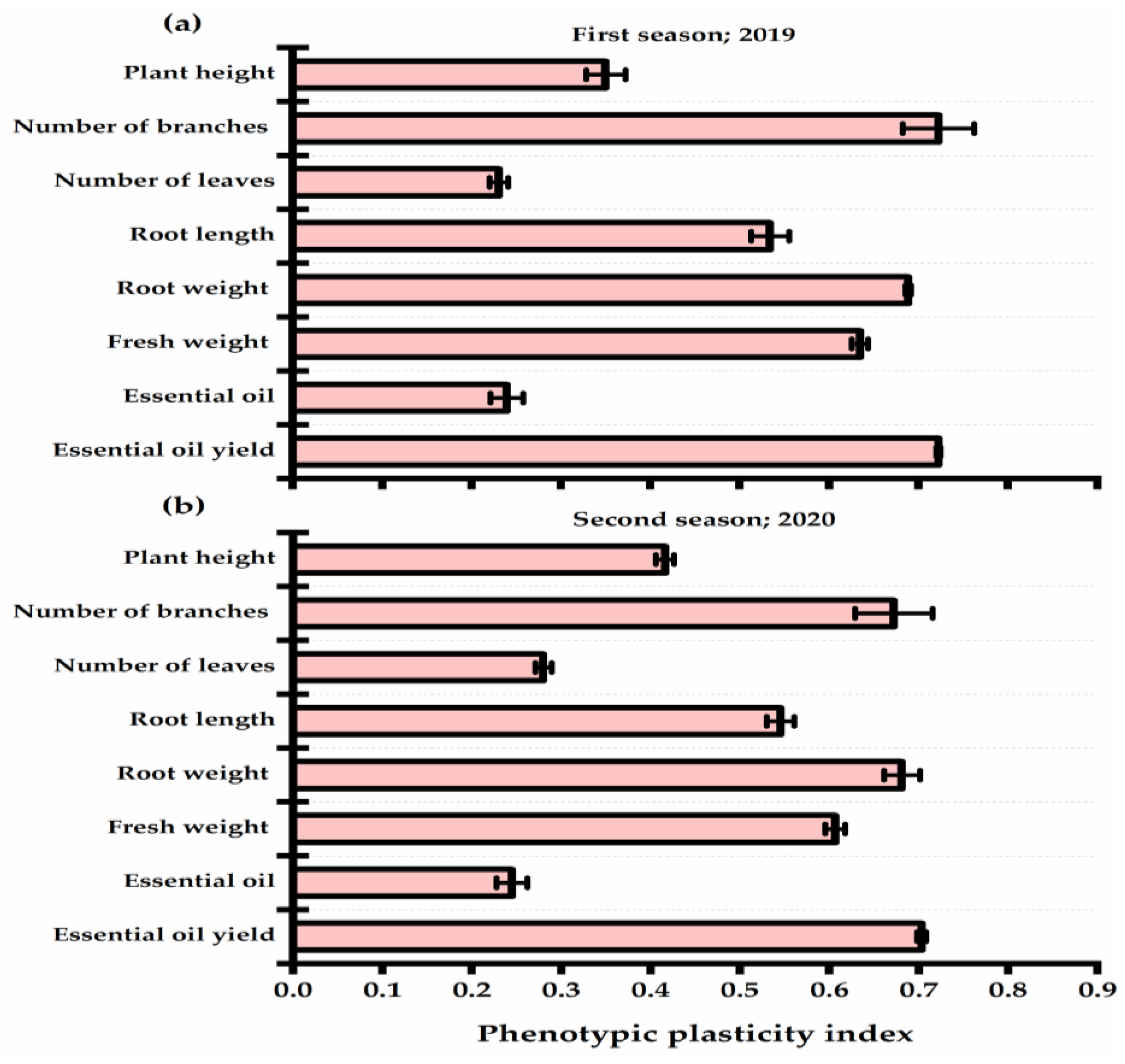
The presented results in Figure 4 revealed the phenotypic plasticity index (PPI) values of P. amboinicus plants in response to yeast strains. Lower PPI values were observed for the number of leaves (0.23 and 0.28 for the first and second seasons, respectively) and oil percent (0.24 and 0.25 for the first and second seasons, respectively). On the other hand, number of branches, essential oil yield, and herb and root weight traits showed great enhancement affected by yeast applications, and they gave the highest PPI values. The root length exhibited a moderate plastic response (0.53 and 0.55 for the first and second seasons, respectively).
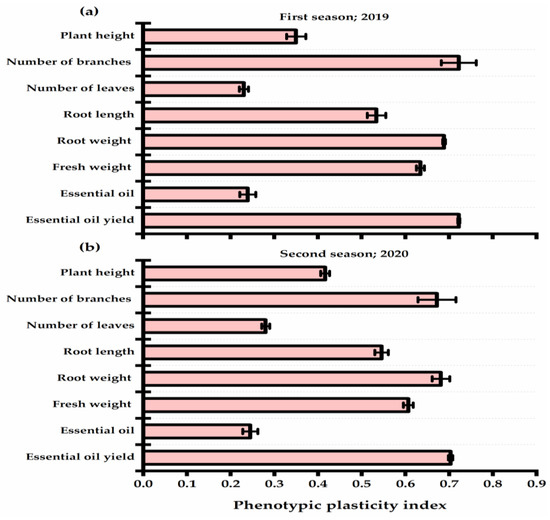
Figure 4.
Phenotypic plasticity index of Plectranthus amboinicus (Lour.) Spreng. traits as affected by Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) yeast strains foliar application at 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL−1 concentrations during 2019 (a) and 2020 (b) seasons. Data are mean value ± SE.
