Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review
Abstract
1. Introduction
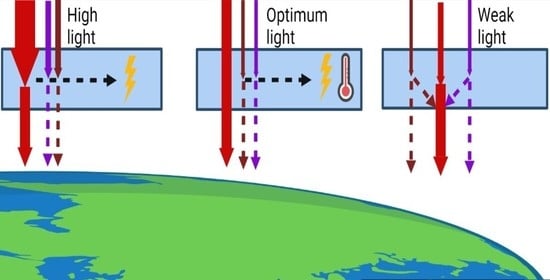
2. First Approach: Change in the Overall Intensity of Natural Light
2.1. Covers That Reduce Overall Light Intensity
2.2. Using Additional Artificial Lighting to Increase Overall Light Intensity
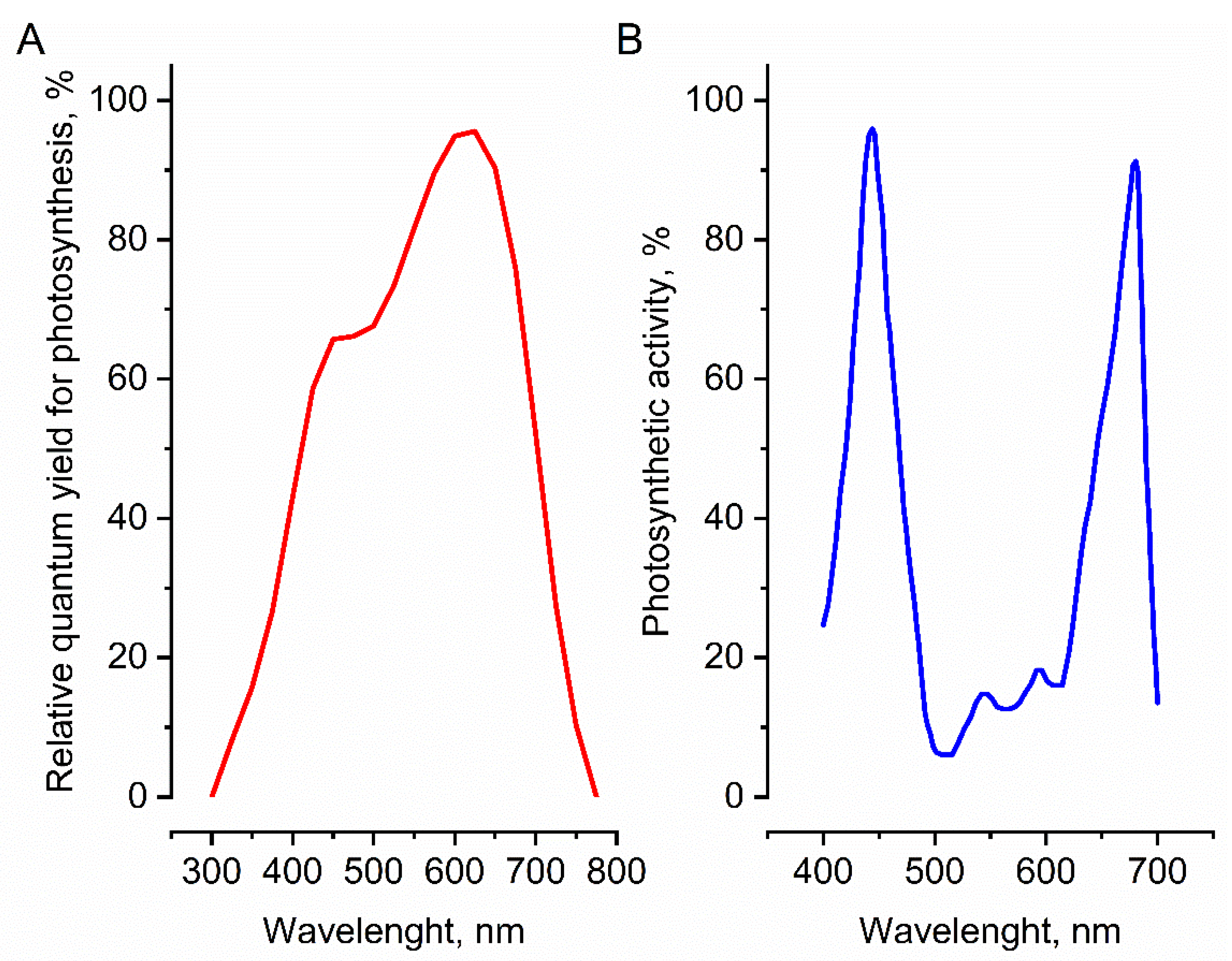
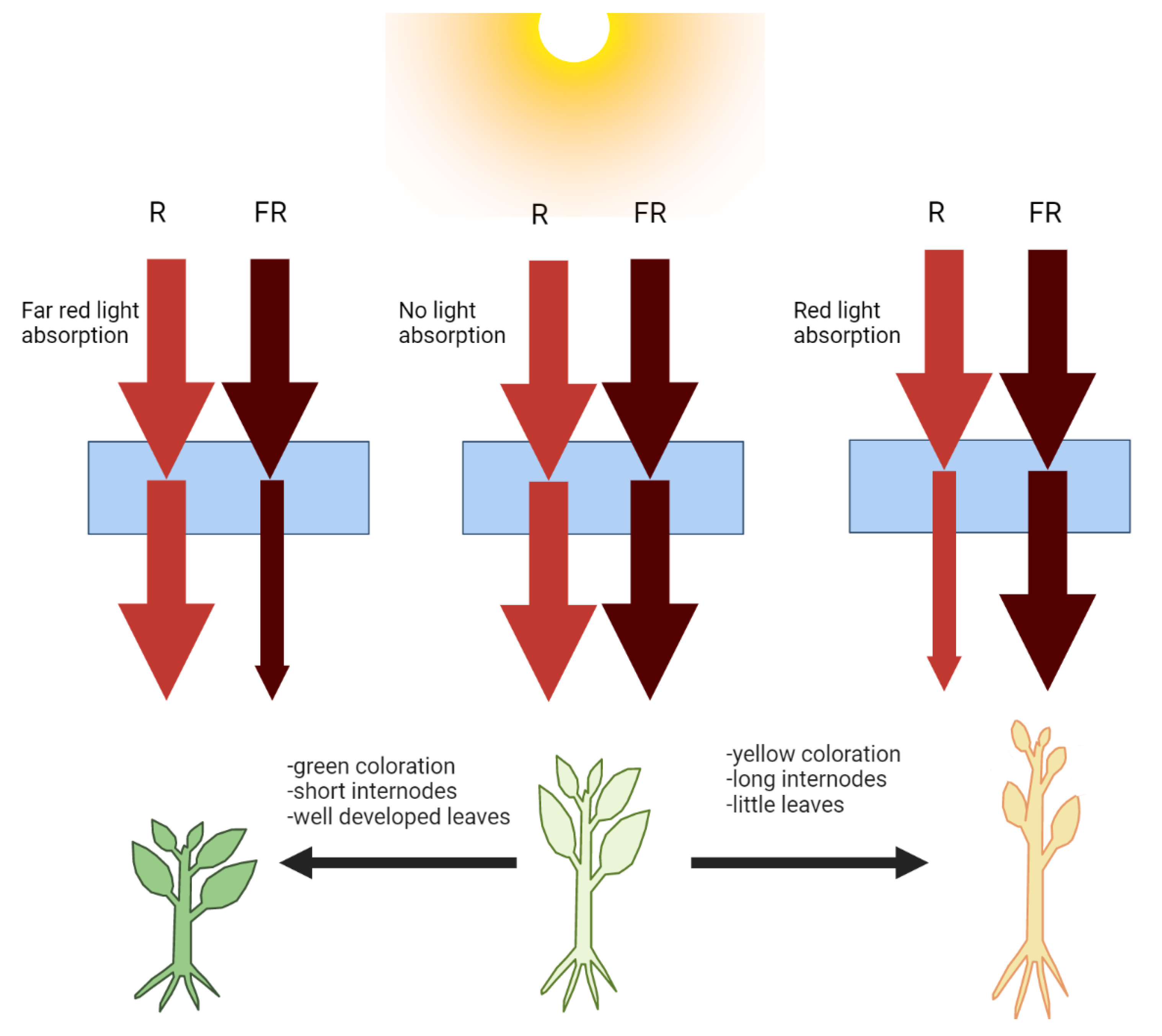
3. Second Approach: Qualitative Transformation of the Sunlight Spectrum Using Special Covers
3.1. Transformation of the Sunlight Spectrum Using Photoselective Covers
3.2. Transformation of the Sunlight Spectrum Using Photoconversion Covers
3.2.1. UV Conversion to PAR
3.2.2. Green and Yellow Light Conversion into Red
3.2.3. Up-Conversion Luminophores
3.3. Transformation of the Sunlight Spectrum into Electricity Using Photoconversion Covers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic Quantum Yield Dynamics: From Photosystems to Leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [PubMed]
- Inada, K. Action Spectra for Photosynthesis in Higher Plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar] [CrossRef]
- Marosvölgyi, M.A.; van Gorkom, H.J. Cost and Color of Photosynthesis. Photosynth. Res. 2010, 103, 105–109. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Karp, G.; Iwasa, J.; Marshall, W. Karp’s Cell and Molecular Biology; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Melis, A.; Spangfort, M.; Andersson, B. Light-absorption and electron-transport balance between photosystem II and photosystem I in spinach chloroplasts. Photochem. Photobiol. 1987, 45, 129–136. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for Energy Efficient Greenhouse Lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; van Grondelle, R.; Govindjee; Scholes, G.D. Light Absorption and Energy Transfer in the Antenna Complexes of Photosynthetic Organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Petroutsos, D.; Tokutsu, R.; Maruyama, S.; Flori, S.; Greiner, A.; Magneschi, L.; Cusant, L.; Kottke, T.; Mittag, M.; Hegemann, P.; et al. A Blue-Light Photoreceptor Mediates the Feedback Regulation of Photosynthesis. Nature 2016, 537, 563–566. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J. Light quality affects water use of sweet basil by changing its stomatal development. Agronomy 2021, 11, 303. [Google Scholar] [CrossRef]
- Smith, H.L.; Mcausland, L.; Murchie, E.H. Don’t Ignore the Green Light: Exploring Diverse Roles in Plant Processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Dougher, T.A.O.; Bugbee, B. Evidence for Yellow Light Suppression of Lettuce Growth. Photochem. Photobiol. 2001, 73, 208–212. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Wang, M.; Liu, H. Green light enhances growth, photosynthetic pigments and CO2 assimilation efficiency of lettuce as revealed by ‘knock out’ of the 480–560 nm spectral waveband. Photosynthetica 2016, 55, 144–152. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Liu, M.; Xu, J.; Xu, Z. Effects of Green and Red Lights on the Growth and Morphogenesis of Potato (Solanum tuberosum L.) Plantlets In Vitro. Sci. Hortic. 2015, 190, 104–109. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves Are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of Green Light to Plant Growth and Development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Shen, W.; Cui, J. Analyzing Photosynthetic Activity and Growth of Solanum lycopersicum Seedlings Exposed to Different Light Qualities. Acta Physiol. Plant. 2014, 36, 1411–1420. [Google Scholar] [CrossRef]
- Kono, M.; Kawaguchi, H.; Mizusawa, N.; Yamori, W.; Suzuki, Y.; Terashima, I. Far-Red Light Accelerates Photosynthesis in the Low-Light Phases of Fluctuating Light. Plant Cell Physiol. 2020, 61, 192–202. [Google Scholar] [CrossRef]
- Laisk, A.; Oja, V.; Eichelmann, H.; Dall’Osto, L. Action Spectra of Photosystems II and I and Quantum Yield of Photosynthesis in Leaves in State 1. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1837, 315–325. [Google Scholar] [CrossRef]
- Pettai, H.; Oja, V.; Freiberg, A.; Laisk, A. Photosynthetic Activity of Far-Red Light in Green Plants. Biochim. Biophys. Acta (BBA) Bioenerg. 2005, 1708, 311–321. [Google Scholar] [CrossRef]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-Red Light Enhances Photochemical Efficiency in a Wavelength-Dependent Manner. Physiol. Plant. 2019, 167, 21–33. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to Lower Red to Far-Red Light Ratios Improve Tomato Tolerance to Salt Stress. BMC Plant Biol. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Kosobryukhov, A.A.; Svidchenko, E.A.; Surin, N.M.; Khramov, R.N.; Kreslavski, V.D. Influence of Photoluminophore-Modified Agro Textile Spunbond on Growth and Photosynthesis of Cabbage and Lettuce Plants. Opt. Express 2019, 27, 31967–31977. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The Impact of the Phytochromes on Photosynthetic Processes. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Tyystjärvi, E. Photoinhibition of Photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef]
