Dose-Dependent Potential of Chitosan to Increase Yield or Bioactive Compound Content in Tomatoes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Fruit Yield
3.2. Fruit Color
3.3. Fruit Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of Chitosan on Plant Responses with Special Reference to Abiotic Stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Parvin, M.A.; Zakir, H.M.; Naznin, S.; Seal, P. Effects of Different Application Methods of Chitosan on Growth, Yield and Quality of Tomato (Lycopersicon esculentum Mill.). J. Arch. Agric. Environ. Sci. 2019, 4, 261–267. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant Activity of Chitosan in Horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Cho, M.H.; No, H.K.; Prinyawiwatkul, W. Chitosan Treatments Affect Growth and Selected Quality of Sunflower Sprouts. J. Food Sci. 2008, 73, S70–S77. [Google Scholar] [CrossRef]
- No, H.K.; Lee, K.S.; Kim, I.D.; Park, M.J.; Kim, S.D.; Meyers, S.P. Chitosan Treatment Affects Yield, Ascorbic Acid Content, and Hardness of Soybean sprouts. J. Food Sci. 2003, 68, 680–685. [Google Scholar] [CrossRef]
- Del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate Change Impacts and Adaptation Strategies of Agriculture in Mediterranean-Climate Regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Gonzalo, M.; Li, Y.C.; Chen, K.Y.; Gil, D.; Montoro, T.; Nájera, I.; Baixauli, C.; Granell, A.; Monforte, A. Genetic Control of Reproductive Traits in Tomatoes Under High Temperature. Front. Plant Sci. 2020, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Shamshiri, R.R.; Jones, J.W.; Thorp, K.R.; Ahmad, D.; Man, H.C.; Taheri, S. Review of Optimum Temperature, Humidity, and Vapour Pressure Deficit for Microclimate Evaluation and Control in Greenhouse Cultivation of Tomato: A Review. Int. Agrophysics 2018, 32, 287–302. [Google Scholar] [CrossRef]
- Islam, M.T. Effect of Temperature on Photosynthesis, Yield Attributes and Yield of Tomato Genotypes. J. Exp. Agric. Int. 2011, 2, 8–11. [Google Scholar]
- Botella, M.A.; Hernandez, V.; Mestre, T.; Hellin, P.; Garcia-Legaz, M.F.; Rivero, R.M.; Martinez, V.; Fenoll, J.; Flores, P. Bioactive Compounds of Tomato Fruit in Response to Salinity, Heat and Their Combination. Agriculture 2021, 11, 534. [Google Scholar] [CrossRef]