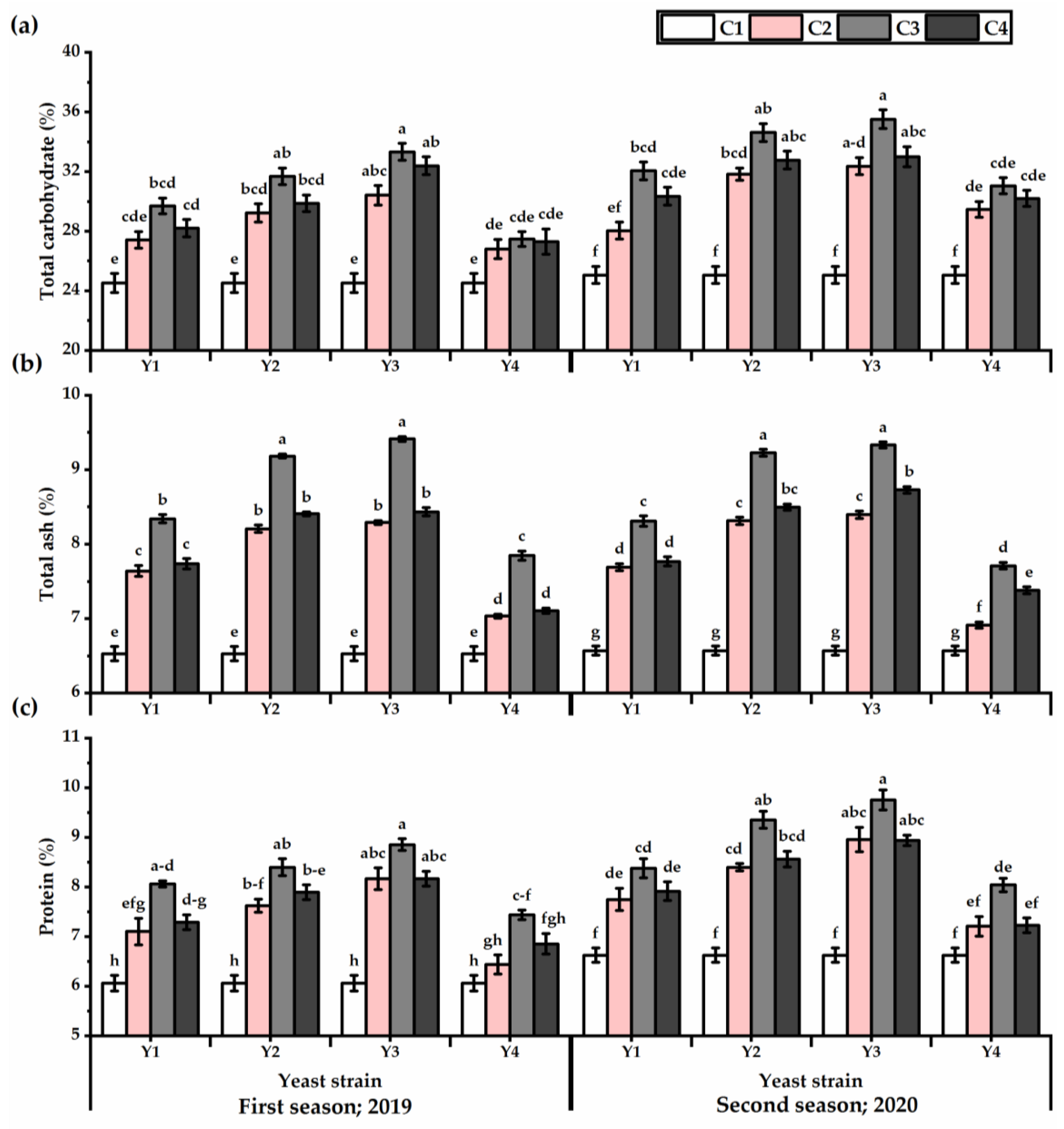
3.4. Carbohydrates, Ash, and Protein
The carbohydrate, ash, and protein levels of P. amboinicus leaves exposed to foliar applications with yeast strains have been improved (Figure 5). The plants subjected to Y3 showed the highest values in this respect, in contrast to the Y4 application, which gained the lowest values in this respect (Table S4). A growing increase was observed in total carbohydrates, ash, and protein values with increasing yeast concentration, as the maximum values were obtained by C3, then declined after that (except of total carbohydrates in the first season) (Table S4). The highest carbohydrate content was observed by Y3 × C3 treatment, which was recorded 26.4 and 29.5% higher for the first and second seasons, respectively, relative to untreated plants. Likewise, the ash and protein content recorded the highest levels following Y3 × C3 application, as they gave 30.6 and 29.6% for ash and 31.7 and 32.1% for protein for the first and second seasons, respectively.
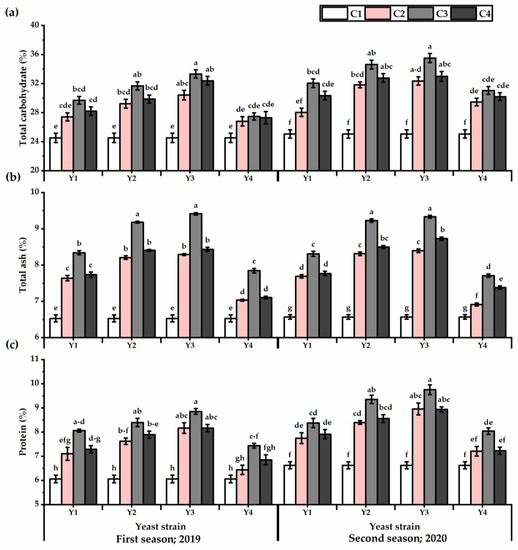
Figure 5.
Total carbohydrates (%) (a), ash (%) (b), and protein (%) (c) of Plectranthus amboinicus (Lour.) Spreng. plants subjected to Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) yeast strains foliar application at 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL−1 concentrations during 2019 and 2020 seasons. Data are mean value ± SE. Means with different letters for each season significantly differed, using Tukey’s test at p ≤ 0.05 level, n = 15.
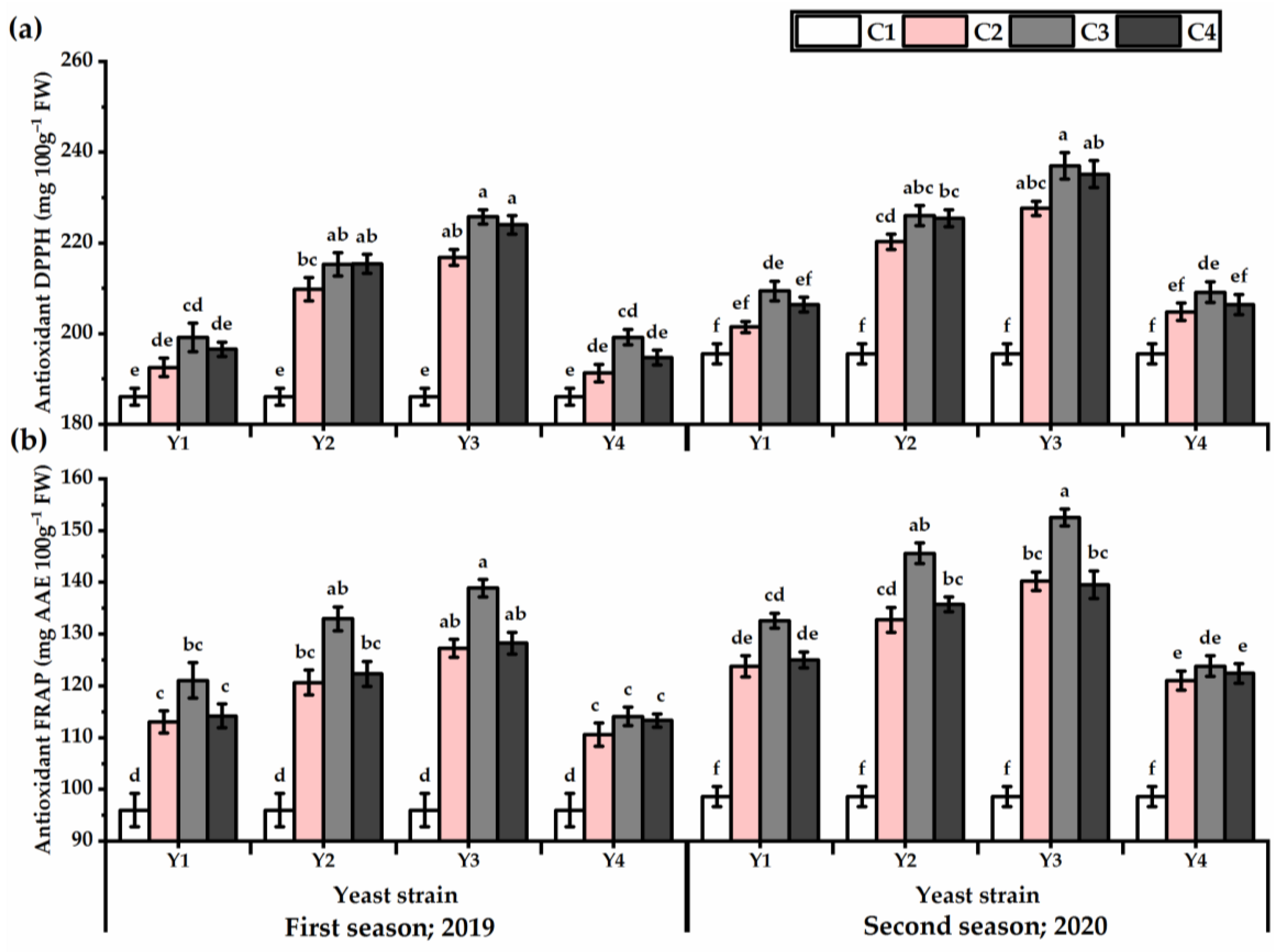
3.5. DPPH and FRAP
Illustrated results in Figure 6 show that the antioxidant activities of DPPH and FRAP were significantly influenced by all yeast strains. Higher antioxidant activities were given by Y3 application against Y4, which recorded the lowest values in this respect (Table S5). The maximum increase in DPPH value was