- Jordan, B.R. Molecular response of plant cells to UV-B stress. Funct. Plant Biol. 2002, 29, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Jansen, M.A.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.R.; Mikkelsen, T.N.; Ro-Poulsen, H.; Arndal, M.F.; Michelsen, A. Ambient UV-B radiation reduces PSII performance and net photosynthesis in high Arctic Salix arctica. Environ. Exp. Bot. 2011, 73, 10–18. [Google Scholar] [CrossRef]
- Neugart, S.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. Structurally different flavonol glycosides and hydroxycinnamic acid derivatives respond differently to moderate UV-B radiation exposure. Physiol. Plant. 2012, 145, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; Krumbein, A. UV-B-induced secondary plant metabolites-potential benefits for plant and human health. Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Kitta, E.; Baille, A.D.; Katsoulas, N.; Rigakis, N.; González-Real, M.M. Effects of Cover Optical Properties on Screenhouse Radiative Environment and Sweet Pepper Productivity. Biosyst. Eng. 2014, 122, 115–126. [Google Scholar] [CrossRef]
- Ewart, A.J. On Assimilatory Inhibition in Plants. Bot. J. Linn. Soc. 1896, 31, 364–461. [Google Scholar] [CrossRef]
- Osmond, C.B. Photorespiration and Photoinhibition: Some Implications for the Energetics of Photosynthesis. Biochim. Biophys. Acta (BBA) Rev. Bioenerg. 1981, 639, 77–98. [Google Scholar] [CrossRef]
- Rabinowitch, E.I. Photosynthesis and Related Processes; Spectroscopy and Fluorescence of Photosynthetic Pigments, Kinetics of Photosynthesis; Interscience Publishers, Inc.: New York, NY, USA, 1951; Volume II, Part I; pp. 603–1208. [Google Scholar]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the Beneficial Effects of Green Light on Lettuce Grown under Short-Term Continuous Red and Blue Light-Emitting Diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Spectral Effects of Light-Emitting Diodes on Plant Growth, Visual Color Quality, and Photosynthetic Photon Efficacy: White versus Blue plus Red Radiation. PLoS ONE 2018, 13, e0202386. [Google Scholar] [CrossRef]
- Semenova, N.A.; Smirnov, A.A.; Grishin, A.A.; Pishchalnikov, R.Y.; Chesalin, D.D.; Gudkov, S.V.; Chilingaryan, N.O.; Skorokhodova, A.N.; Dorokhov, A.S.; Izmailov, A.Y. The Effect of Plant Growth Compensation by Adding Silicon-Containing Fertilizer under Light Stress Conditions. Plants 2021, 10, 1287. [Google Scholar] [CrossRef]
- Tang, Y.; Mao, R.; Guo, S. Effects of LED Spectra on Growth, Gas Exchange, Antioxidant Activity and Nutritional Quality of Vegetable Species. Life Sci. Space Res. 2020, 26, 77–84. [Google Scholar] [CrossRef]
- Tewolde, F.T.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Supplemental LED Inter-Lighting Compensates for a Shortage of Light for Plant Growth and Yield under the Lack of Sunshine. PLoS ONE 2018, 13, e0206592. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, J.; Zhang, D.; Cai, Q.; Zhou, D.; Tu, S.; Liu, Q.; Tu, K. Effects of White LED Light and UV-C Radiation on Stilbene Biosynthesis and Phytochemicals Accumulation Identified by UHPLC-MS/MS during Peanut (Arachis hypogaea L.) Germination. J. Agric. Food Chem. 2020, 68, 5900–5909. [Google Scholar] [CrossRef]
- Van Haeringen, C.J.; West, J.S.; Davis, F.J.; Gilbert, A.; Hadley, P.; Pearson, S.; Wheldon, A.E.; Henbest, R.G.C. The Development of Solid Spectral Filters for the Regulation of Plant Growth. Photochem. Photobiol. 1998, 67, 407–413. [Google Scholar] [CrossRef]
- Li, E.H. Material Parameters of InGaAsP and InAlGaAs Systems for Use in Quantum Well Structures at Low and Room Temperatures. Phys. E Low-Dimens. Syst. Nanostructures 2000, 5, 215–273. [Google Scholar] [CrossRef]
- Han, T.; Vaganov, V.A.; Cao, S.; Li, Q.; Ling, L.; Cheng, X.; Peng, L.; Zhang, C.; Yakovlev, A.N.; Zhong, Y.; et al. Improving “Color Rendering” of LED Lighting for the Growth of Lettuce. Sci. Rep. 2017, 7, 45944. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Pollock, R.K.; McMahon, M.J.; Kelly, J.W.; Young, R.E. Interpretation of Light Quality Measurements and Plant Response in Spectral Filter Research. HortScience 1992, 27, 1208–1211. [Google Scholar] [CrossRef]
- Wilson, S.B.; Rajapakse, N.C. Growth Regulation of Sub-Tropical Perennials by Photoselective Plastic Films. J. Environ. Hortic. 2001, 19, 65–68. [Google Scholar] [CrossRef]
