GC-MS Characterization, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Anvillea radiata Essential Oils
Abstract
:1. Introduction
2. Material and Method
2.1. Chemicals Used
2.2. Plant Material
2.3. GC-MS Identification of A. radiata Essential Oil
2.4. Evaluation of Antioxidant Potential of EOAR
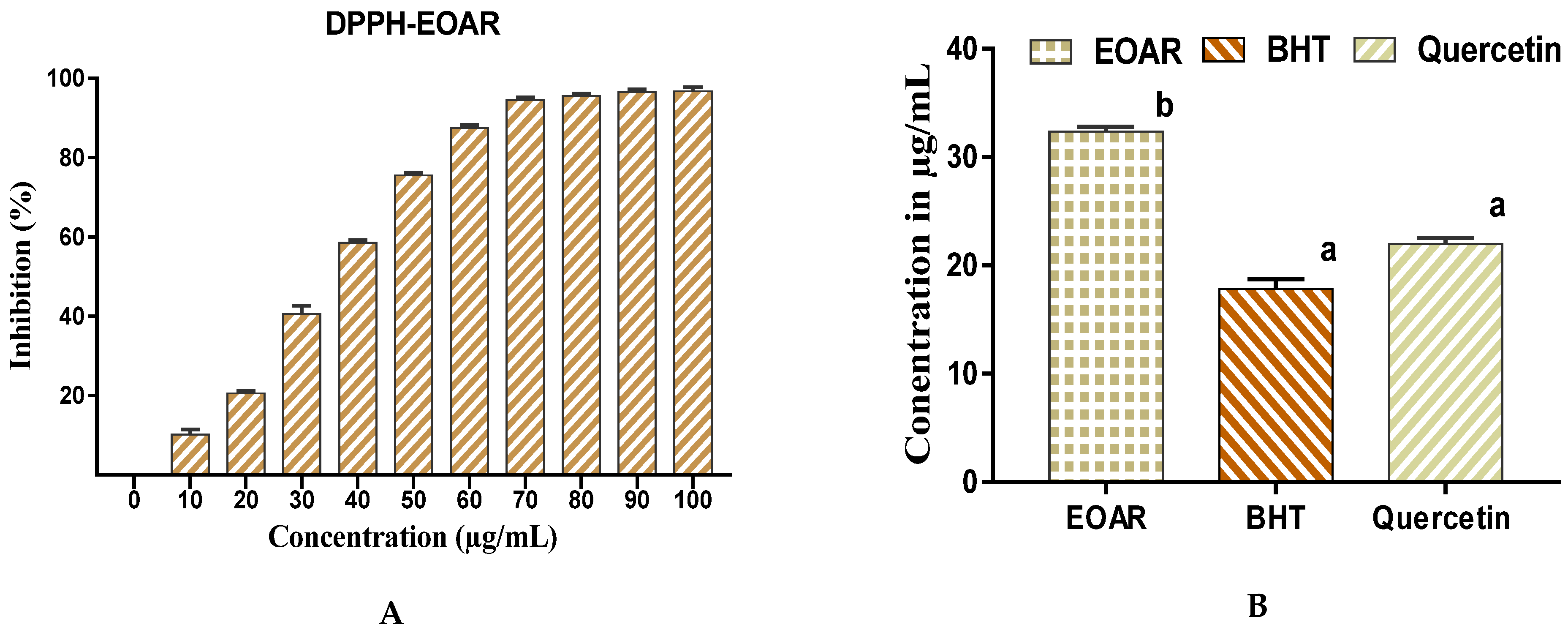
2.4.1. DPPH Test
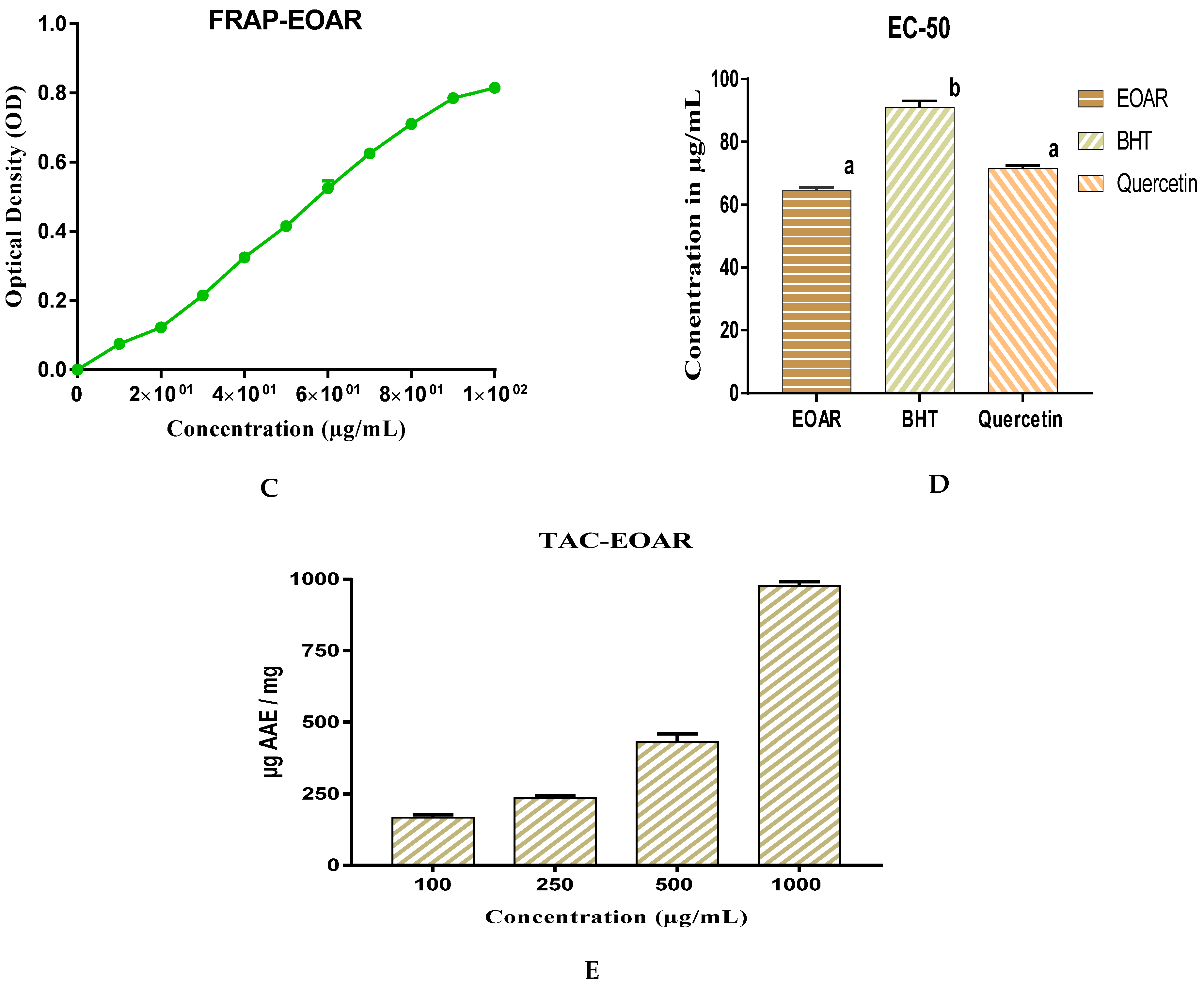
2.4.2. FRAP Test
2.4.3. TAC Test
2.5. Antimicrobial Activity
2.5.1. Stains Used for Testing
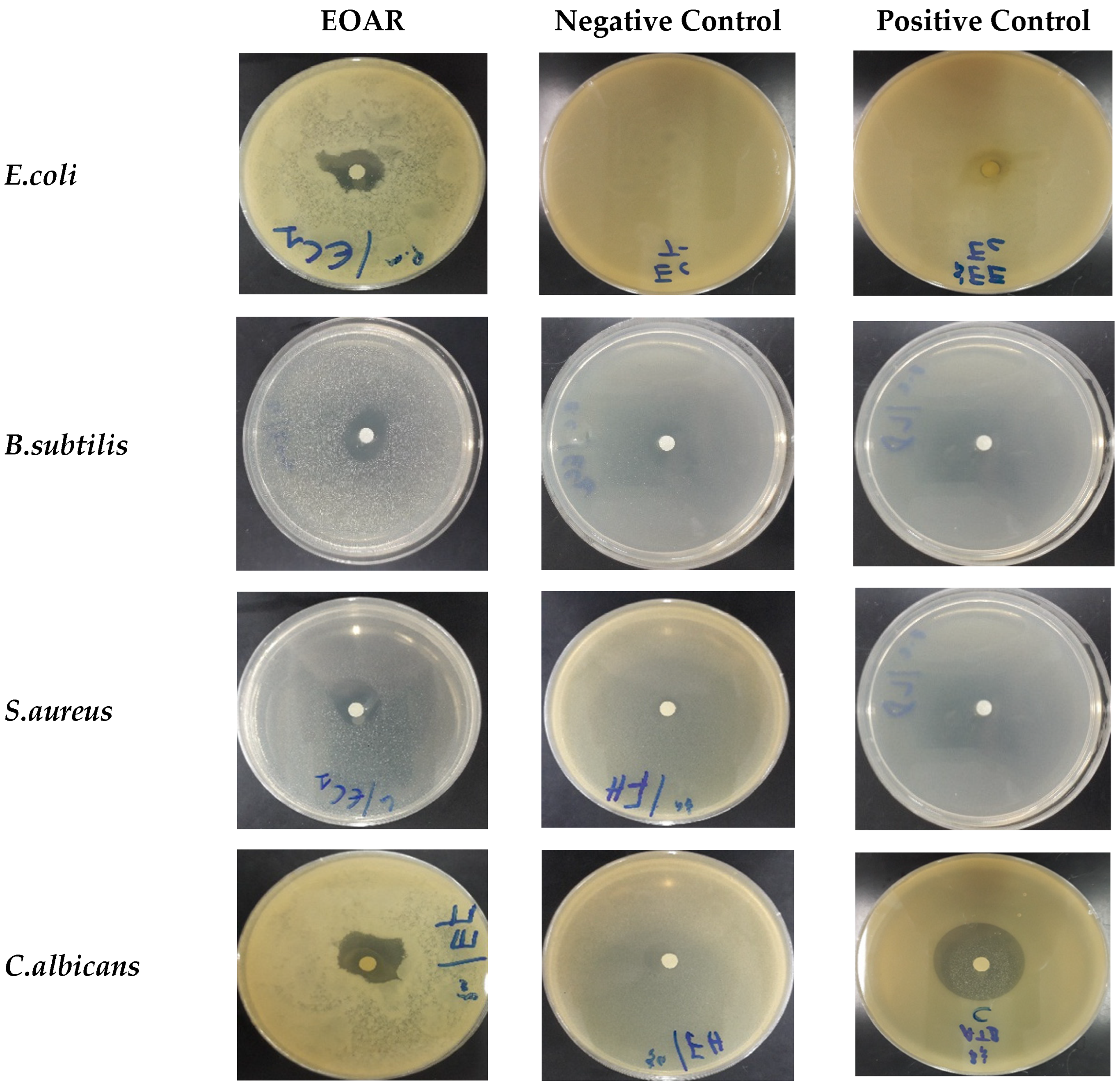
2.5.2. Antimicrobial Power on Solid Medium
2.5.3. Antimicrobial Power on Liquid Medium
2.6. Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
3.1. EOAR GC/MS Analysis
3.2. Antioxydant Activity of EOAR
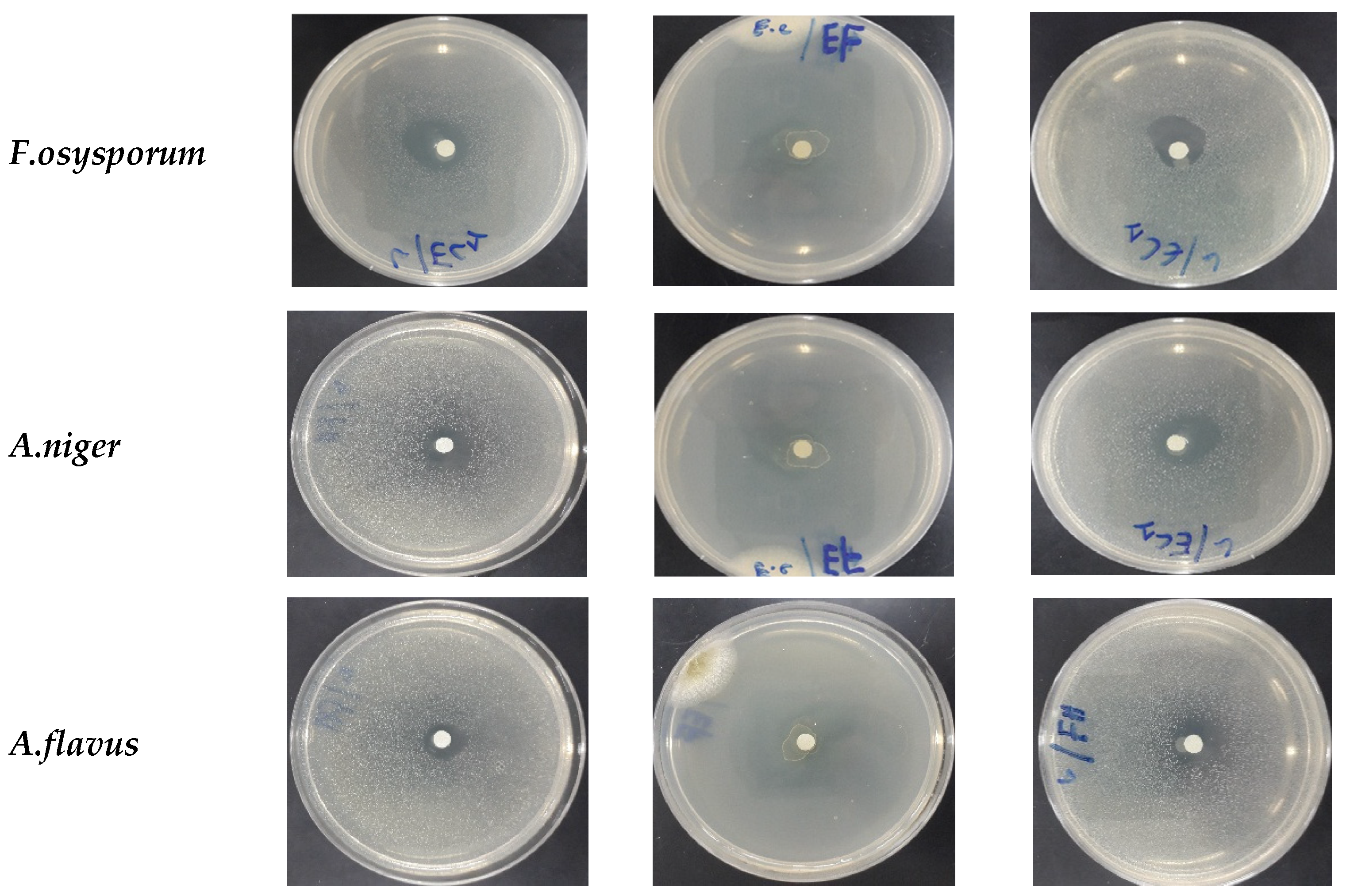
3.3. Antimicrobial Power of EOAR
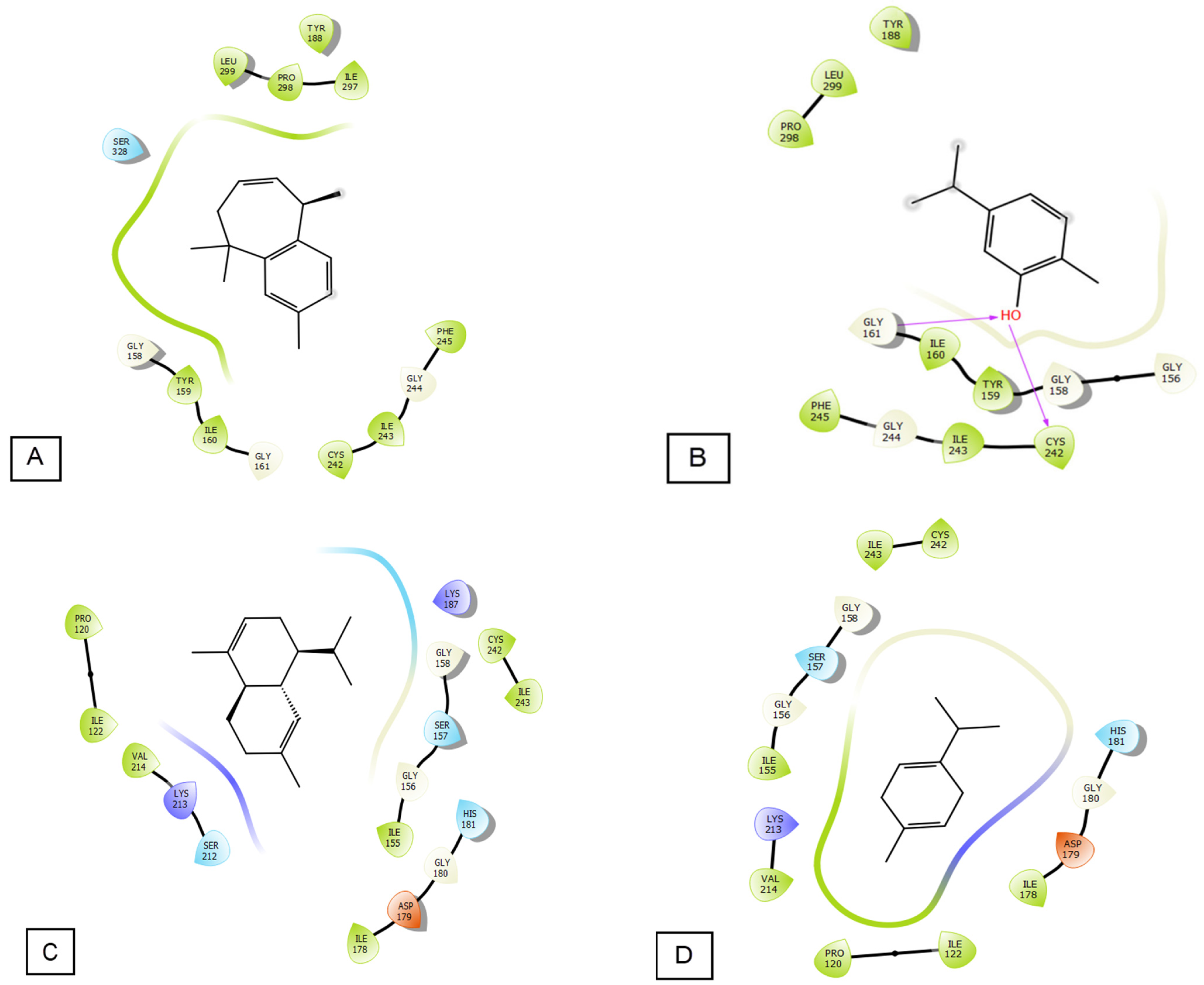
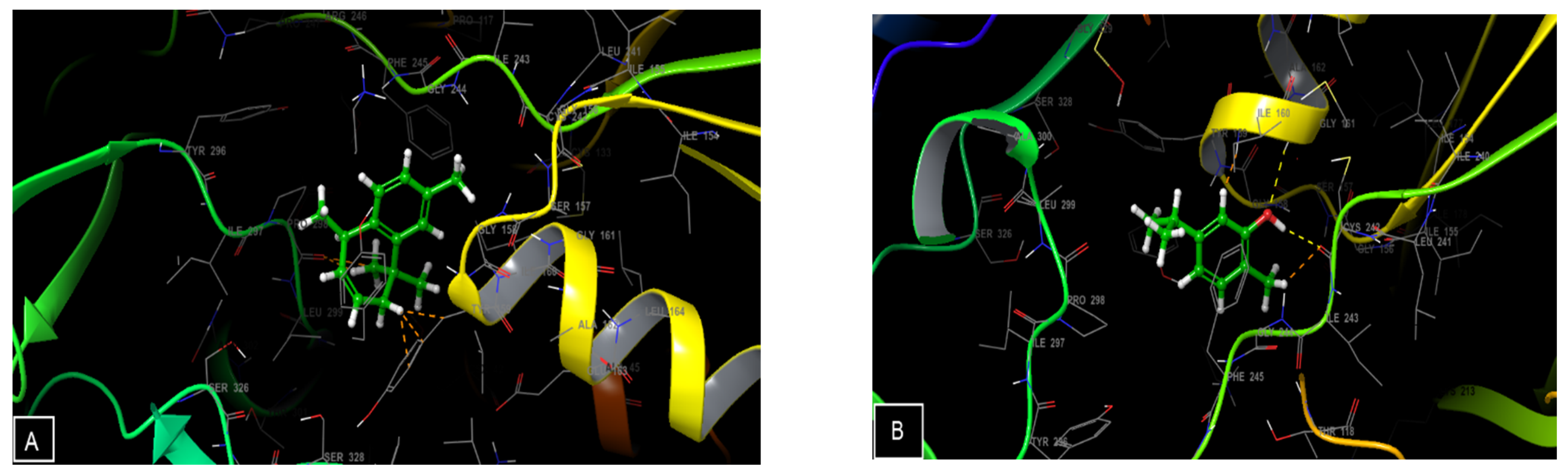
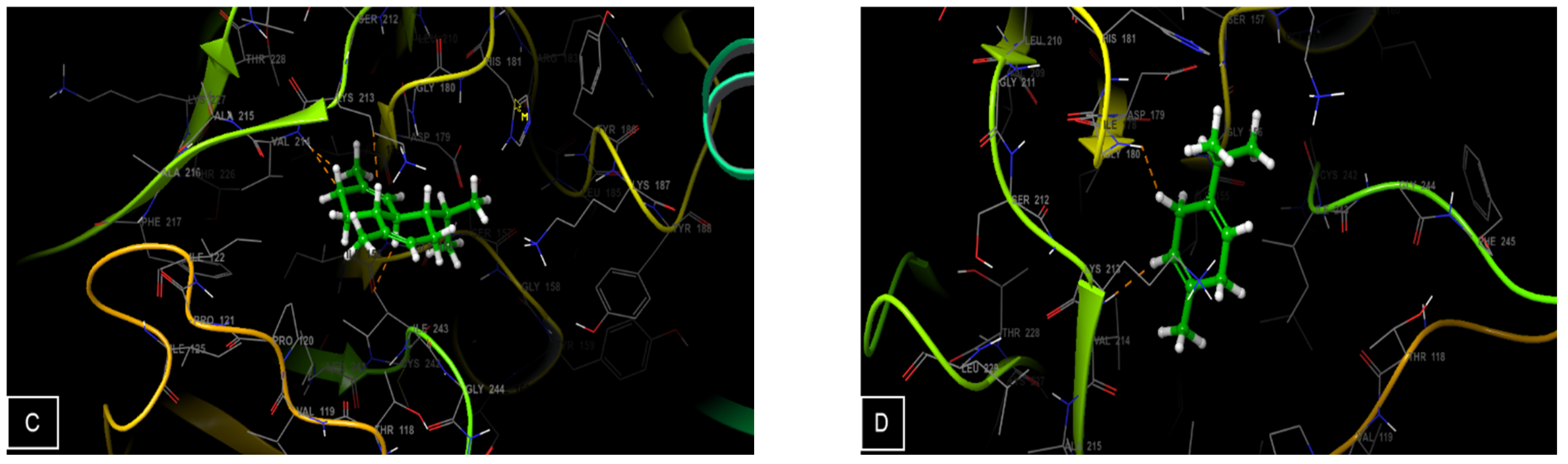
3.4. Molecular Docking
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rollinger, B.J.M.; Stuppner, H.; Hofbauergasse, C.M. Virtual screening for the discovery of bioactive natural products. In Natural Compounds in Drugs Volume I; Springer: Berlin/Heidelberg, Germany, 2008; Volume 65, pp. 211–249. [Google Scholar]
- Mahomoodally, M.F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evid.-Based Complementary Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Ghanmi, M.; Aafi, A.; Satrani, B.; Aberchane, M. Aromatic and Medicinal Plants in North Africa: Opportunities, Constraints and Prospects. In Novel Plant Bioresources: Applications in Food, Medicine and Cosmetics; Wiler Online Library: Hoboken, NJ, USA, 2014; pp. 411–423. [Google Scholar]