noticed by plants grown under the Y3 strain application at the concentration of C3 (225.8 and 237.6 mg 100 g−1 for the first and second seasons, respectively) and C4 (224 and 235.2 mg 100 g−1 for the first and second seasons, respectively). Whereas, the lowest DPPH value (186 and 195.5 mg 100 g−1 for the first and second seasons, respectively) was given by untreated plants. Regarding FRAP, the Y3 × C3 treatment resulted in 44.6 and 54.7% higher in FRAP value for the first and second seasons, respectively, as compared with untreated plants.
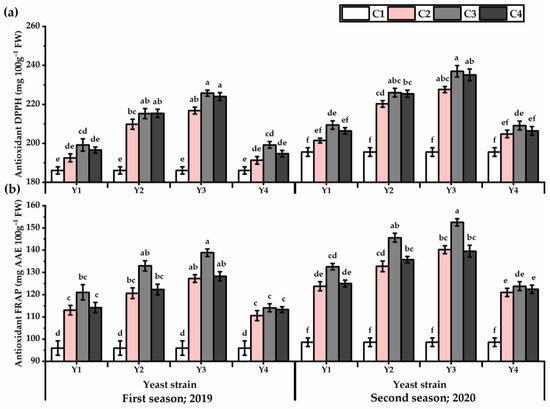
Figure 6.
DPPH (mg 100 g−1) (a) and FRAP (mg AAE 100 g−1 FW) (b) of Plectranthus amboinicus (Lour.) Spreng. plants subjected to Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) yeast strains foliar application at 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL−1 concentrations during 2019 and 2020 seasons. Data are mean value ± SE. Means with different letters for each season significantly differed, using Tukey’s test at p ≤ 0.05 level, n = 15.
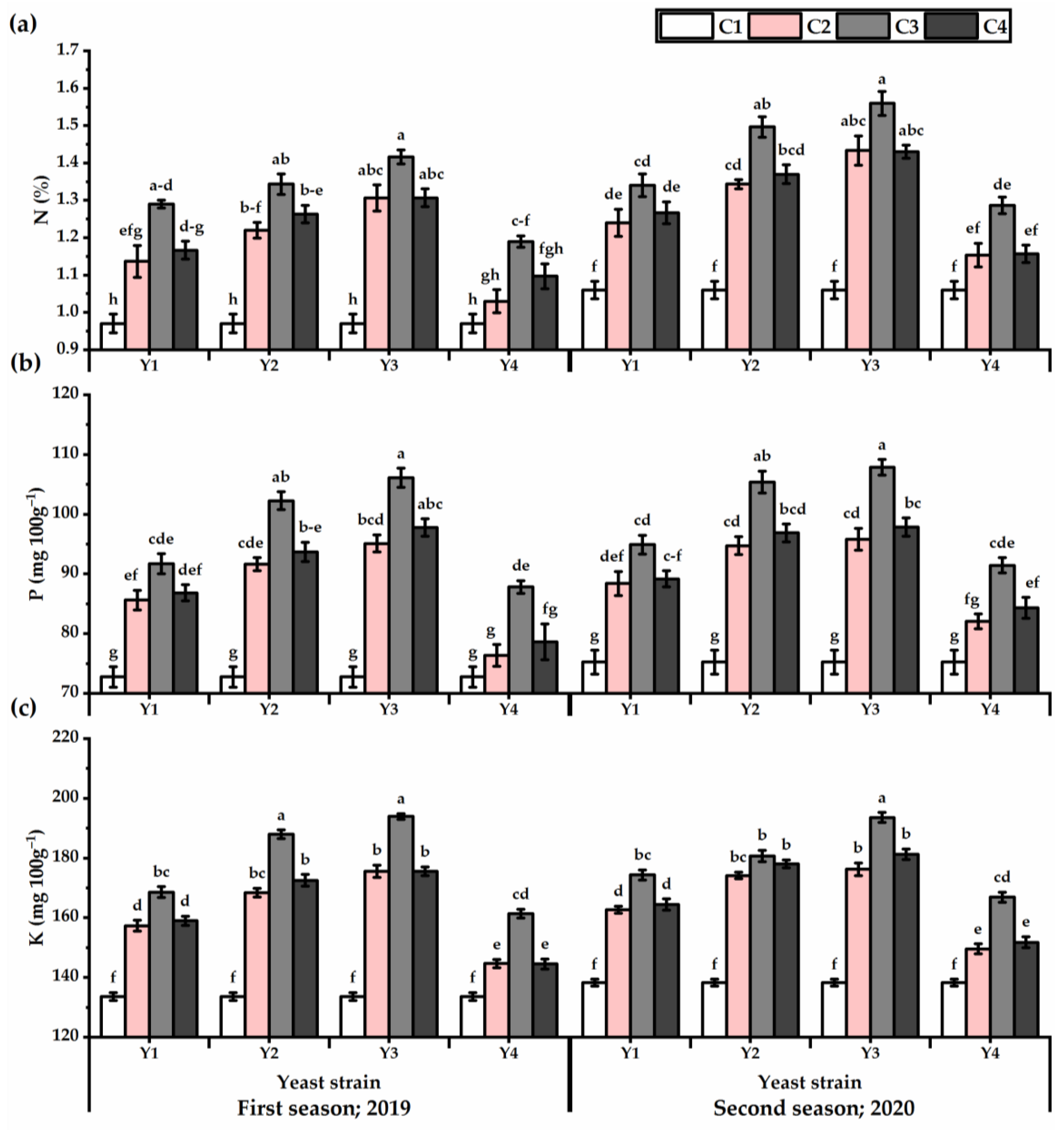
3.6. Nutrients Content
Data in Table S6 clearly indicate that P. amboinicus leaves nutrient contents (i.e., N, P, and K) were significantly (p ≤ 0.05) impacted following foliar application of all yeast strains. Generally, increasing the Y concentration caused a gradual increase in the P. amboinicus leaf nutrients (Figure 7). The highest levels of the investigated elements were noticed at 1 × 107 concentration. By applying Y3 at the 1 × 107 concentration, the improvements in nutrient content, as compared with the control, were 46.1%, 45.82 mg 100 g−1, and 45.1 mg 100 g−1 for N, P, and K, respectively, for the first season, and 47.14%, 43.27 mg 100 g−1, and 40 mg 100 g−1 for N, P, and K, respectively, for the second season). However, the lowest levels of the investigated elements were noticed in untreated plants.