- de Salvador, F.R.; Mugnozza, G.S.; Vox, G.; Schettini, E.; Mastrorilli, M.; Bou Jaoudé, M. Innovative Photoselective and Photoluminescent Plastic Films for Protected Cultivation. Acta Hortic 2008, 801, 115–121. [Google Scholar] [CrossRef]
- González, A.; Rodríguez, R.; Bañón, S.; Franco, J.A.; Fernández, J.A.; Salmerón, A.; Espí, E. Strawberry and Cucumber Cultivation under Fluorescent Photoselective Plastic Films Cover. Acta Hortic. 2003, 614, 407–413. [Google Scholar] [CrossRef]
- Kosobryukhov, A.A.; Kreslavskii, V.D.; Khramov, R.N. Effect of Additional Low Intensity Luminescence Radiation 625 Nm on Plant Growth and Photosynthesis Useful Sun for Crop Yields View Project ROS Sensing and Monitoring View Project. Biotronics 2000, 29, 23–31. [Google Scholar]
- Minich, A.S.; Minich, I.B.; Shaitarova, O.V.; Permyakova, N.L.; Zelenchukova, N.S.; Ivanitskiy, A.E.; Ivlev, G.A. Vital activity of Lactuca sativa and soil microorganisms under fluorescent films. TSPU Bull. 2011, 8, 78–84. [Google Scholar]
- Parrish, C.H.; Hebert, D.; Jackson, A.; Ramasamy, K.; McDaniel, H.; Giacomelli, G.A.; Bergren, M.R. Optimizing Spectral Quality with Quantum Dots to Enhance Crop Yield in Controlled Environments. Commun. Biol. 2021, 4, 124. [Google Scholar] [CrossRef]
- Elza Joseph, R. Photophysics of Upconversion: Towards Application in Upconversion Displays. Ph.D. Thesis, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 2021. [Google Scholar] [CrossRef]
- Powles, S.B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 1984, 35, 15–44. [Google Scholar] [CrossRef]
- Wilson, D.A.; Weigel, R.C.; Wheeler, R.M.; Sager, J.C. Light Spectral Quality Effects on the Growth of Potato (Solanum tuberosum L.) Nodal Cuttings In Vitro. In Vitr. Cell. Dev. Biol. Plant 1993, 29, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xia, J.; Lv, Y.; Chen, Z.; Xu, K. Effect of a Mist Culture System on Photosynthesis and Nitrogen Metabolism in Ginger. Protoplasma 2020, 257, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Siwek, P.; Wojciechowska, R.; Kalisz, A.; Libik, A.; Gryza, I. Effect of Shading with Various Coloured Films on the Yield and Quality of Celery and Butterhead Lettuce. Ecol. Chem. Eng. 2010, 17, 1619–1627. [Google Scholar]
- Holcman, E.; Sentelhas, P.C.; Conceição, M.A.F.; Couto, H.T.Z. Vineyard Microclimate and Yield under Different Plastic Covers. Int. J. Biometeorol. 2018, 62, 925–937. [Google Scholar] [CrossRef]
- Cathey, H.M.; Campbell, L.E. Light and Lighting Systems for Horticultural Plants. Hortic.-Rev. 1980, 2, 491–537. [Google Scholar]
- Kong, Y.; Nemali, A.; Mitchell, C.; Nemali, K. Spectral Quality of Light Can Affect Energy Consumption and Energy-Use Efficiency of Electrical Lighting in Indoor Lettuce Farming. HortScience 2019, 54, 865–872. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. LED Light Sources Improved the Essential Oil Components and Antioxidant Activity of Two Genotypes of Lemon Balm (Melissa officinalis L.). Bot. Stud. 2021, 62, 9. [Google Scholar] [CrossRef]
- Hwang, O.J.; Kang, K.; Back, K. Effects of Light Quality and Phytochrome Form on Melatonin Biosynthesis in Rice. Biomolecules 2020, 10, 523. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kitaya, Y.; Seoka, M.; Kudaka, R.; Yahata, M.; Yamawaki, K.; Shimada, T.; Fujii, H.; Endo, T.; et al. Blue LED Light Induces Regreening in the Flavedo of Valencia Orange In Vitro. Food Chem. 2021, 335, 127621. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED Photoperiods and Light Qualities on In Vitro Growth and Chlorophyll Fluorescence of Cunninghamia Lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Lu, L.; Effendy Ya’acob, M.; Shamsul Anuar, M.; Nazim Mohtar, M. Comprehensive Review on the Application of Inorganic and Organic Photovoltaics as Greenhouse Shading Materials. Sustain. Energy Technol. Assess. 2022, 52, 102077. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Wu, G.; Yang, Q.; Yuan, Y.; Cheng, R.; Tong, Y.; Fang, H. Photovoltaic/Spectrum Performance Analysis of a Multifunctional Solid Spectral Splitting Covering for Passive Solar Greenhouse Roof. Energy Convers. Manag. 2022, 251, 114955. [Google Scholar] [CrossRef]
- Ravishankar, E.; Charles, M.; Xiong, Y.; Henry, R.; Swift, J.; Rech, J.; Calero, J.; Cho, S.; Booth, R.E.; Kim, T.; et al. Balancing Crop Production and Energy Harvesting in Organic Solar-Powered Greenhouses. Cell Rep. Phys. Sci. 2021, 2, 100381. [Google Scholar] [CrossRef]
- Tomar, A. Addressing Virtual Asymmetry of Photovoltaic Greenhouse with Comprehensive AOMH Based SWAPP Approach. Sustain. Energy Technol. Assess. 2021, 47, 101512. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and Application of Photoconversion Fluoropolymer Films for Greenhouses Located at High or Polar Latitudes. J. Photochem. Photobiol. B Biol. 2020, 213, 112056. [Google Scholar] [CrossRef]