- Idm’Hand, E.; Msanda, F.; Cherifi, K. Ethnobotanical study and biodiversity of medicinal plants used in the Tarfaya Province, Morocco. Acta Ecol. Sin. 2020, 40, 134–144. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A.M.; Yusufoglu, H.S.; Fawzy, G.A.; Foudah, A.; Abdel-Kader, M.S. Hepatoprotective and cytotoxic activities of Anvillea garcinii and isolation of four new secondary metabolites. J. Nat. Med. 2017, 72, 106–117. [Google Scholar] [CrossRef]
- Hebi, M.; Eddouks, M. Glucose Lowering Activity of Anvillea Radiata Coss & Durieu in Diabetic Rats. Cardiovasc. Haematol. Disord.-Drug Targets 2018, 18, 71–80. [Google Scholar] [CrossRef]
- Kandouli, C.; Cassien, M.; Mercier, A.; Delehedde, C.; Ricquebourg, E.; Stocker, P.; Mekaouche, M.; Leulmi, Z.; Mechakra, A.; Thétiot-Laurent, S.; et al. Antidiabetic, antioxidant and anti inflammatory properties of water and n-butanol soluble extracts from Saharian Anvillea radiata in high-fat-diet fed mice. J. Ethnopharmacol. 2017, 207, 251–267. [Google Scholar] [CrossRef]
- Akdad, M.; Ajebli, M.; Breuer, A.; Khallouki, F.; Owen, R.W.; Eddouks, M. Study of Antihypertensive Activity of Anvillea radiata in L-Name-Induced Hypertensive Rats and HPLC-ESI-MS Analysis. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1059–1072. [Google Scholar] [CrossRef]
- Gutteridge, J.C.M.; Halliwell, B. Free Radicals and Antioxidants in the Year 2000: A Historical Look to the Future. Ann. N. Y. Acad. Sci. 2006, 899, 136–147. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S. Free Radicals and Antioxidants in Human Health: Current Status and Future Prospects R ESEARCH. Japi 2004, 52, 4. [Google Scholar]
- Barber, D.A.; Harris, S.R. Oxygen Free Radicals and Antioxidants: A Review. Am. Pharm. 1994, 34, 26–35. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative da and pathogenesis. Curr. Sci. 1999, 77, 658–666. [Google Scholar]
- Nath, K.A.; Norby, S.M. Reactive Oxygen Species and Acute Renal Failure of reactive oxygen species. Am. J. Med. 2000, 109, 665–678. [Google Scholar] [CrossRef]