- Arena, C.; Conti, S.; Francesca, S.; Melchionna, G.; Hájek, J.; Barták, M.; Barone, A.; Rigano, M.M. Eco-Physiological Screening of Different Tomato Genotypes in Response to High Temperatures: A Combined Field-to-Laboratory Approach. Plants 2020, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wolters-Ars, M.; Mariani, C.; Huber, H.; Rieu, I. Heat Stress Affects Vegetative and Reproductive Performance and Trait Correlations in Tomato (Solanum lycopersicum). Euphytica 2017, 213, 156. [Google Scholar] [CrossRef]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Increased Temperature Produces Changes in the Bioactive Composition of Tomato, Depending on its Developmental Stage. J. Agric. Food Chem. 2015, 63, 2378–2382. [Google Scholar] [CrossRef]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W. The Tomato as a Functional Food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef]
- Shah, K.; Modi, B.; Lamsal, B.; Shrestha, J.; Aryal, S.; Shah, K. Bioactive Compounds in Tomato and their Roles in Disease Prevention. Fundam. Appl. Agric. 2021, 6, 210–224. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Doorenbos, J.; Pruitt, W.O. Crop Water Requirements; FAO Irrigation and Drainage Paper; FAO: Rome, Italy, 1977; p. 144. [Google Scholar]
- Keller, J.; Bliesner, R.D. Sprinkle and Trickle Irrigation; Blackburn Press: Caldwell, NJ, USA, 2001. [Google Scholar]
- Flores, P.; Hernandez, V.; Hellin, P.; Fenoll, J.; Cava, J.; Mestre, T.; Martinez, V. Metabolite Profile of the Tomato Dwarf Cultivar Micro-Tom and Comparative Response to Saline and Nutritional Stresses with Regard to a Commercial Cultivar. J. Sci. Food Agric. 2016, 96, 1562–1570. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Ramadan, W.A. Effect of Zinc Foliar Spray Alone and Combined with Humic Acid or/and Chitosan on Growth, Nutrient Elements Content and Yield of Dry Bean (Phaseolus vulgaris L.) Plants Sown at Different Dates. Sci. Hortic. 2015, 184, 101–105. [Google Scholar] [CrossRef]
- El-Miniawy, S.; Ragab, M.; Youssef, S.; Metwally, A. Response of Strawberry Plants to Foliar Spraying of Chitosan. Res. J. Agric. Biol. Sci. 2013, 9, 366–372. [Google Scholar]
- Mahmood, N.; Abbasi, N.A.; Hafiz, I.A.; Ali, I.; Zakia, S. Effect of Biostimulants on Growth, Yield and Quality of Bell Pepper cv. Yolo Wonder. Pak. J. Agric. Sci. 2017, 54, 311–318. [Google Scholar] [CrossRef]
- Sultana, S.; Islam, M.; Khatun, A.; Huque, R. Effect of Foliar Application of Oligo-Chitosan on Growth, Yield and Quality of Tomato and Eggplant. Asian J. Agric. Res. 2017, 11, 36–42. [Google Scholar] [CrossRef][Green Version]
- Mondal, M.M.A.; Puteh, A.B.; Dafader, N.C. Foliar Application of Chitosan Improved Morpho-Physiological Attributes and Yield in Summer Tomato (Solanum lycopersicum). Pak. J. Agric. Sci. 2016, 53, 339–344. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Baños, S.; Hernández-Lauzardo, A.N.; Velázquez-del Valle, M.G.; Hernández-López, M.; Ait-Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a Potencial Natural Compounds to Control Pre and Postharvest Diseases of Horticultural Commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Akila, G.; Einstein Charles, R. Chitosan-Induced Defence Responses in Tomato Plants Against Early Blight Disease Caused by Alternaria solani (Ellis and Martin) Sorauer. Arch. Phytopatholy Plant Prot. 2014, 47, 1777–1787. [Google Scholar] [CrossRef]