- McMahon, M.J.; Kelly, J.W.; Decoteau, D.R.; Young, R.E.; Pollock, R.K. Growth of Dendranthema × grandiflorum (Ramat.) Kitamura under Various Spectral Filters. J. Am. Soc. Hortic. Sci. 1991, 116, 950–954. [Google Scholar] [CrossRef]
- Wilson, S.B.; Rajapakse, N.C. Growth Control of Lisianthus by Photoselective Plastic Films. Horttechnology 2001, 11, 581–584. [Google Scholar] [CrossRef]
- Díaz, B.M.; Biurrún, R.; Moreno, A.; Nebreda, M.; Fereres, A. Impact of Ultraviolet-Blocking Plastic Films on Insect Vectors of Virus Diseases Infesting Crisp Lettuce. HortScience 2006, 41, 711–716. [Google Scholar] [CrossRef]
- Doukas, D.; Payne, C.C. The Use of Ultraviolet-Blocking Films in Insect Pest Management in the UK; Effects on Naturally Occurring Arthropod Pest and Natural Enemy Populations in a Protected Cucumber Crop. Ann. Appl. Biol. 2007, 151, 221–231. [Google Scholar] [CrossRef]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef]
- González, A.; García-Alonso, Y.; Espí, E.; Fontecha, A.; Salmerón, A. Viral Diseases Control with UV-Blocking Films in Greenhouses of Southern Spain. Acta Hortic. 2004, 659, 331–338. [Google Scholar] [CrossRef]
- Kittas, C.; Tchamitchian, M.; Katsoulas, N.; Karaiskou, P.; Papaioannou, C. Effect of Two UV-Absorbing Greenhouse-Covering Films on Growth and Yield of an Eggplant Soilless Crop. Sci. Hortic. 2006, 110, 30–37. [Google Scholar] [CrossRef]
- Lamnatou, C.; Chemisana, D. Solar Radiation Manipulations and Their Role in Greenhouse Claddings: Fresnel Lenses, NIR- and UV-Blocking Materials. Renew. Sustain. Energy Rev. 2013, 18, 271–287. [Google Scholar] [CrossRef]
- López-Marin, J.; Rodríguez, M.; González, A. Effect of Ultraviolet-Blocking Plastic Films on Insect Vectors of Virus Diseases Infesting Tomato (Lycopersicon esculentum) in Greenhouse. Acta Hortic. 2011, 914, 175–179. [Google Scholar] [CrossRef]
- Vakalonakis, D.J. Control of Early of Greenhouse Tomato, Caused Alternaria Solani by Inhibiting Sporulation with Ultraviolet-Absorbing Vinyl Film. Plant Dis. 1991, 75, 795–797. [Google Scholar] [CrossRef]
- Cerny, T.A.; Faust, J.E.; Layne, D.R.; Rajapakse, N.C. Influence of Photoselective Films and Growing Season on Stem Growth and Flowering of Six Plant Species. J. Am. Soc. Hortic. Sci. 2003, 128, 486–491. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Tatsiopoulou, A.; Mpezamihigo, M.; Carew, J.G.; Henbest, R.G.C.; Hadley, P. Far-Red Light Filtering by Plastic Film, Greenhouse-Cladding Materials: Effects on Growth and Flowering in Petunia and Impatiens. J. Hortic. Sci. Biotechnol. 2015, 80, 303–306. [Google Scholar] [CrossRef]
- Patil, G.G.; Oi, R.; Gissinger, A.; Moe, R. Plant Morphology Is Affected by Light Quality Selective Plastic Films and Alternating Day and Night Temperature. Gartenbauwissenschaft 2001, 66, 53–60. [Google Scholar]
- Kambalapally, V.R.; Rajapakse, N.C. Spectral Filters Affect Growth, Flowering, and Postharvest Quality of Easter Lilies. HortScience 1998, 33, 1028–1029. [Google Scholar] [CrossRef]
- Kang, S.K.; Motosugi, H.; Yonemori, K.; Sugiura, A. Supercooling Characteristics of Some Deciduous Fruit Trees as Related to Water Movement within the Bud. J. Hortic. Sci. Biotechnol. 2015, 73, 165–172. [Google Scholar] [CrossRef]
- Magnani, G.; Filippi, F.; Borghesi, E.; Vitale, M. Impact of Sunlight Spectrum Modification on Yield and Quality of Ready-to-Use Lettuce and Rocket Salad Grown on Floating System. Acta Hortic. 2008, 801 Pt 1, 163–169. [Google Scholar] [CrossRef]
- Murakami, K.; Cui, H.; Kiyota, M.; Aiga, I.; Yamane, T. Control of Plant Growth by Covering Materials for Greenhouses Which Alter the Spectral Distribution of Transmitted Light. Acta Hortic. 1997, 435, 123–130. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Kelly, J.W. Regulation of Chrysanthemum Growth by Spectral Filters. J. Am. Soc. Hortic. Sci. 1992, 117, 481–485. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Kelly, J.W. Influence of Spectral Filters on Growth and Postharvest Quality of Potted Miniature Roses. Sci. Hortic. 1994, 56, 245–255. [Google Scholar] [CrossRef]
- Ward, K.D.; Eissenberg, T.; Gray, J.N.; Srinivas, V.; Wilson, N.; Maziak, W. Characteristics of U.S. Waterpipe Users: A Preliminary Report. Nicotine Tob. Res. 2007, 9, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Causin, H.F.; Jauregui, R.N.; Barneix, A.J. The Effect of Light Spectral Quality on Leaf Senescence and Oxidative Stress in Wheat. Plant Sci. 2006, 171, 24–33. [Google Scholar] [CrossRef]
- Noè, N.; Eccher, T.; del Signore, E.; Montoldi, A. Growth and Proliferation In Vitro of Vaccinium Corymbosum under Different Irradiance and Radiation Spectral Composition. Biol. Plant. 1998, 41, 161–167. [Google Scholar] [CrossRef]