- Wolff, S.P.; Garner, A.; Dean, R.T. Free radicals, lipids and protein degradation. Trends Biochem. Sci. 1986, 11, 27–31. [Google Scholar] [CrossRef]
- Hensley, K.; Floyd, R.A. Reactive Oxygen Species and Protein Oxidation in Aging: A Look Back, A Look Ahead. Arch. Biochem. Biophys. 2002, 397, 377–383. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Talbaoui, A.; Khouchlaa, A.; Charfi, S.; Abrini, J.; Dakka, N. Résistance aux antibiotiques et mécanismes d’action des huiles essentielles contre les bactériesResistance to antibiotics and mechanisms of action of essential oils against bacteria. Phytothérapie 2017, 1–11. [Google Scholar] [CrossRef]
- Chaudhary, A.S. A review of global initiatives to fight antibiotic resistance and recent antibiotics discovery. Acta Pharm. Sin. B 2016, 6, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Dung, N.T.; Kim, J.M.; Kang, S.C. Chemical composition, antimicrobial and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb.) Merr and Perry buds. Food Chem. Toxicol. 2008, 46, 3632–3639. [Google Scholar] [CrossRef]
- Et-Touys, A.; Fellah, H.; Mniouil, M.; Bouyahya, A.; Dakka, N.; Abdennebi, E.H.; Sadak, A.; Bakri, Y. Screening of Antioxidant, Antibacterial and Antileishmanial Activities of Salvia officinalis L. Extracts from Morocco. Br. Microbiol. Res. J. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; El-Baabou, A.; Dakka, Y.B.A.N. Determination of Phenol Content and Antibacterial Activity of Five Medicinal Plants Ethanolic Extracts from North-West of Morocco. J. Plant Pathol. Microbiol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Moussaoui, N.; Abrini, J.; Bakri, Y.; Dakka, N. Determination of Phenolic Contents, Antioxidant and Antibacterial Activities of Strawberry Tree (Arbutus unedo L.) Leaf Extracts. Br. Biotechnol. J. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2015, 32, 117–160. [Google Scholar] [CrossRef]