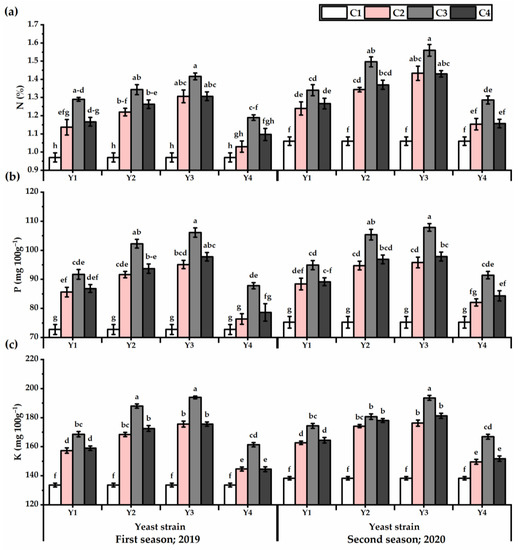
Figure 7.
N (%) (a), P (mg 100 g−1) (b), and K (mg 100 g−1) (c) of Plectranthus amboinicus (Lour.) Spreng. plants subjected to Rhodotorula muciligenese (Y1), Candida sake (Y2), Candida apicola (Y3), and Candida kunwiensis (Y4) yeast strains foliar application at 0 (C1), 1 × 104 (C2), 1 × 107 (C3), and 1 × 109 (C4) CFU mL−1 concentrations during 2019 and 2020 seasons. Data are mean value ± SE. Means with different letters for each season significantly differed, using Tukey’s test at p ≤ 0.05 level, n = 15.
4. Discussion
Yeast application is a biostimulation technique performed in the agriculture sector for enhancing growth performance and productivity by amending plants with the metabolic substances essential for plant growth [3,50]. Obtained results indicated that P. amboinicus growth was significantly improved as a response to yeast applications, particularly C. apicola. Moreover, root weight and length values showed a great enhancement following all yeast strains. The enhancement in root growth is a good indicator of productivity improvement, soil nutrient acquisition, and water uptake efficiency [51], so the root system has the ability to improve its competitiveness under specific conditions [52]. Indole-3-acetic acid (IAA) is one of the most widespread phytohormones released by yeasts [53], which stimulates cell division and root growth, causing more root tip generation and branching, leading to an increase in root systems under the soil surface [54]. R. mucilaginosa is a good-producing IAA and has a high plant growth-promoting ability [19]; moreover, Candida species may produce gibberellins, IAA, and other plant growth promoters [55]. Yeast provides plants with indole-3-pyruvic acid, polyamines, gibberellins, and other plant growth-promoting substances that contribute to improving plant performance [7]. During reproductive growth, the competition between roots and shoots for photosynthates is the dominant factor controlling the growth of roots [56]. Yeast foliar applications increased the root and shoot length of P. amboinicus plants, especially Candida sp. Similar results were noticed by Sannazzaro [57], who illustrated that yeast supplementation improved the root: shoot length ratio of lotus plants under saline conditions. Furthermore, Alwhib [58] reported that yeast application improved the root: shoot weight ratio of tomato plants grown under drought conditions.
Plant growth occurs naturally as a result of cell divisions, elongation, and differentiation, these functions are controlled by a group of naturally occurring compounds (plant growth regulators) with hormonal action [9,59,60]. Yeasts may directly promote plant growth by generating a wide range of biologically active substances, such as amino acids, enzymes, vitamins, etc., which have an efficient stimulating influence on plant cells and development during the life cycle and help to raise their productivity [7,13], or indirectly by releasing antimicrobial compounds that assist in reducing phytopathogenic infection [7,61,62]. The improvement observed in P. amboinicus growth following Candida treatments may be due to the capability of Candida for fixing nitrogen [18]. Yeast species are able to produce the plant hormone zeatin [63,64]. Zeatin, a member of cytokinin family, is vital in embryogenesis, shoot and root meristem maintenance, and vascular development during plant growth and development [65]. Seed inoculation with yeast strains promotes the length and biomass of seedlings and stimulates plant growth [8,13,66].
In this study, plants subjected to R. mucilaginosa application exhibited growth stimulation. R. mucilaginosa has a significant growth-promoting influence of many agricultural crops [67,68]. Various genes in the R. mucilaginosa genome have the potential to impact plant hormonal reactions, which positively impact plant growth [69], including abscisic acid pathway genes, the auxin pathway genes, the gene (g288.t1) necessary to change the inactive form of the plant hormone cytokinin into the active form [70], and the first gene (g5608.t1) in the cytokinin pathway production [69]. C. apicola is considered a sophorolipid-producing member [71]. Sophorolipids are glycolipid microbial surfactant molecules, which are connected to cell membranes. Glycolipids have an extensive range of biological functions, including generating and storing energy, creating, and maintaining the structural components of cell membranes, producing vitamins and hormones, absorbing vitamins, as well as providing insulation and protection [72].
The ability of a plant to change its single genotype to exhibit different phenotypes in response to environmental factors is known as phenotypic plasticity [73]. Various plant features exhibit a diverse degree of plasticity with several environmental conditions. Interestingly, the plant displays a plastic reaction to biostimulant application [51]. The phenotypic plasticity index describes the grade of phenotypic plasticity on a scale from 0 (no plastic response) to 1 (high plasticity). Higher PPI values were exhibited by branches number, herb, and root weights, as well oil yield traits, which indicates that plant productivity reached its maximum when yeast supplementation was applied. High PPI values of the roots are a good predictor of plant health, as it is important for nutrient absorption under unfavorable environmental conditions that influence plant species distribution and plant reaction to low soil resources [74]. Hill et al. [75] and Bossdorf et al. [76] describe the alteration in root/shoot allocation and root architecture associated with nutrient availability.