- Ali, M.; Muhammad, C.; Zahoor, A.; Rashid, H.; Rashid, S. Optimization of Chitosan Level to Alleviate the Drastic Effects of Heat Stress in Cucumber (Cucumis sativus L.). J. Pure Appl. Agric. 2020, 5, 30–38. [Google Scholar]
- Bittelli, M.; Flury, M.; Campbell, G.S.; Nichols, E.J. Reduction of Transpiration through Foliar Application of Chitosan. Agric. For. Meteorol. 2001, 107, 167–175. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Kabelka, E.; Yang, W.C.; Francis, D.M. Improved Tomato Fruit Color within an Inbred Backcross Line Derived from Lycopersicon esculentum and L. hirsutum Involves the Interaction of Loci. J. Am. Soc. Hortic. Sci. 2004, 129, 250–257. [Google Scholar] [CrossRef]
- Montesano, D.; Cossignani, L.; Bosi, A.; Simonetti, M.S.; Damiani, P. Innovative Extraction Procedure for Obtaining High Pure Lycopene from Tomato. Eur. Food Res. Technol. 2008, 226, 327–335. [Google Scholar] [CrossRef]
- López-Camelo, A.F.; Gómez, P.A. Comparison of Color Indexes for Tomato Ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending Shelf Life and Maintaining Quality of Tomato Fruit by Calcium Chloride, Hydrogen Peroxide, Chitosan, and Ozonated Water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Safari, Z.S.; Ding, P.; Nakasha, J.J.; Yusoff, S.F. Combining Chitosan and Vanillin to Retain Postharvest Quality of Tomato Fruit During Ambient Temperature Storage. Coatings 2020, 10, 1222. [Google Scholar] [CrossRef]
- Almunqedhi, B.; Kassem, H.; Al-Harbi, A.R. Effect of Preharvest Chitosan and/or Salicylic acid Spray on Quality and Shelf Life of Tomato Fruits. J. Sci. Eng. Res. 2017, 4, 114–122. [Google Scholar]
- Zhao, J.; Xu, Y.; Ding, Q.; Huang, X.; Zhang, Y.; Zou, Z.; Li, M.; Cui, L.; Zhang, J. Association mapping of Main Tomato Fruit Sugars and Organic Acids. Front. Plant Sci. 2016, 7, 1286. [Google Scholar] [CrossRef]
- El Amerany, F.; Meddich, A.; Wahbi, S.; Porzel, A.; Taourirte, M.; Rhazi, M.; Hause, B. Foliar Application of Chitosan Increases Tomato Growth and Influences Mycorrhization and Expression of Endochitinase-Encoding Genes. Int. J. Mol. Sci. 2020, 21, 535. [Google Scholar] [CrossRef]