- Petrovi, J.; Stojkovi, D.; Sokovi, M. Terpene Core in Selected Aromatic and Edible Plants: Natural Health Improving Agents, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- EL Moussaoui, A.; Bourhia, M.; Jawhari, F.; Salamatullah, A.; Ullah, R.; Bari, A.; Mahmood, H.M.; Sohaib, M.; Serhii, B.; Rozhenko, A.; et al. Chemical Profiling, Antioxidant, and Antimicrobial Activity against Drug-Resistant Microbes of Essential Oil from Withania frutescens L. Appl. Sci. 2021, 11, 5168. [Google Scholar] [CrossRef]
- Chebbac, K.; EL Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Guemmouh, R. Chemical Analysis and Antioxidant and Antimicrobial Activity of Essential oils from Artemisia negrei L. against Drug-Resistant Microbes. Evid. Based Complement. Altern. Med. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Benbrahim, K.F.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens L. Bioorganic Chem. 2019, 93, 103337. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Kadiri, M.; Bourhia, M.; Agour, A.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Chedadi, M.; Sfaira, M.; et al. Promising Antioxidant and Anticorrosion Activities of Mild Steel in 1.0 M Hydrochloric Acid Solution by Withania frutescens L. Essential Oil. Front. Chem. 2021, 9, 760. [Google Scholar] [CrossRef]
- El Abdali, Y.; Agour, A.; Allali, A.; Bourhia, M.; El Moussaoui, A.; Eloutassi, N.; Salamatullah, A.M.; Alzahrani, A.; Ouahmane, L.; Aboul-Soud, M.A.M.; et al. Lavandula dentata L.: Phytochemical Analysis, Antioxidant, Antifungal and Insecticidal Activities of Its Essential Oil. Plants 2022, 11, 311. [Google Scholar] [CrossRef]