- Stagnari, F.; Galieni, A.; Cafiero, G.; Pisante, M. Application of Photo-Selective Films to Manipulate Wavelength of Transmitted Radiation and Photosynthate Composition in Red Beet (Beta vulgaris var. conditiva Alef.). J. Sci. Food Agric. 2014, 94, 713–720. [Google Scholar] [CrossRef]
- Casal, J.J.; Sánchez, R.A. Phytochromes and Seed Germination. Seed Sci. Res. 1998, 8, 317–329. [Google Scholar] [CrossRef]
- Franklin, K.A.; Quail, P.H. Phytochrome Functions in Arabidopsis Development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef]
- Halliday, K.J.; Koornneef, M.; Whitelam, G.C. Phytochrome B and at Least One Other Phytochrome Mediate the Accelerated Flowering Response of Arabidopsis thaliana L. to Low Red/Far-Red Ratio. Plant Physiol. 1994, 104, 1311–1315. [Google Scholar] [CrossRef]
- Penfield, S.; King, J. Towards a Systems Biology Approach to Understanding Seed Dormancy and Germination. Proc. R. Soc. B Biol. Sci. 2009, 276, 3561–3569. [Google Scholar] [CrossRef]
- Wilson, S.B.; Rajapakse, N.C. Use of Photoselective Plastic Films to Control Growth of Three Perennial Salvias. J. Appl. Hort 2002, 3, 71–74. [Google Scholar] [CrossRef]
- Raviv, M. The use of photoselective cladding materials as modifiers of morphogenesis of plants and pathogens. Acta Hortic. 1989, 246, 275–284. [Google Scholar] [CrossRef]
- Aguyoh, J.N.; Gaoquiong, L.; Ochieng, J. Influence of greenhouse cover material on light transmission, aphids, mites, powdery mildew and rose petal colour. Agric. Trop. Subtrop. 2010, 43, 232–235. [Google Scholar]
- Katz, A.; Weiss, D. Light regulation of anthocyanin accumulation and chalcone synthase gene expression in Petunia flowers. Israel J. Plant Sci. 1999, 47, 225–229. [Google Scholar] [CrossRef]
- Antignus, Y. Manipulation of Wavelength-Dependent Behaviour of Insects: An IPM Tool to Impede Insects and Restrict Epidemics of Insect-Borne Viruses. Virus Res. 2000, 71, 213–220. [Google Scholar] [CrossRef]
- Wargent, J.J.; Taylor, A.; Paul, N.D. UV Supplementation for Growth Regulation and Disease Control. Acta Hortic. 2006, 711, 333–338. [Google Scholar] [CrossRef]
- Barbosa, G.L.; Almeida Gadelha, F.D.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Bot, G.; Van De Braak, N.; Challa, H.; Hemming, S.; Rieswijk, T.H.; Van Straten, G.; Verlodt, I. The Solar Greenhouse: State of the Art in Energy Saving and Sustainable Energy Supply. Acta Hortic. 2005, 691, 501–508. [Google Scholar] [CrossRef]
- Nadal, A.; Llorach-Massana, P.; Cuerva, E.; López-Capel, E.; Montero, J.I.; Josa, A.; Rieradevall, J.; Royapoor, M. Building-Integrated Rooftop Greenhouses: An Energy and Environmental Assessment in the Mediterranean Context. Appl. Energy 2017, 187, 338–351. [Google Scholar] [CrossRef]
- Edser, C. Light Manipulating Additives Extend Opportunities for Agricultural Plastic Films. Plast. Addit. Compd. 2002, 4, 20–24. [Google Scholar] [CrossRef]
- Golovatskaya, I.F.; Minich, A.S.; Bolshakova, M.A. Regulation and development of Brassica oleracea plants growth with the help of sunlight correction. Tomsk State Univ. J. Biol. 2012, 2, 151–165. [Google Scholar]
- Hamada, K.; Shimasaki, K.; Nishimura, Y.; Oyama-Egawa, H.; Yoshida, K. Effects of Red, Blue and Yellow Fluorescent Films on Proliferation and Organogenesis in Cymbidium and Phalaenopsis PLB In Vitro. Acta Hortic. 2011, 907, 381–384. [Google Scholar] [CrossRef]
- Hamada, K.; Shimasaki, K.; Ogata, T.; Nishimura, Y.; Nakamura, K.; Oyama-Egawa, H.; Yoshida, K. Effects of Spectral Composition Conversion Film and Plant Growth Regulators on Proliferation of Cymbidium Protocorm Like Body (PLB) Cultured In Vitro. Environ. Control Biol. 2010, 48, 127–132. [Google Scholar] [CrossRef][Green Version]
- Hemming, S.; Van Os, E.A.; Hemming, J.; Dieleman, J.A. The Effect of New Developed Fluorescent Greenhouse Films on the Growth of Fragaria x Ananassa “Elsanta”. Eur. J. Hortic. Sci. 2006, 71, 145–154. [Google Scholar]
- Hidaka, K.; Yoshida, K.; Shimasaki, K.; Murakami, K.; Yasutake, D.; Kitano, M. Spectrum Conversion Film for Regulation of Plant Growth. J. Fac. Agric. Kyushu Univ. 2008, 53, 549–552. [Google Scholar] [CrossRef]
- Ivanyuk, V.V.; Shkirin, A.V.; Belosludtsev, K.N.; Dubinin, M.V.; Kozlov, V.A.; Bunkin, N.F.; Dorokhov, A.S.; Gudkov, S.V. Influence of Fluoropolymer Film Modified with Nanoscale Photoluminophor on Growth and Development of Plants. Front. Phys. 2020, 8, 616040. [Google Scholar] [CrossRef]
- Ke-li, Z.; Liang-jie, Y.; Mei-yun, X.; You-zu, Y.; Ju-tang, S. The Application of Lights-Conversed Polyethylene Film for Agriculture. Wuhan Univ. J. Nat. Sci. 2002, 7, 365–367. [Google Scholar] [CrossRef]