All yeast applications significantly increased the essential oil productivity of P. amboinicus plants. Yeast showed significant differences in essential oil percent and yield of Zinnia elegans [77], as well as foliar application with yeast, increased oil yield, and quality [3]. The improvements in oil quantity and quality of lovage plants following yeast supplementation were also noticed by Złotek et al. [78].
In the current study, total carbohydrates, ash, and protein content were increased following yeast applications, and their levels were elevated with increasing yeast concentration until C3 (1 × 107 CFU mL−1), and they declined after that. These results are in harmony with Taha et al. [79] who stated that using the high rate of yeast application did not result in any additional beneficial improvement, and they indicated that this may be owing to the adverse impact of the highest yeast level. The enhancements in vegetative growth are an indicator of more photosynthetic pigments, more carbohydrates, and protein synthesis. Yeast extracts stimulated protein, amino acid, and total carbohydrate levels in caraway, faba bean, sugarbeet, and wheat plants [66,80,81,82,83,84]. C. apicola is one of the yeasts with significant biological potential for protein production [85]. The improvements in the vegetative growth of P. amboinicus following yeast treatments mean a favorable impact on the biological activities and plant metabolism, moreover, a stimulating effect on the antioxidant activity [77,78]. Fungi have considerable antioxidant activity and perfect activity versus the DPPH radical [86]. Candida sp. has the ability to produce antioxidants [87]. Fungi are a potent antioxidant source [88]. Abbas [80] stated that yeast enhanced plant metabolism and boosted antioxidant activity in faba beans; moreover, Złotek [78] reported that yeast caused a significant improvement in the antioxidant enzyme activities of the lovage plants.
Root growth are increased by an increase in the root length, branching, mass, and root hair amount, which causes greater root surface area and more nutrient absorption. The increase in root traits of P. amboinicus, which are affected by yeast application, is followed by an increase in the leaf macronutrients. Yeast is an important source of amino acids and protein, and both act as an extra nitrogen source. R. mucilaginosa has the capability to enhance urea utilization in plants [68,69]. Phosphorus is a vital element for cell growth and plant development and has a critical role in many functions, including cell division, photosynthesis, nutrient uptake, and biological oxidation [89]. Phosphate-solubilizing fungi can convert the insoluble P form to the soluble form, causing an improvement in soil fertility [11]. The efficiency of P solubilizing properties of fungal strains differs [90]. Both C. tropicalis and Rhodotorula sp. yeast strains are able to provide plants with soluble inorganic P [18,91]. Sen et al. [69] found that R. mucilaginosa increased the growth of rice plants grown in low-phosphate conditions and that R. mucilaginosa can solubilize insoluble calcium phosphate in the media. Rhodotorula glutinis showed a high K concentration that was released from mica in the soil [92]. The macronutrient levels of onion bulbs showed an increase, as affected by yeast extract foliar spray [17,93]. Furthermore, similar findings were observed by Ahmed et al. [94] on potatoes and by Mahmoud et al. [95] on xerophytic plants.
5. Conclusions
The effects of Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis yeast strains foliar application at 0, 1 × 104, 1 × 107, and 1 × 109 CFU mL−1 concentrations on the growth and productivity of Plectranthus amboinicus plants were investigated in this study. Yeast applications had a marked influence on growth and nutritional quality, as well as a significant impact on the antioxidant activities of P. amboinicus leaves, in particular, Candida apicola. Herein, Candida apicola application increased plant performance and quality, especially at 1 × 107 concentration, which presented the heaviest fresh herb (342.9 and 334.7 g for the first and second seasons, respectively) and maximum essential oil yield (72.2 and 70% higher relative to untreated plants for the first and second seasons, respectively), with an increase in DPPH value by 17.6 and 17.8% and by 44.6 and 54.7% higher in FRAP value for the first and second seasons, respectively. Additional studies are required to investigate if mixing the yeast strains could give better growth.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8100887/s1. Table S1. Effects of yeast strains of Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis yeast strains at 0, 1 × 104, 1 × 107, and 1 × 109 concentrations on plant height (cm), branches number, and leaves number of Plectranthus amboinicus (Lour.) Spreng. in 2019 and 2020 growing seasons. Table S2. Effects of yeast strains of Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis yeast strains at 0, 1 × 104, 1 × 107, and 1 × 109 concentrations on root length (cm), root weight (g), and root:shoot length ratio of Plectranthus amboinicus (Lour.) Spreng. in 2019 and 2020 growing seasons. Table S3. Effects of yeast strains of Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis yeast strains at 0, 1 × 104, 1 × 107, and 1 × 109 concentrations on fresh weight (g), essential oil (%), and essential oil yield (mL) of Plectranthus amboinicus (Lour.) Spreng. in 2019 and 2020 growing seasons. Table S4. Effects of yeast strains of Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis yeast strains at 0, 1 × 104, 1 × 107, and 1 × 109 concentrations on total carbohydrate (%), total ash (%), and protein (%) of Plectranthus amboinicus (Lour.) Spreng. in 2019 and 2020 growing seasons. Table S5. Effects of yeast strains of Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis yeast strains at 0, 1 × 104, 1 × 107, and 1 × 109 concentrations on antioxidant DPPH, and antioxidant FRAP of Plectranthus amboinicus (Lour.) Spreng. in 2019 and 2020 growing seasons. Table S6. Effects of yeast strains of Rhodotorula muciligenese, Candida sake, Candida apicola, and Candida kunwiensis yeast strains at 0, 1 × 104, 1 × 107, and 1 × 109 concentrations N (%), P (mg 100 mL-1), and K (mg 100 mL-1) of Plectranthus amboinicus (Lour.) Spreng. in 2019 and 2020 growing seasons.