- Zouirech, O.; Alyousef, A.A.; El Barnossi, A.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Ouahmane, L.; Giesy, J.P.; Aboul-Soud, M.A.M.; Lyoussi, B.; et al. Phytochemical Analysis and Antioxidant, Antibacterial, and Antifungal Effects of Essential Oil of Black Caraway (Nigella sativa L.) Seeds against Drug-Resistant Clinically Pathogenic Microorganisms. BioMed Res. Int. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Saghrouchni, H.; El Barnossi, A.; Chefchaou, H.; Mzabi, A.; Tanghort, M.; Remmal, A.; Fouzia, C. Study the effect of carvacrol, eugenol and thymol on fusariums sp responsible for lolium perenne fusariosis. Ecol. Environ. Conserv. 2020, 26, 1059–1067. [Google Scholar]
- Remmal, A.; Bouchikhi, T.; Rhayour, K.; Ettayebi, M.; Tantaoui-Elaraki, A. Improved Method for the Determination of Antimicrobial Activity of Essential Oils in Agar Medium. J. Essent. Oil Res. 1993, 5, 179–184. [Google Scholar] [CrossRef]
- Saghrouchni, H.; El Barnossi, A.; Salamatullah, A.; Bourhia, M.; Alzahrani, A.; Alkaltham, M.; Alyahya, H.; Tahiri, N.; Imtara, H.; Var, I. Carvacrol: A Promising Environmentally Friendly Agent to Fight Seeds Damping-Off Diseases Induced by Fungal Species. Agronomy 2021, 11, 985. [Google Scholar] [CrossRef]
- Chalkha, M.; El Moussaoui, A.; Ben Hadda, T.; Berredjem, M.; Bouzina, A.; Almalki, F.A.; Saghrouchni, H.; Bakhouch, M.; Saadi, M.; El Ammari, L.; et al. Crystallographic study, biological evaluation and DFT/POM/Docking analyses of pyrazole linked amide conjugates: Identification of antimicrobial and antitumor pharmacophore sites. J. Mol. Struct. 2021, 1252, 131818. [Google Scholar] [CrossRef]
- El Hanbali, F.; El Hakmaoui, A.; Mellouki, F.; El Rhaffari, L.; Akssira, M. Chemical Composition and Antibacterial Activity of Essential Oil of Anvillea radiata Coss. & Dur. Nat. Prod. Commun. 2007, 2, 595–597. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Bourhia, M.; Jawhari, F.Z.; Khalis, H.; Chedadi, M.; Agour, A.