- Minich, A.S.; Minich, I.B.; Zelenchukova, N.S.; Raida, V.S. Feature of growth and productivity in cucumber hybrids during growing under phololuminescent and hydrophilic films. Agric. Biol. 2010, 1, 81–85. [Google Scholar]
- Nishimura, Y.; Wada, E.; Fukumoto, Y.; Aruga, H.; Shimoi, Y. The Effect of Spectrum Conversion Covering Film on Cucumber in Soilless Culture. Acta Hortic. 2012, 956, 481–487. [Google Scholar] [CrossRef]
- Novoplansky, A.; Sachs, T.; Cohen, D.; Bar, R.; Bodenheimer, J.; Reisfeld, R. Increasing Plant Productivity by Changing the Solar Spectrum. Sol. Energy Mater. 1990, 21, 17–23. [Google Scholar] [CrossRef]
- Sánchez-Lanuza, M.B.; Menéndez-Velázquez, A.; Peñas-Sanjuan, A.; Navas-Martos, F.J.; Lillo-Bravo, I.; Delgado-Sánchez, J.M. Advanced Photonic Thin Films for Solar Irradiation Tuneability Oriented to Greenhouse Applications. Materials 2021, 14, 2357. [Google Scholar] [CrossRef]
- Schettini, E.; de Salvador, F.R.; Scarascia-Mugnozza, G.; Vox, G. Radiometric Properties of Photoselective and Photoluminescent Greenhouse Plastic Films and Their Effects on Peach and Cherry Tree Growth. J. Hortic. Sci. Biotechnol. 2015, 86, 79–83. [Google Scholar] [CrossRef]
- Simakin, A.V.; Ivanyuk, V.V.; Dorokhov, A.S.; Gudkov, S.V. Photoconversion Fluoropolymer Films for the Cultivation of Agricultural Plants Under Conditions of Insufficient Insolation. Appl. Sci. 2020, 10, 8025. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Z.; Dong, R.; Xie, G.; Zhou, J.; Wu, K.; Zhang, H.; Cai, Q.; Lei, B. Characterization and Properties of a Sr2Si5N8:Eu2+-Based Light-Conversion Agricultural Film. J. Rare Earths 2020, 38, 539–545. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kim, J.H.; Park, K.S.; Namgoong, J.W.; Hwang, T.G.; Kim, J.P.; Son, J.E. Quantitative Methods for Evaluating the Conversion Performance of Spectrum Conversion Films and Testing Plant Responses under Simulated Solar Conditions. Hortic. Environ. Biotechnol. 2020, 61, 999–1009. [Google Scholar] [CrossRef]
- Zelenchukova, N.S.; Ivanitsky, A.E.; Agaeva, S.A.; Tishkina, V.N. Productivity, ascorbic acid synthesis and catalase activity in Lactuca sativa L. leaves under Plastic films. TSPU Bull. 2013, 8, 55–59. [Google Scholar]
- Zhang, Z.; Zhao, Z.; Lu, Y.; Wang, D.; Wang, C.; Li, J. One-Step Synthesis of Eu3+-Modified Cellulose Acetate Film and Light Conversion Mechanism. Polymers 2020, 13, 113. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kang, J.H.; Kim, D.; Son, J.E. Seedling Quality and Photosynthetic Characteristic of Vegetables Grown Under a Spectrum Conversion Film. J. Bio-Environ. Control 2021, 30, 110–117. [Google Scholar] [CrossRef]
- Stokes, G.G. On the change in the refractivity of light. Ann. Phys. 1852, 163, 480–490. [Google Scholar] [CrossRef]
- Pogreb, R.; Finkelshtein, B.; Shmukler, Y.; Musina, A.; Popov, O.; Stanevsky, O.; Yitzchaik, S.; Gladkikh, A.; Schulzinger, A.; Streltsov, V.; et al. Low-Density Polyethylene Films Doped with Europium(III) Complex: Their Properties and Applications. Polym. Adv. Technol. 2004, 15, 414–418. [Google Scholar] [CrossRef]
- Palkina, K.K.; Kuz’mina, N.E.; Shchelokov, R.N.; Strashnova, S.B.; Zaitsev, B.E.; Koval’chukova, O.V.; Nikitin, S.V. Synthesis and structure of the 2-Amino-3-Hydroxypyridine complexes with trivalent praseodymium, neodymium, samarium, and europium nitrates: Crystal structure of Tris (2-Amino-3-Hydroxypyridine) trinitratosamarium (III) monohydrate. Russ. J. Inorg. Chem. 2000, 45, 515–520.117. [Google Scholar]
- Schelokov, R. Polysvetanes and polysvetane effect. Her. Russ. Acad. Sci. 1986, 10, 50–55. [Google Scholar]
- Ziessel, R.; Diring, S.; Kadjane, P.; Charbonnière, L.; Retailleau, P.; Philouze, C. Highly Efficient Blue Photoexcitation of Europium in a Bimetallic Pt–Eu Complex. Chem. Asian J. 2007, 2, 975–982. [Google Scholar] [CrossRef]
- Fitzmorris, B.C.; Pu, Y.C.; Cooper, J.K.; Lin, Y.F.; Hsu, Y.J.; Li, Y.; Zhang, J.Z. Optical Properties and Exciton Dynamics of Alloyed Core/Shell/Shell Cd 1-XZnxSe/ZnSe/ZnS Quantum Dots. ACS Appl. Mater. Interfaces 2013, 5, 2893–2900. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Lin, W.H.; Lu, Y.H.; de Chiou, Y.; Hsu, Y.J. First Demonstration of Rainbow Photocatalysts Using Ternary Cd1-XZnxSe Nanorods of Varying Compositions. Appl. Catal. A Gen. 2014, 476, 140–147. [Google Scholar] [CrossRef]