- García-Mier, L.; Jimenez-Garcia, S.; Guevara-Gonzalez, R.; Feregrino-Perez, A.; Contreras-Medina, L.; Torres-Pacheco, I. Elicitor Mixtures Significantly Increase Bioactive Compounds, Antioxidant Activity, and Quality Parameters in Sweet Bell Pepper. J. Chem. 2015, 2015, 269296. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Bondok, A.M. Response of Tomato Plants to Salicylic Acid and Chitosan under Infection with Tomato Mosaic Virus. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 1520–1529. [Google Scholar] [CrossRef]
- Shehata, S.A.; Fawzy, Z.F.; El-Ramady, H.R. Response of Cucumber Plants to Foliar Application of Chitosan and Yeast under Greenhouse Conditions. Aust. J. Basic Appl. Sci. 2012, 6, 63–71. [Google Scholar]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors that Can Affect their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Perez-Balibrea, S.; Moreno, D.A.; Garcia-Viguera, C. Improving the Phytochemical Composition of Broccoli Sprouts by Elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Khan, W.M.; Prithiviraj, B.; Smith, D.L. Effect of Foliar Application of Chitin and Chitosan Oligosaccharides on Photosynthesis of Maize and Soybean. Photosynthetica 2002, 40, 621–624. [Google Scholar] [CrossRef]
- Inestroza-Lizardo, C.; Mattiuz, B.H.; da Silva, J.P.; Voigt, V.; Muniz, A.C.; Pinsetta Junior, J.S. Effect of Hyperbaric Pressure on the Activity of Antioxidant Enzymes and Bioactive Compounds of cv. ‘Debora’ Tomatoes. Sci. Hortic. 2019, 249, 340–346. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of Chitosan on Control of Postharvest Diseases and Physiological Responses of Tomato Fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Coqueiro, D.S.O.; Maraschin, M.; Di Piero, R.M. Chitosan Reduces Bacterial Spot Severity and Acts in Phenylpropanoid Metabolism in Tomato Plants. J. Phytopathol. 2011, 159, 7–8, 488–494. [Google Scholar] [CrossRef]
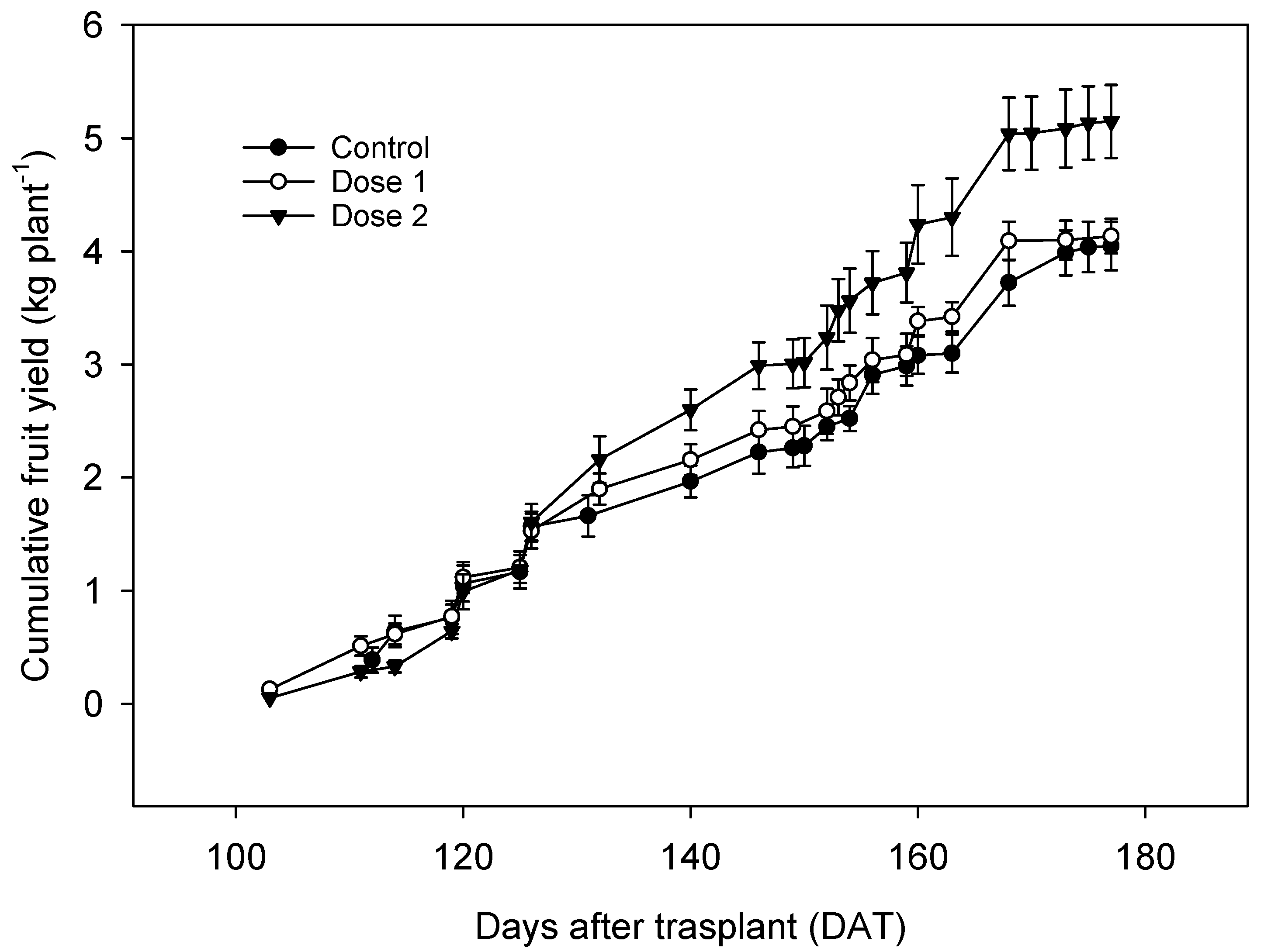
| Chitosan Dose (g L−1) | Total Yield (kg plant−1) | Fruit Number | Fruit Mean Weight (g) |
|---|---|---|---|
| Water | 3.98 a | 44.7 a | 99.1 b |
| 0 (control) | 4.05 a | 47.8 a | 86.5 ab |
| 0.1 | 4.13 a | 52.8 ab | 80.2 a |
| 1 | 5.15 b | 56.0 b | 95.5 b |
| ** | ** | ** |
| Chroma | Hue | Glucose (mg g−1) | Fructose (mg g−1) | Vitamin C (mg g−1) | ||
|---|---|---|---|---|---|---|
| Doses | 0 | 25.4 b | 43.7 a | 17.4 | 16.4 | 25.1 b |
| 0.1 | 25.0 b | 45.3 ab | 17.6 | 15.4 | 24.1 b | |
| 1 | 23.9 a | 46.5 b | 15.9 | 14.4 | 19.2 a | |
| *** | ** | n.s. | n.s. | *** | ||
| Truss | 2 | 24.2 | 45.3 | 16.2 | 14.9 | 22.5 |
| 7 | 25.2 | 45.1 | 17.8 | 15.9 | 23.2 | |
| *** | n.s. | n.s. | n.s. | n.s. | ||
| INTERACTION | ||||||
| Truss | Dose | |||||
| 2 | 0 | 25.1 | 43.5 | 17.8 | 16.7 | 24.0 c |
| 0.1 | 24.7 | 45.4 | 16.1 | 14.6 | 22.7 bc | |
| 1 | 23.0 | 47 | 14.6 | 13.3 | 20.7 ab | |
| 7 | 0 | 25.6 | 43.8 | 16.9 | 16.1 | 26.3 c |