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Alotaibi, A.; et al. Responses of Withania frutescens (L.) Pauquy (Solanaceae) Growing in the Mediterranean Area to Changes in the Environmental Conditions: An Approach of Adaptation. Front. Ecol. Evol. 2021, 9, 666005. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The Alarming Antimicrobial Resistance in ESKAPEE Pathogens: Can Essential Oils Come tothe Rescue? Fitoterapia 2020, 140, 10443. [Google Scholar] [CrossRef] [PubMed]
- Mssillou, I.; Agour, A.; Allali, A.; Saghrouchni, H.; Bourhia, M.; El Moussaoui, A.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant, Antimicrobial, and Insecticidal Properties of a Chemically Characterized Essential Oil from the Leaves of Dittrichia viscosa L. Molecules 2022, 27, 2282. [Google Scholar] [CrossRef] [PubMed]
- Lafraxo, S.; El Barnossi, A.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Akka, A.A.; Choubbane, A.; Akhazzane, M.; Aboul-Soud, M.A.M.; et al. Essential Oils from Leaves of Juniperus thurifera L., Exhibiting Antioxidant, Antifungal and Antibacterial Activities against Antibiotic-Resistant Microbes. Horticulturae 2022, 8, 321. [Google Scholar] [CrossRef]
- Benslama, A.; Harrar, A.; Gül, F.; Demirtaş, I. In vitro Antioxidant, Antibacterial Activities and HPLC-TOF/MS Analysis of Anvillea radiata (Asteraceae) Extracts. Curr. Nutr. Food Sci. 2019, 15, 376–383. [Google Scholar] [CrossRef]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Ouaritini, Z.B.; Salamatullah, A.M.; Alzahrani, A.; Aboul-soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef]
- Lu, M.; Dai, T.; Murray, C.K.; Wu, M.X. Bactericidal Property of Oregano Oil against Multidrug-Resistant Clinical Isolates. Front. Microbiol. 2018, 9, 2329. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.; Donnell, C.P.O.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef]