Author Contributions
Conceptualization, A.A.D., R.S.E.-S. and M.F.M.; methodology, A.A.D., R.S.E.-S., M.F.M. and H.S.A.E.-S.; software, R.S.E.-S., A.A.D., M.F.M. and H.S.A.E.-S.; validation, R.S.E.-S., A.A.D., M.F.M. and H.S.A.E.-S.; formal analysis, R.S.E.-S., A.A.D., M.F.M. and H.S.A.E.-S.; investigation, R.S.E.-S., A.A.D., M.F.M., K.S.A. and H.S.A.E.-S.; resources, R.S.E.-S., A.A.D., M.F.M. and H.S.A.E.-S.; data curation, R.S.E.-S., A.A.D., M.F.M. and H.S.A.E.-S.; writing—original draft preparation, R.S.E.-S., A.A.D., M.F.M., K.S.A. and H.S.A.E.-S.; writing—review and editing, R.S.E.-S., A.A.D., M.F.M.,K.S.A. and H.S.A.E.-S.; visualization, R.S.E.-S., A.A.D., M.F.M. and H.S.A.E.-S.; supervision, R.S.E.-S., A.A.D., M.F.M., K.S.A. and H.S.A.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
First author Khalid S. Alshallash from Saudi Arabia would like to express his gratitude to the deanship of scientific research at Imam Mohammed Bin Saud Islamic University for supporting publishing this study work. The authors gratefully acknowledge Abdel Nasser El-Sheshtawy professor in Environment and Bio-Agriculture Department, Faculty of Agriculture, Al-Azhar University for scientific revision of this manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Yadav, P.; Jaiswal, D.K.; Sinha, R.K. Climate change: Impact on agricultural production and sustainable mitigation. In Global Climate Change; Singh, S., Singh, P., Rangabhashiyam, S., Srivastava, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 151–174. [Google Scholar]
- El-Serafy, R.S.; El-Sheshtawy, A.A.; Abd El-Razek, U.A.; Abd El-Hakim, A.F.; Hasham, M.M.A.; Sami, R.; Khojah, E.; Al-Mushhin, A.A.M. Growth, yield, quality, and phytochemical behavior of three cultivars of quinoa in response to moringa and Azolla extracts under organic farming conditions. Agronomy 2021, 11, 2186. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.A.; Dahab, A.A.; Al-Ashkar, I. Can yeast extract and chitosan-oligosaccharide improve fruit yield and modify the pharmaceutical active ingredients of organic fennel? Ind. Crops Prod. 2021, 173, 114130. [Google Scholar] [CrossRef]
- Rosa-Magri, M.M.; Avansini, S.H.; Lopes-Assad, M.L.; Tauk-Tornisielo, S.M.; Ceccato-Antonini, S.R. Release of potassium from rock powder by the yeast Torulaspora globosa. Braz. Arch. Biol. Technol. 2012, 55, 577–582. [Google Scholar] [CrossRef]
- El-Maraghy, S.S.; Tohamy, T.A.; Hussein, K.A. Expression of SidD gene and physiological characterization of the rhizosphere plant growth-promoting yeasts. Heliyon 2020, 6, e04384. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W. Yeast systematics and phylogeny—Implications of molecular identification methods for studies in ecology. In Biodiversity and Ecophysiology of Yeasts; Péter, G., Rosa, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 11–30. [Google Scholar]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Amprayn, K.O.; Rose, M.T.; Kecskés, M.; Pereg, L.; Nguyen, H.T.; Kennedy, I.R. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl. Soil Ecol. 2012, 61, 295–299. [Google Scholar] [CrossRef]
- Silambarasan, S.; Vangnai, A.S. Plant-growth promoting Candida sp. AVGB4 with capability of 4-nitroaniline biodegradation under drought stress. Ecotoxicol. Environ. Saf. 2017, 139, 472–480. [Google Scholar] [CrossRef]
- Cloete, K.J.; Valentine, A.J.; Stander, M.A.; Blomerus, L.M.; Botha, A. Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a Sclerophyllous medicinal shrub, Agathosma betulina (Berg.). Pillans. Microb. Ecol. 2009, 57, 624–632. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, K.P. Enriching vermicompost by nitrogen fixing and phosphate solubilizing bacteria. Bioresour. Technol. 2001, 76, 173–175. [Google Scholar] [CrossRef]
- Sansone, G.; Rezza, I.; Calvente, V.; Benuzzi, D.; de Tosetti, M.I.S. Control of Botrytis cinerea strains resistant to iprodione in apple with rhodotorulic acid and yeasts. Postharvest Biol. Technol. 2005, 35, 245–251. [Google Scholar] [CrossRef]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils 2005, 42, 97–108. [Google Scholar] [CrossRef]
- Diouf, D.; Diop, T.A.; Ndoye, I. Actinorhizal, mycorrhizal and rhizobial symbioses: How much do we know? Afr. J. Biotechnol. 2003, 2, 1–7. [Google Scholar]
- El-Tarabily, K.A.; Sivasithamparam, K. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience 2006, 47, 25–35. [Google Scholar] [CrossRef]
- Saad-Allah, K.M.; Fetouh, M.I.; Elhaak, M.A. Induction of milk thistle (Silybum marianum L. Gaertn) growth and phytochemicals production by natural stimulants. J. Appl. Res. Med. Arom. Plants 2017, 6, 101–110. [Google Scholar] [CrossRef]
- Abdel-Moneim, M.M.; El-Mazny, M.Y.; Abdel-Mageed, Y.T.; Moustala, Y.M.M.; Yamani, S.H.S. Effect of some natural antioxidants on the productivity and storage ability of Egyptian onion grown in sandy soil. In Proceedings of the Minia 2nd International Conference for Agriculture and Irrigation in the Nile Basin Countries, Minia, Egypt, 23–25 March 2015; pp. 411–429. [Google Scholar]
- Mukherjee, S.; Sen, S.K. Exploration of novel rhizospheric yeast isolate as fertilizing soil inoculant for improvement of maize cultivation. J. Sci. Food Agric. 2015, 95, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, L.V.; Brazhnikova, Y.V.; Berzhanova, R.Z.; Mukasheva, T.D. Plant growth-promoting and antifungal activity of yeasts from dark chestnut soil. Microbiol. Res. 2015, 175, 78–83. [Google Scholar] [CrossRef]
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, phytochemical, pharmacological and nutritional significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef]
- Castillo, R.A.M.; Gonzalez, V.P. Plecthranthus amboinicus (Lour.) Spreng. Rev. Cuba. Plant Med. 1999, 4, 110–115. [Google Scholar]
- Singh, G.; Singh, O.P.; Prasad, Y.R.; de Lamposona, M.P.; Catalan, C. Studies on essential oils. Part 33. Chemical and insecticidal investigations on leaf oil of Coleus amboinicus (Lour). Flavour Fragr. J. 2002, 17, 440–442. [Google Scholar] [CrossRef]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Venkatesalu, V. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi: A malarial vector mosquito. Parasitol. Res. 2010, 107, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.B.; Braga, M.A.; de Oliveira, F.F.M.; Santiago, G.M.P.; Carvalho, C.B.M.; Cabral, P.B.; Santiago, T.M.; Sousa, J.S.; Barros, E.B.; do Nascimento, R.F.; et al. Effect of subinihibitory and inhibitory concentrations of Plectranthus amboinicus (Lour.) Spreng essential oil on Klebsiella pneumoniae. Phytomedicine 2012, 19, 962–968. [Google Scholar] [CrossRef]