| 0.1 | 25.2 | 45.1 | 19.0 | 16.2 | 25.6 c | |
| 1 | 24.8 | 46.4 | 17.3 | 15.4 | 17.7 a | |
| n.s. | n.s. | n.s. | n.s. | *** |
| Viol | Lutein | Phyto | Phytof | β-Carot | Lycop | ||
|---|---|---|---|---|---|---|---|
| Dose | 0 | 0.96 | 2.2 b | 5.6 b | 4.5 | 9.7 b | 22.0 |
| (g L−1) | 0.1 | 0.92 | 2.1 ab | 4.5 ab | 3.5 | 8.9 ab | 20.3 |
| 1 | 0.89 | 1.8 a | 3.5 a | 2.8 | 7.8 a | 19.6 | |
| n.s. | * | *** | n.s. | * | n.s. | ||
| Truss | 2 | 0.97 | 2.2 | 5.7 | 4.6 | 9.7 | 20.2 |
| 7 | 0.87 | 1.9 | 3.3 | 2.6 | 8.0 | 21.1 | |
| n.s. | ** | *** | *** | ** | n.s. | ||
| INTERACTION SECCION | |||||||
| Truss | Dose | ||||||
| 2 | 0 | 1.07 | 2.5 | 7.5 b | 6.0 c | 11.0 | 22.6 b |
| 0.1 | 1.01 | 2.2 | 5.9 b | 4.5 bc | 9.8 | 19.8 ab | |
| 1 | 0.85 | 1.9 | 3.9 a | 3.1 ab | 8.2 | 18.4 a | |
| 7 | 0 | 0.85 | 2.0 | 3.7 a | 3.0 a | 8.5 | 21.3 ab |
| 0.1 | 0.98 | 1.9 | 3.1 a | 2.4 a | 8.0 | 21.2 ab | |
| 1 | 0.84 | 1.8 | 3.0 a | 2.4 a | 7.5 | 20.9 ab | |
| n.s. | n.s. | ** | * | n.s. | * | ||
| Flavanones | Hydroxi. | Flavonols | Phloretin | ||
|---|---|---|---|---|---|
| Dose | 0 | 36.5 a | 29.8 b | 19.0 b | 3.3 b |
| 0.1 | 51.3 b | 26.6 b | 17.8 b | 3.4 b | |
| 1 | 35.1 a | 19.9 a | 12.5 a | 2.6 a | |
| *** | *** | *** | * | ||
| Truss | 2 | 30.7 | 24.1 | 11.1 | 2.2 |
| 7 | 51.3 | 28.2 | 22.5 | 4.0 | |
| *** | * | *** | *** | ||
| INTERACTION | |||||
| Truss | Dose | ||||
| 2 | 0 | 23.9 | 25.4 | 11.6 ab | 1.9 a |
| 0.1 | 44.2 | 25.4 | 12.8 ab | 2.6 b | |
| 1 | 23.9 | 20.2 | 9.4 a | 2.0 ab | |
| 7 | 0 | 49.1 | 34.2 | 25.9 c | 4.7 d |
| 0.1 | 58.5 | 27.9 | 23.6 c | 4.2 cd | |
| 1 | 46.2 | 19.6 | 16.2 b | 3.1 bc | |
| n.s. | n.s. | * | * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, V.; Botella, M.Á.; Hellín, P.; Fenoll, J.; Flores, P. Dose-Dependent Potential of Chitosan to Increase Yield or Bioactive Compound Content in Tomatoes. Horticulturae 2022, 8, 1152. https://doi.org/10.3390/horticulturae8121152
Hernández V, Botella MÁ, Hellín P, Fenoll J, Flores P. Dose-Dependent Potential of Chitosan to Increase Yield or Bioactive Compound Content in Tomatoes. Horticulturae. 2022; 8(12):1152. https://doi.org/10.3390/horticulturae8121152
Chicago/Turabian StyleHernández, Virginia, María Ángeles Botella, Pilar Hellín, José Fenoll, and Pilar Flores. 2022. "Dose-Dependent Potential of Chitosan to Increase Yield or Bioactive Compound Content in Tomatoes" Horticulturae 8, no. 12: 1152. https://doi.org/10.3390/horticulturae8121152
APA StyleHernández, V., Botella, M. Á., Hellín, P., Fenoll, J., & Flores, P. (2022). Dose-Dependent Potential of Chitosan to Increase Yield or Bioactive Compound Content in Tomatoes. Horticulturae, 8(12), 1152. https://doi.org/10.3390/horticulturae8121152