- Bouhdid, S.; Abrini, J.; Amensour, M.; Zhiri, A.; Espuny, M.J.; Manresa, A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. J. Appl. Microbiol. 2010, 109, 1139–1149. [Google Scholar] [CrossRef]
- Hamada, D.; Ladjel, S. Chemical Composition, In-Vitro Anti-microbial and Antioxidant Activities of the Methanolic Extract of Anvillea radiata Asteraceae. Res. J. Pharm. Biol. Chem. 2015, 6, 1367. [Google Scholar]
- Mastelić, J.; Jerković, I.; Blažević, I.; Poljak-Blaži, M.; Borović, S.; Ivančić-Baće, I.; Smrěcki, V.; Žarković, N.; Brčić-Kostic, K.; Vikić-Topić, D.; et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
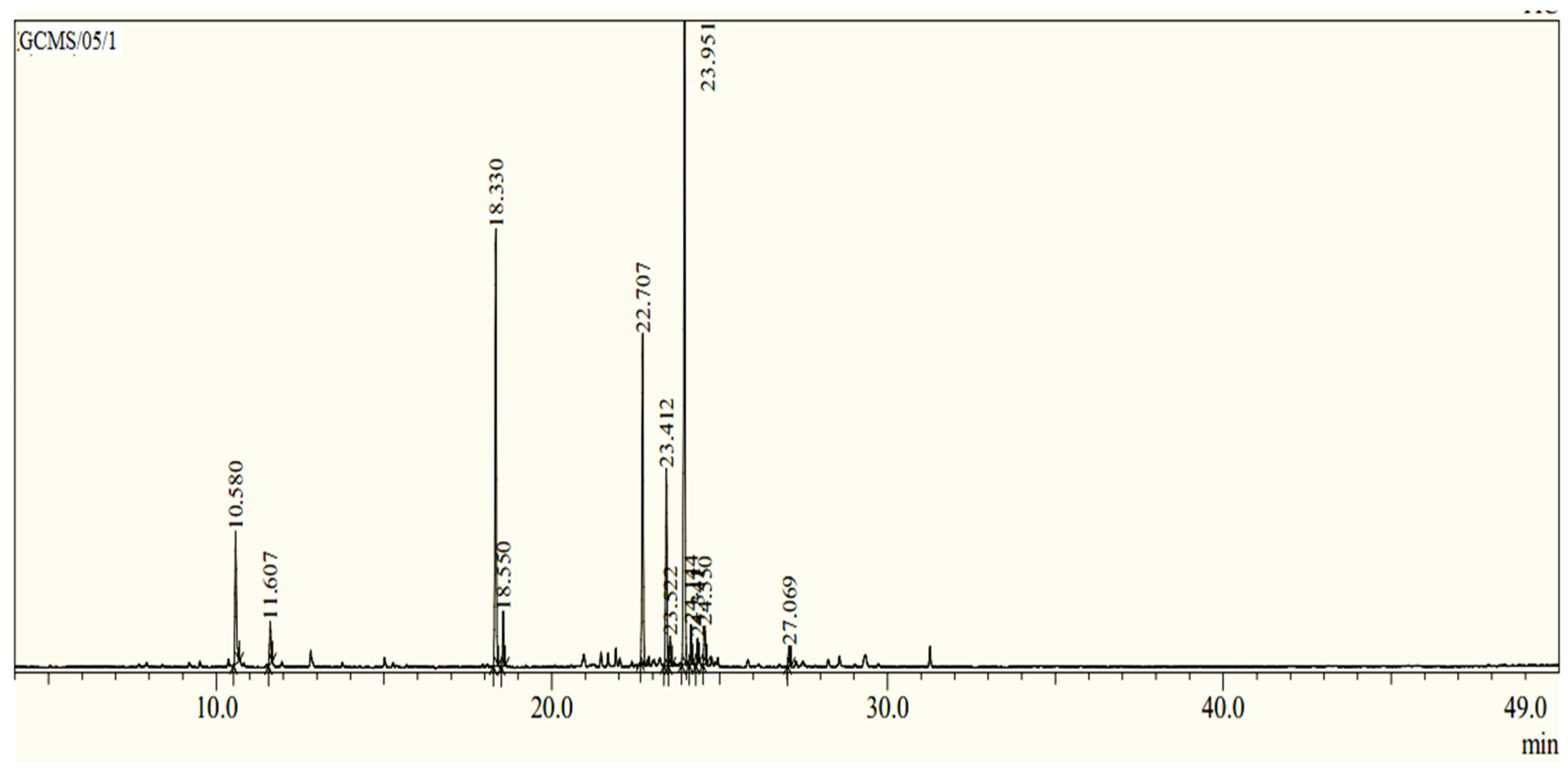


| Peaks | Retention Time | Compound | Chemical Class | Kovats Index | Area (%) |
|---|---|---|---|---|---|
| 1 | 10.580 | O-Cymene | MOH | 1026 | 7.06 |
| 2 | 11.607 | ϒ-Terpinene | MOH | 1056 | 2.05 |
| 3 | 18.330 | Camphor | MOO | 1290 | 21.41 |
| 4 | 18.550 | Carvacrol | MOO | 1299 | 2.54 |
| 5 | 22.707 | α-Himachalene | SEH | 1451 | 15.88 |
| 6 | 23.412 | Longifolene | SEH | 1407 | 9.77 |
| 7 | 23.522 | α-Cedrene | SEH | 1411 | 1.42 |
| 8 | 23.951 | α-Cuprenene | SEH | 1505 | 33.48 |
| 9 | 24.144 | Isolongifolene | SEH | 1390 | 1.82 |
| 10 | 24.341 | α-Cadinen | SEH | 1538 | 1.15 |
| 11 | 24.550 | ϒ-Dehydro-ar himachalene | SEH | 1517 | 2.31 |
| 12 | 27.069 | β-Himachalene oxide | SEO | 1616 | 1.09 |
| Monoterpene hydrocarbon (MOH) | 9.11% | ||||
| Monoterpene oxygene (MOO) | 23.95% | ||||
| Sesquiterpene hydrocarbon (SEH) | 65.83 | ||||
| Sesquiterpene oxygene (SEO) | 1.09% | ||||
| Total | 99.98% | ||||
| Glide Gscore | Glide Emodel | Glide Energy | |
|---|---|---|---|
| γ-dehydro-ar-himachalene | −6.233 | −31.939 | −22.777 |
| Carvacrol | −6.082 | −32.356 | −23.536 |
| α-cadinene | −5.057 | −27.232 | −21.43 |
| γ-terpinene | −4.91 | −24.19 | −19.057 |