- Pu, Y.C.; Chen, W.T.; Fang, M.J.; Chen, Y.L.; Tsai, K.A.; Lin, W.H.; Hsu, Y.J. Au–Cd1−xZnxS Core–Alloyed Shell Nanocrystals: Boosting the Interfacial Charge Dynamics by Adjusting the Shell Composition. J. Mater. Chem. A 2018, 6, 17503–17513. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. ROS Generation in Plants: Boon or Bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Bernal, M.; Llorens, L.; Badosa, J.; Verdaguer, D. Interactive Effects of UV Radiation and Water Availability on Seedlings of Six Woody Mediterranean Species. Physiol. Plant. 2013, 147, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhang, Y.; Zhang, Y.; Zou, J.; Yang, Q.; Li, T. Ultraviolet-A Radiation Stimulates Growth of Indoor Cultivated Tomato (Solanum lycopersicum) Seedlings. HortScience 2018, 53, 1429–1433. [Google Scholar] [CrossRef]
- Tezuka, T.; Hotta, T.; Watanabe, I. Growth Promotion of Tomato and Radish Plants by Solar UV Radiation Reaching the Earth’s Surface. J. Photochem. Photobiol. B Biol. 1993, 19, 61–66. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; Ai, X.; Pan, H.; Zhang, H.; Dong, L. Polylactide/Poly(Butylene Adipate-Co-Terephthalate)/Rare Earth Complexes as Biodegradable Light Conversion Agricultural Films. Polym. Adv. Technol. 2019, 30, 203–211. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, P.; Jia, S.; Pan, H.; Zhang, H.; Wang, D.; Dong, L. Exploring Polylactide/Poly(Butylene Adipate-Co-Terephthalate)/Rare Earth Complexes Biodegradable Light Conversion Agricultural Films. Int. J. Biol. Macromol. 2019, 127, 210–221. [Google Scholar] [CrossRef]
- Auzel, F. Quantum counter by energy transfer from Yb3+ to Tm3+ in a mixed tungstate and a germanate glass. C. R. Acad. Sci. 1966, 263, 819–821. [Google Scholar]
- Ovsyankin, V.V.; Feofilov, P.P. Mechanism of summation of electronic excitations in activated crystals. Sov. J. Exp. Theor. Phys. Lett. 1966, 3, 322. [Google Scholar]
- Mendez-Gonzalez, D.; Calderón, O.G.; Melle, S.; González-Izquierdo, J.; Bañares, L.; López-Díaz, D.; Velázquez, M.M.; López-Cabarcos, E.; Rubio-Retama, J.; Laurenti, M. Contribution of Resonance Energy Transfer to the Luminescence Quenching of Upconversion Nanoparticles with Graphene Oxide. J. Colloid Interface Sci. 2020, 575, 119–129. [Google Scholar] [CrossRef]
- He, C.; Qin, G.; Zhao, D.; Chuai, X.; Wang, L.; Zheng, K.; Qin, W. Upconversion luminescence properties of Yb3+ and Tm3+ codoped amorphous fluoride ZrF4-BaF2-LaF 3-AlF3-NaF thin film prepared by pulsed laser deposition. J. Nanosci. Nanotechnol. 2014, 14, 3831–3833. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Ji, Y.; Xu, J.; Li, D.; Xu, L.; Xu, J.; Chen, K. Plasmon-Enhanced Upconversion Luminescence in Pyrochlore Phase YbxEr2-XTi2O7 Thin Film. Nanotechnology 2018, 30, 085701. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Yanykin, D.V.; Simakin, A.V.; Paskhin, M.O.; Ivanyuk, V.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; Gudkov, S.V. Cultivation of Solanum lycopersicum under Glass Coated with Nanosized Upconversion Luminophore. Appl. Sci. 2021, 11, 10726. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Burmistrov, D.E.; Simakin, A.V.; Ermakova, J.A.; Gudkov, S.V. Effect of Up-Converting Luminescent Nanoparticles with Increased Quantum Yield Incorporated into the Fluoropolymer Matrix on Solanum lycopersicum Growth. Agronomy 2022, 12, 108. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Paskhin, M.O.; Simakin, A.V.; Burmistrov, D.E.; Pobedonostsev, R.V.; Vyatchinov, A.A.; Vedunova, M.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; et al. Plant Photochemistry under Glass Coated with Upconversion Luminescent Film. Appl. Sci. 2022, 12, 7480. [Google Scholar] [CrossRef]
- Ouazzani Chahidi, L.; Fossa, M.; Priarone, A.; Mechaqrane, A. Energy Saving Strategies in Sustainable Greenhouse Cultivation in the Mediterranean Climate—A Case Study. Appl. Energy 2021, 282, 116156. [Google Scholar] [CrossRef]
- Ben Amara, H.; Bouadila, S.; Fatnassi, H.; Arici, M.; Allah Guizani, A. Climate Assessment of Greenhouse Equipped with South-Oriented PV Roofs: An Experimental and Computational Fluid Dynamics Study. Sustain. Energy Technol. Assess. 2021, 45, 101100. [Google Scholar] [CrossRef]
- Campiotti, C.; Dondi, Y.; Genovese, A.; Alonzo, G.; Catanese, V.; Incrocci, L.; Bibbiani, C. Photovoltaic as Sustainable Energy for Greenhouse and Closed Plant Production System. Acta Hortic. 2008, 797, 373–380. [Google Scholar] [CrossRef]
- Gorjian, S.; Calise, F.; Kant, K.; Ahamed, M.S.; Copertaro, B.; Najafi, G.; Zhang, X.; Aghaei, M.; Shamshiri, R.R. A Review on Opportunities for Implementation of Solar Energy Technologies in Agricultural Greenhouses. J. Clean. Prod. 2021, 285, 124807. [Google Scholar] [CrossRef]
- Sethi, V.P.; Sumathy, K.; Lee, C.; Pal, D.S. Thermal Modeling Aspects of Solar Greenhouse Microclimate Control: A Review on Heating Technologies. Sol. Energy 2013, 96, 56–82. [Google Scholar] [CrossRef]
- Santolini, E.; Pulvirenti, B.; Benni, S.; Barbaresi, L.; Torreggiani, D.; Tassinari, P. Numerical Study of Wind-Driven Natural Ventilation in a Greenhouse with Screens. Comput. Electron. Agric. 2018, 149, 41–53. [Google Scholar] [CrossRef]