- Bhatt, P.; Negi, P.S. Antioxidant and antibacterial activities in the leaf extracts of Indian borage (Plectranthus amboinicus). Food Nutr. Sci. 2012, 3, 146–152. [Google Scholar]
- Valera, D.; Rivas, R.; Avila, J.L.; Aubert, L.; Amelot, M.A.; Usubillaga, A.M. The essential oil of Coleus amboinicus Lour., chemical composition and evaluation of insect antifeedant effects. Ciencia 2003, 11, 113–118. [Google Scholar]
- Koba, K.; Grade, D.; Sanda, K.; Raynaud, C.; Chaumont, J.P. Chemical composition and antimicrobial properties of the leaf essential oil of Coleus aromaticus Benth. from Cambodia. Int. J. Essent. Oil Ther. 2007, 1, 16–20. [Google Scholar]
- El-Hawary, S.S.; El-Sofany, R.H.; Abdel-Monem, A.R.; Ashour, R.S.; Sleem, A.A. Seasonal variation in the composition of Plectranthus amboinicus (Lour.) Spreng essential oil and its biological activities. Am. J. Essent. Oil Nat. Prod. 2013, 1, 11–18. [Google Scholar]
- Erny, S.M.N.; Razali, M.; Mirfat, A.H.S.; Mohd Shukri, M.A. Antimicrobial activity and bioactive evaluation of Plectranthus amboinicus essential oil. Am. J. Res. Commun. 2014, 2, 121–127. [Google Scholar]
- El-Gohary, A.E.; Amer, H.M.; Salem, S.H.; Hussein, M.S. Foliar application of selenium and humic acid changes yield, essential oil, and chemical composition of Plectranthus amboinicus (Lour.) plant and its antimicrobial effects. Egypt. Pharm. J. 2019, 18, 365–367. [Google Scholar]
- Muniandy, K.; Hassan, Z.; Isa, M.H. The action of Coleus aromaticus as a potential wound healing agent in experimentally induced diabetic mice. PERINTIS E-J. 2014, 4, 1–30. [Google Scholar]
- Saraswati, S.; Jatinder, K.K.; Avinash, K.N. Analytical techniques for phytochemicals screening and bioactivities of some Coleus species: A review. J. Pharm. Sci. Res. 2016, 8, 227–237. [Google Scholar]
- Grayer, R.J.; Eckert, M.R.; Lever, A.; Veitch, N.C.; Kite, G.C.; Paton, A.J. Distribution of exudate flavonoids in the genus Plectranthus. Biochem. Syst. Ecol. 2010, 38, 335–341. [Google Scholar] [CrossRef]
- Lonhienne, T.; Mason, M.G.; Ragan, M.A.; Hugenholtz, P.; Schmidt, S.; Paungfoo Lonhienne, C. Yeast as a biofertilizer alters plant growth and morphology. Crop Sci. 2014, 54, 785–790. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, U.; Agrawal, V. Isolation and optimization of plumbagin production in root callus of Plumbago zeylanica L. augmented with chitosan and yeast extract. Ind. Crops Prod. 2020, 151, 112446. [Google Scholar] [CrossRef]
- Difco Manual. Dehydrated Culture Media and Reagents for Microbiology; Laboratories Incorporated Detroit: Detroit, MI, USA, 1984; Volume 48232, p. 1027. [Google Scholar]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecological 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.A.; Fernández-López, J.; ElRazik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A.; Sendra, E. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Protein; USDA Circular Series; US Department of Agriculture: Washington, DC, USA, 1931; Volume 183, pp. 1–21.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Badhani, A.; Sakalani, S.; Mishra, A.P. Variation in biochemical’s and antioxidant activity of some wild edible fruits of Uttarakhand. Rep. Opin. 2011, 3, 1–10. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Jackson, W.A. Nitrate acquisition and assimilation by higher plants: Processes in the root system. In Nitrogen in the Environment; Nielsen, D.R., MacDonald, J.G., Eds.; Academic Press: New York, NY, USA, 1978; Volume 2. [Google Scholar]
- Black, C.A.; Evans, D.D.; Ensminger, L.E. Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Jackson, B.L.J.; During, C.M. Studies of slowly available potassium in soils of New Zealand. I. Effects of leaching, temperature and potassium depletion on the equilibrium concentration of potassium in solution. Plant Soil 1979, 51, 197–204. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Hernández-Fernández, M.; Cordero-Bueso, G.; Ruiz-Muñoz, M.; Cantoral, J.M. Culturable Yeasts as Biofertilizers and Biopesticides for a Sustainable Agriculture: A Comprehensive Review. Plants 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Crescente, M.F.; Frattaroli, A.R.; Gratani, L. Morphological, anatomical and physiological leaf trait plasticity of Sesleria nitida (Poaceae) in open vs shaded conditions. Pol. J. Ecol. 2015, 63, 10–22. [Google Scholar]
- Sheha, A.M.; Abou El-Enin, M.M.; El-Hashash, E.F.; Rady, M.M.; El-Serafy, R.S.; Shaaban, A. The productivity and overall benefits of faba bean-sugar beet intercropping systems interacted with foliar-applied nutrients. J. Plant Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Fu, S.F.; Wei, J.Y.; Chen, H.W.; Liu, Y.Y.; Lu, H.Y.; Chou, J.Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.P.; Hunter, A.; Kashpur, O.; Normanly, J. Aberrant synthesis of indole-3-acetic acid in Saccharomyces cerevisiae triggers morphogenic transition, a virulence trait of pathogenic fungi. Genetics 2010, 185, 211–220. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J. Appl. Microbiol. 2004, 96, 69–75. [Google Scholar] [CrossRef] [PubMed]
- French, R.J.; Buirchell, B.J. Lupin: The largest grain legume crop in Western Australia, its adaptation and improvement through plant breeding. Aust. J. Agric. Res. 2005, 56, 1169–1180. [Google Scholar] [CrossRef]
- Sannazzaro, A.I.; Ruiz, O.A.; Albertó, E.O.; Menéndez, A.B. Alleviation of salt stress in Lotus glaber by Glomus intraradices. Plant Soil 2006, 285, 279–287. [Google Scholar] [CrossRef]
- Alwhib, M.S.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar]
- Sharma, I. Phytopathogenic fungi and their biocontrol applications. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nanotechnology; Sharma, V.K., Shah, M.P., Kumar, A., Eds.; Fungal Diversity of Sustainable Agriculture; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 155–188. [Google Scholar]
- Atteya, A.K.G.; El-Serafy, R.S.; El-Zabalawy, K.M.; Elhakem, A.; Genaidy, E.A.E. Brassinolide maximized the fruit and oil yield, induced the secondary metabolites, and stimulated linoleic acid synthesis of Opuntia ficus-indica oil. Horticulturae 2022, 8, 452. [Google Scholar] [CrossRef]
- Dixon, G.R.; Tilston, E.L. Soil Microbiology and Sustainable Crop Production; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Streletskii, R.A.; Kachalkin, A.V.; Glushakova, A.M.; Yurkov, A.M.; Demin, V.V. Yeasts producing zeatin. PeerJ 2019, 7, e6474. [Google Scholar] [CrossRef] [PubMed]