| β-himachalene oxide | −4.415 | −17.108 | −15.532 |
| O-cymen | −4.121 | −35.819 | −29.056 |
| Longifolene | −3.861 | −29.233 | −23.893 |
| α-himachalene | −3.859 | −19.536 | −11.804 |
| Camphor | −3.845 | −21.596 | −17.537 |
| α-cedrene | −3.841 | −9.574 | −0.657 |
| β-himachalene oxide | −3.752 | −19.554 | −15.836 |
| Longifolene | −3.097 | −12.851 | −10.753 |
| Isolongifolene | −2.759 | −13.925 | −4.531 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beniaich, G.; Hafsa, O.; Maliki, I.; Bin Jardan, Y.A.; El Moussaoui, A.; Chebaibi, M.; Agour, A.; Zouirech, O.; Nafidi, H.-A.; Khallouki, F.; et al. GC-MS Characterization, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Anvillea radiata Essential Oils. Horticulturae 2022, 8, 886. https://doi.org/10.3390/horticulturae8100886
Beniaich G, Hafsa O, Maliki I, Bin Jardan YA, El Moussaoui A, Chebaibi M, Agour A, Zouirech O, Nafidi H-A, Khallouki F, et al. GC-MS Characterization, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Anvillea radiata Essential Oils. Horticulturae. 2022; 8(10):886. https://doi.org/10.3390/horticulturae8100886
Chicago/Turabian StyleBeniaich, Ghada, Ouattar Hafsa, Imane Maliki, Yousef A. Bin Jardan, Abdelfattah El Moussaoui, Mohamed Chebaibi, Abdelkrim Agour, Otmane Zouirech, Hiba-Allah Nafidi, Farid Khallouki, and et al. 2022. "GC-MS Characterization, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Anvillea radiata Essential Oils" Horticulturae 8, no. 10: 886. https://doi.org/10.3390/horticulturae8100886
APA StyleBeniaich, G., Hafsa, O., Maliki, I., Bin Jardan, Y. A., El Moussaoui, A., Chebaibi, M., Agour, A., Zouirech, O., Nafidi, H.-A., Khallouki, F., Bourhia, M., & Taleb, M. (2022). GC-MS Characterization, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Anvillea radiata Essential Oils. Horticulturae, 8(10), 886. https://doi.org/10.3390/horticulturae8100886