- Banda, M.H.; Nyeinga, K.; Okello, D. Performance Evaluation of 830 KWp Grid-Connected Photovoltaic Power Plant at Kamuzu International Airport-Malawi. Energy Sustain. Dev. 2019, 51, 50–55. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The Role of Renewable Energy in the Global Energy Transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Shubbak, M.H. Advances in Solar Photovoltaics: Technology Review and Patent Trends. Renew. Sustain. Energy Rev. 2019, 115, 109383. [Google Scholar] [CrossRef]
- Hassanien, R.H.E.; Li, M. Influences of greenhouse-integrated semi-transparent photo-voltaics on microclimate and lettuce growth. Int. J. Agric. Biol. Eng. 2017, 10, 11–22. [Google Scholar]
- Hassanien, R.H.E.; Li, M.; Yin, F. The integration of semi-transparent photovoltaics on greenhouse roof for energy and plant production. Renew. Energy 2018, 121, 377–388. [Google Scholar] [CrossRef]
- Aira, J.R.; Gallardo-Saavedra, S.; Eugenio-Gozalbo, M.; Alonso-Gómez, V.; Muñoz-Garcia, M.A.; Hernandez-Callejo, L. Analysis of the Viability of a Photovoltaic Greenhouse with Semi-Transparent Amorphous Silicon (a-Si) Glass. Agronomy 2021, 11, 1097. [Google Scholar] [CrossRef]
- Marrou, H.; Dufour, L.; Guilioni, L.; Salles, J.; Loisel, P.; Nogier, A.; Wery, J. Designing Farming Systems Combining Food and Electricity Production; Gansu Science and Technology Press: Lanzhou, China, 2014. [Google Scholar]
- Kadowaki, M.; Yano, A.; Ishizu, F.; Tanaka, T.; Noda, S. Effects of Greenhouse Photovoltaic Array Shading on Welsh Onion Growth. Biosyst. Eng. 2012, 111, 290–297. [Google Scholar] [CrossRef]
- Marrou, H.; Wery, J.; Dufour, L.; Dupraz, C. Productivity and Radiation Use Efficiency of Lettuces Grown in the Partial Shade of Photovoltaic Panels. Eur. J. Agron. 2013, 44, 54–66. [Google Scholar] [CrossRef]
- Bensink, J. On Morphogenesis of Lettuce Leaves in Relation to Light and Temperature; Wageningen University and Research: Wageningen, The Netherlands, 1971. [Google Scholar]
- Murphy, C.J.; Jana, N.R. Controlling the Aspect Ratio of Inorganic Nanorods and Nanowires. Adv. Mater. 2002, 14, 80–82. [Google Scholar] [CrossRef]
- Vakili, S.; Samare-Najaf, M.; Dehghanian, A.; Tajbakhsh, A.; Askari, H.; Tabrizi, R.; Saadi, M.I.; Movahedpour, A.; Alizadeh, M.; Samareh, A.; et al. Gold Nanobiosensor Based on the Localized Surface Plasmon Resonance is Able to Diagnose Human Brucellosis, Introducing a Rapid and Affordable Method. Nanoscale Res. Lett. 2021, 16, 144. [Google Scholar] [CrossRef]
- Matsuo, Y.; Sasaki, K. Time-Resolved Laser Scattering Spectroscopy of a Single Metallic Nanoparticle. Jpn. J. Appl. Phys. 2001, 40, 6143–6147. [Google Scholar] [CrossRef]
- Khattak, A.M.; Pearson, S.; Johnson, C.B. The Effect of Spectral Filters and Nitrogen Dose on the Growth of Chrysanthemum (Chrysanthemum Morifolium Ramat., Cv. Snowdon). J. Hortic. Sci. Biotechnol. 2015, 74, 206–212. [Google Scholar] [CrossRef]
- Palonen, P.; Karhu, S.; Savelainen, H.; Rantanen, M.; Junttila, O. Growth and Cropping of Primocane and Biennial Raspberry Cultivars Grown under a Film Absorbing Far-Red Light. J. Hortic. Sci. Biotechnol. 2015, 86, 113–119. [Google Scholar] [CrossRef]
- Li, Y.; Tu, W.; Liu, C.; Liu, W.; Hu, G.; Liu, X.; Chen, Z.; Yang, C. Light conversion film promotes CO2 assimilation by increasing cyclic electron flow around Photosystem I in Arabidopsis thaliana. Int. J. Hydrog. Energy 2017, 42, 8545–8553. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kang, J.H.; Kang, W.H.; Son, J.E. Subtle changes in solar radiation under a green-to-red conversion film affect the photosynthetic performance and chlorophyll fluorescence of sweet pepper. Photosynthetica 2020, 58, 1107–1115. [Google Scholar] [CrossRef]

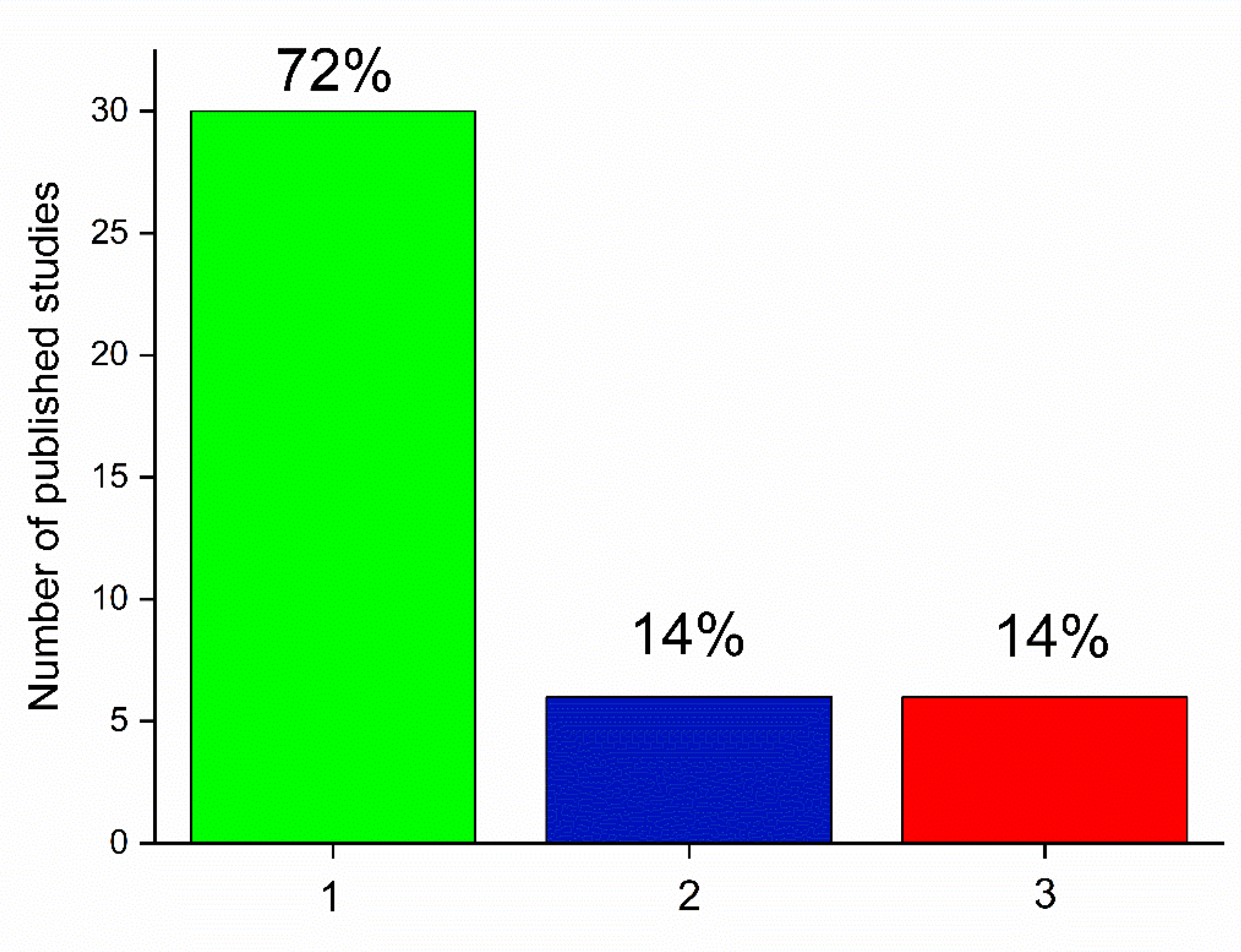
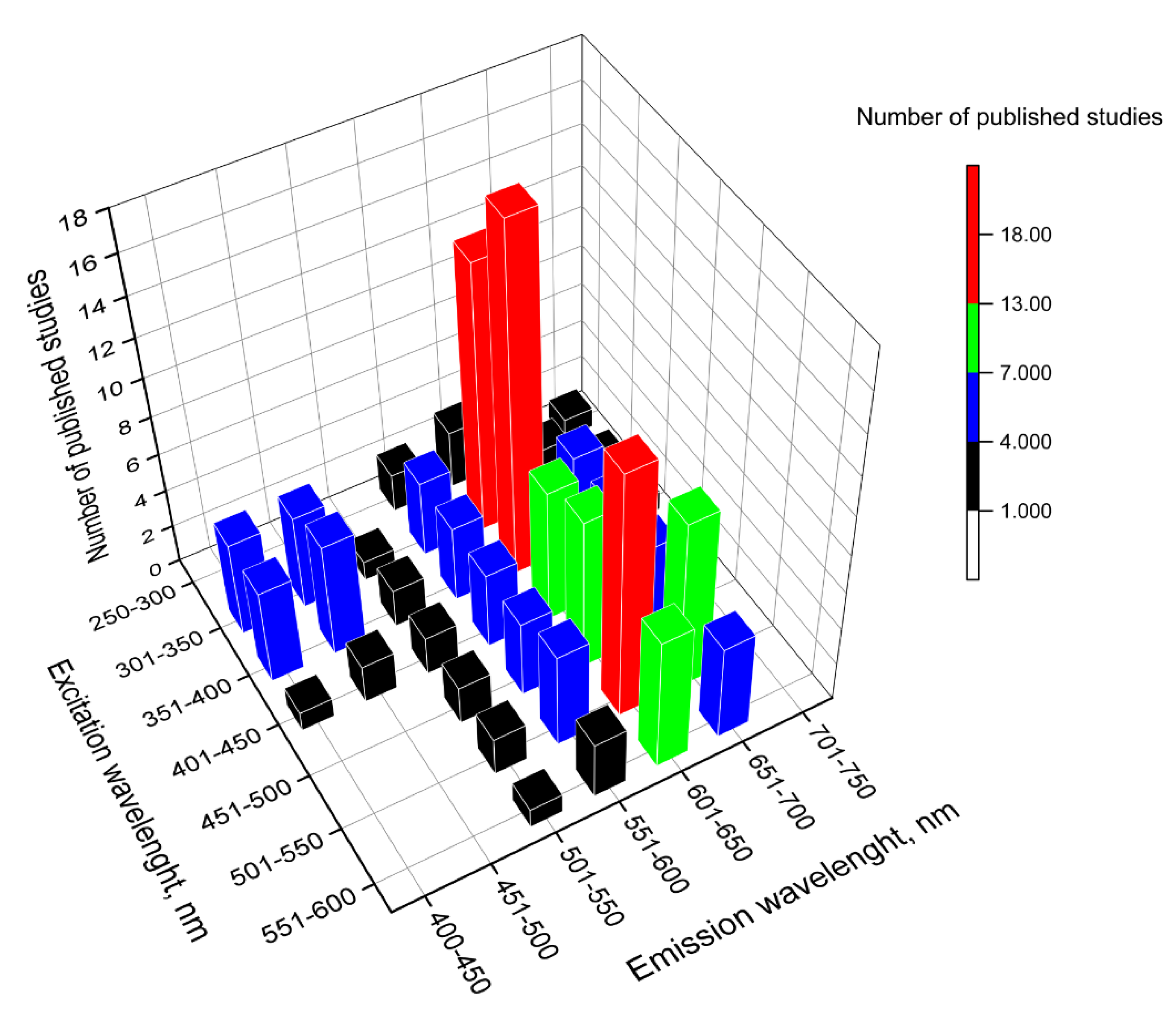
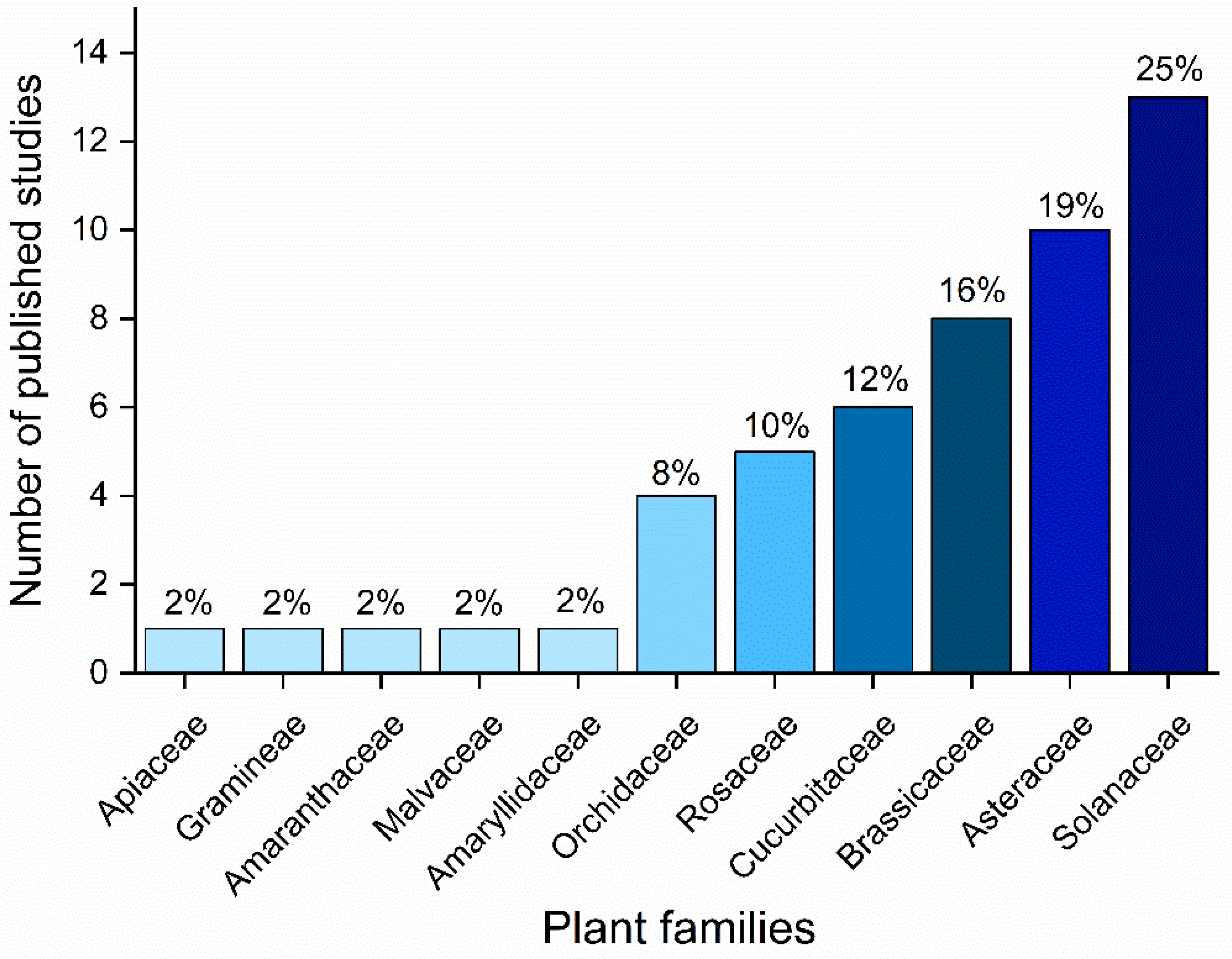
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskhin, M.O.; Yanykin, D.V.; Gudkov, S.V. Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review. Horticulturae 2022, 8, 885. https://doi.org/10.3390/horticulturae8100885
Paskhin MO, Yanykin DV, Gudkov SV. Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review. Horticulturae. 2022; 8(10):885. https://doi.org/10.3390/horticulturae8100885
Chicago/Turabian StylePaskhin, Mark O., Denis V. Yanykin, and Sergey V. Gudkov. 2022. "Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review" Horticulturae 8, no. 10: 885. https://doi.org/10.3390/horticulturae8100885
APA StylePaskhin, M. O., Yanykin, D. V., & Gudkov, S. V. (2022). Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review. Horticulturae, 8(10), 885. https://doi.org/10.3390/horticulturae8100885