- El-Mehy, A.A.; El-Gendy, H.M.; Aioub, A.A.A.; Mahmoud, S.F.; Abdel-Gawad, S.; Elesawy, A.E.; Elnahal, A.S.M. Response of faba bean to intercropping, biological and chemical control against broomrape and root rot diseases. Saudi J. Biol. Sci. 2022, 29, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Osugi, A.; Sakakibara, H. Q&A: How do plants respond to cytokinins and what is their importance? BMC Boil. 2015, 13, 102. [Google Scholar]
- Agamy, R.; Hashem, M.; Alamri, S. Effect of soil amendment with yeasts as bio-fertilizers on the growth and productivity of sugar beet. Afr. J. Agric. Res. 2013, 8, 46–56. [Google Scholar]
- Akhtyamova, N.; Sattarova, R.K. Endophytic yeast Rhodotorula rubra strain TG-1: Antagonistic protection activities. Biochem. Physiol. 2013, 2, 1000104. [Google Scholar]
- Rakib, A.A.; Athab, M.A.; Matny, O.N. Management of potato virus Y (PVY) in potato by some biocontrol agents under field conditions. Adv. Environ. Biol. 2013, 7, 441–444. [Google Scholar]
- Sen, D.; Paul, K.; Saha, C.; Mukherjee, G.; Nag, M.; Ghosh, S.; Das, A.; Seal, A.; Tripathy, S. A unique life-strategy of an endophytic yeast Rhodotorula mucilaginosa JGTA-S1—A comparative genomics viewpoint. DNA Res. 2019, 26, 131–146. [Google Scholar] [CrossRef]
- Mou, H.; Lu, J.; Zhu, S.; Lin, C.; Tian, G.; Xu, X.; Zhao, W. Transcriptomic analysis of Paulownia infected by paulownia witches’-broom Phytoplasma. PLoS ONE 2013, 8, e77217. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.M. Production of sophorolipid biosurfactants by multiple species of the Starmerella (Candida) bombicola yeast clade. FEMS Microbiol. Lett. 2010, 311, 140–146. [Google Scholar] [CrossRef]
- Desai, S.N.; Farris, F.F.; Ray, S.D. Lipid Peroxidation, Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 89–93. [Google Scholar]
- Arnold, P.A.; Kruuk, L.E.B.; Nicotra, A.B. How to analyse plant phenotypic plasticity in response to a changing climate. New Phytol. 2019, 222, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- El-Serafy, R.S. Phenotypic plasticity, biomass allocation, and biochemical analysis of cordyline seedlings in response to oligo-Chitosan foliar spray. J. Soil Sci. Plant Nutr. 2020, 20, 1503–1514. [Google Scholar] [CrossRef]
- Hill, J.O.; Simpson, R.J.; Moore, A.D.; Chapman, D.F. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 2006, 286, 7–19. [Google Scholar] [CrossRef]
- Bossdorf, O.; Lipowsky, A.; Prati, D. Selection of readapted populations allowed Senecio inaequidens to invade Central Europe. Divers. Distrib. 2008, 14, 676–685. [Google Scholar] [CrossRef]
- Atteya, A.K.; El Gendy, A.E.N.G. Impact of actosol and yeast extract on productivity and essential oil constituents of Zinnia elegans plants. Biosci. Res. 2018, 15, 1542–1558. [Google Scholar]
- Złotek, U.; Szymanowska, U.; Rybczynska-Tkaczyk, K.; Jakubczyk, A. Effect of jasmonic acid, yeast extract elicitation, and drying methods on the main bioactive compounds and consumer quality of lovage (Levisticum Officinale Koch). Foods 2020, 9, 323. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Alhammad, B.A.; Alkahtani, J.; Alwahibi, M.S.; Mahdi, A.H.A. Activated yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy 2021, 11, 74. [Google Scholar] [CrossRef]
- Abbas, S.M. The influence of biostimulants on the growth and on the biochemical composition of Vicia faba cv. Giza 3 beans. Rom. Biotechnol. Lett. 2013, 18, 8061–8068. [Google Scholar]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Neseim, M.R.; Amin, A.Y.; El-Mohammady, M.M.S. Effect of potassium applied with foliar spray of yeast on sugar beet growth and yield under drought stress. Glob. Adv. Res. J. Agric. Sci. 2014, 3, 211–222. [Google Scholar]
- Medani, R.A.; Taha, R.S. Improving growth and yield of caraway (Carum carvi L.) plants by decapitation and/or active dry yeast application. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 47–60. [Google Scholar]
- Abdallah, M.M.S.; El Habbasha, S.F.; El Sebai, T. Comparison of yeast extract and Nicotinaminde foliar applications effect on quinoa plants grown under sandy soil condition. Int. J. PharmTech Res. 2016, 9, 24–32. [Google Scholar]
- Sugiah, S.; Balia, R.L.; Utama, G.L. The potential of mannoprotein extracted from candida apicola cell wall as emulsification agent. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2019, 19, 335–340. [Google Scholar]
- Chandra, P.; Arora, D.S. Antioxidant activity of fungi isolated from soil of different areas of Punjab, India. J. Appl. Nat. Sci. 2009, 1, 123–128. [Google Scholar] [CrossRef][Green Version]
- Rios, M.F.; Pajan, C.M.G.; Galan, R.H.; Sanchez, A.J.M.; Callado, I.G. Synthesis and free radical scavenging activity of a novel metabolite from the fungus Colletotrichum gloeosporioides. Bioorg. Med. Chem. Lett. 2006, 16, 5836–5839. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Oyebanji, E.; Fowora, M.; Aiyeolemi, A.; Orabuchi, C.; Akinnawo, B.; Adekunle, A.A. Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Complement. Med. Ther. 2021, 21, 98. [Google Scholar] [CrossRef]
- Atteya, A.K.; Albalawi, A.N.; El-Serafy, R.S.; Albalawi, K.N.; Bayomy, H.M.; Genaidy, E.A.E. Response of Moringa oleifera seeds and fixed oil production to vermicompost and NPK fertilizers under calcareous soil conditions. Plants 2021, 10, 1998. [Google Scholar] [CrossRef]
- Scervino, J.M.; Mesa, M.P.; Monica, I.D.; Recchi, M.; Moreno, N.S.; Godeas, A. Soil fungal isolates produce different organic acid patterns involved in phosphate salts solubilization. Biol. Fertil. Soil 2010, 46, 755–763. [Google Scholar] [CrossRef]
- Millan, A.F.-S.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef]
- Mohamed, H.M.; El-Homosy, R.F.; Abd-Ellatef, A.-E.H.; Salh, F.M.; Hussein, M.Y. Identification of yeast strains isolated from agricultural soils for releasing potassium-bearing minerals. Geomicrobiol. J. 2017, 34, 261–266. [Google Scholar] [CrossRef]
- Ahmed, M.E.M. Response of garlic plants (Allium sativum L.) to foliar application of some bio-stimulants. Egypt. J. Hortic. 2015, 42, 613–625. [Google Scholar]
- Ahmed, A.A.; El-Baky, M.A.; Zaki, M.F.; El-Aal, F.S.A. Effect of foliar application of active yeast extract and zinc on growth, yield and quality of potato plant (Solanum tuberosum L.). J. Appl. Sci. Res. 2011, 7, 2479–2488. [Google Scholar]
- Mahmoud, H.I.; Azzaz, N.A.; Khalifa, Y.A.; Mahmoud, M.A.; Fakhry, G. Effect of foliar application with active yeast extract and benzyladenine on some vegetative growth criteria and chemical composition of lupine (Lupinus termis L.) plants. Minia J. Agric. Res. Dev. 2016, 36, 193–214. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).