Strawberry Tree Fruits and Leaves (Arbutus unedo L.) as Raw Material for Sustainable Functional Food Processing: A Review
Abstract
1. Introduction
2. Agriculture Perspective of Strawberry Tree Cultivation
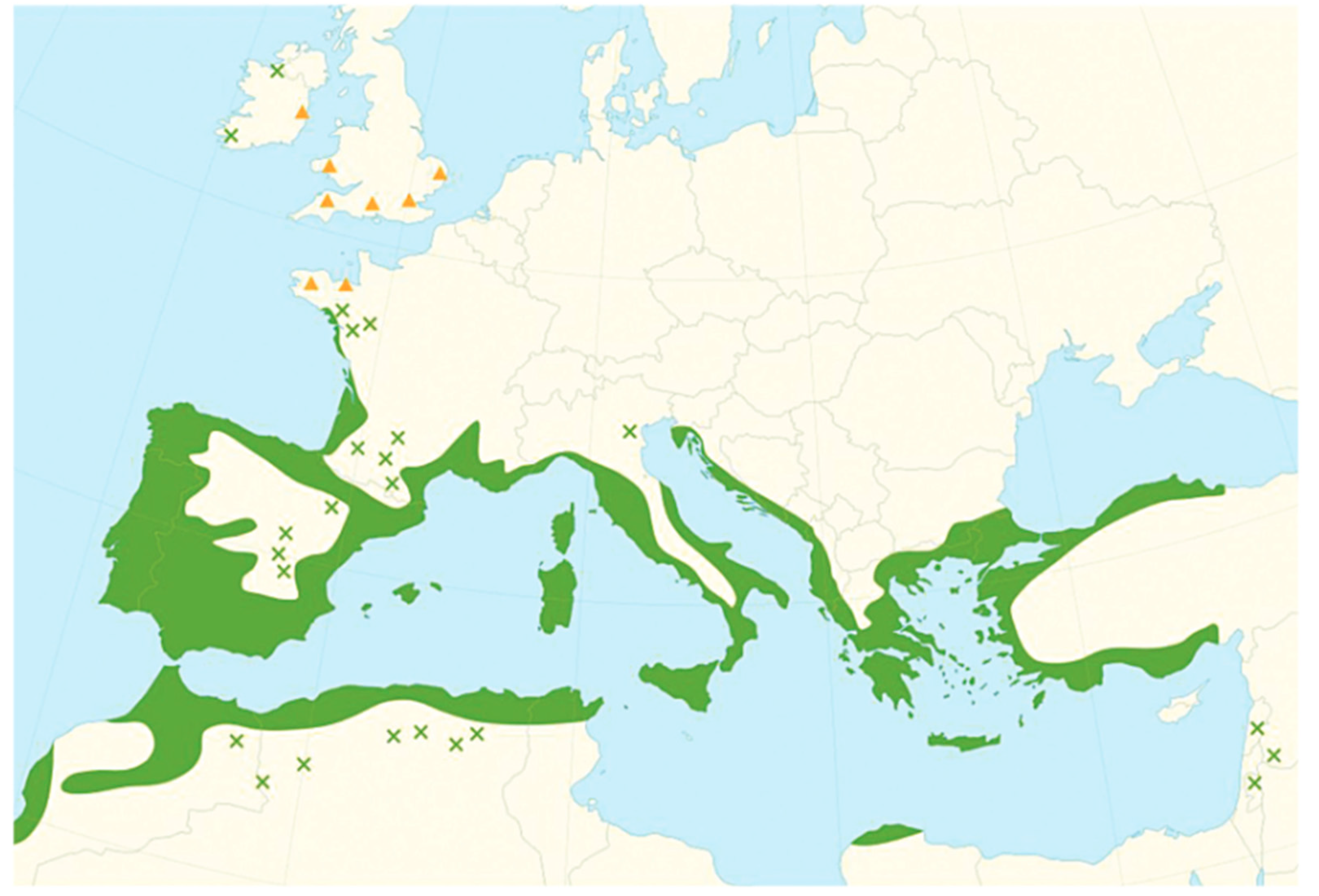
Geographic Distribution of A. unedo L.
3. Nutritive Value of Strawberry Tree
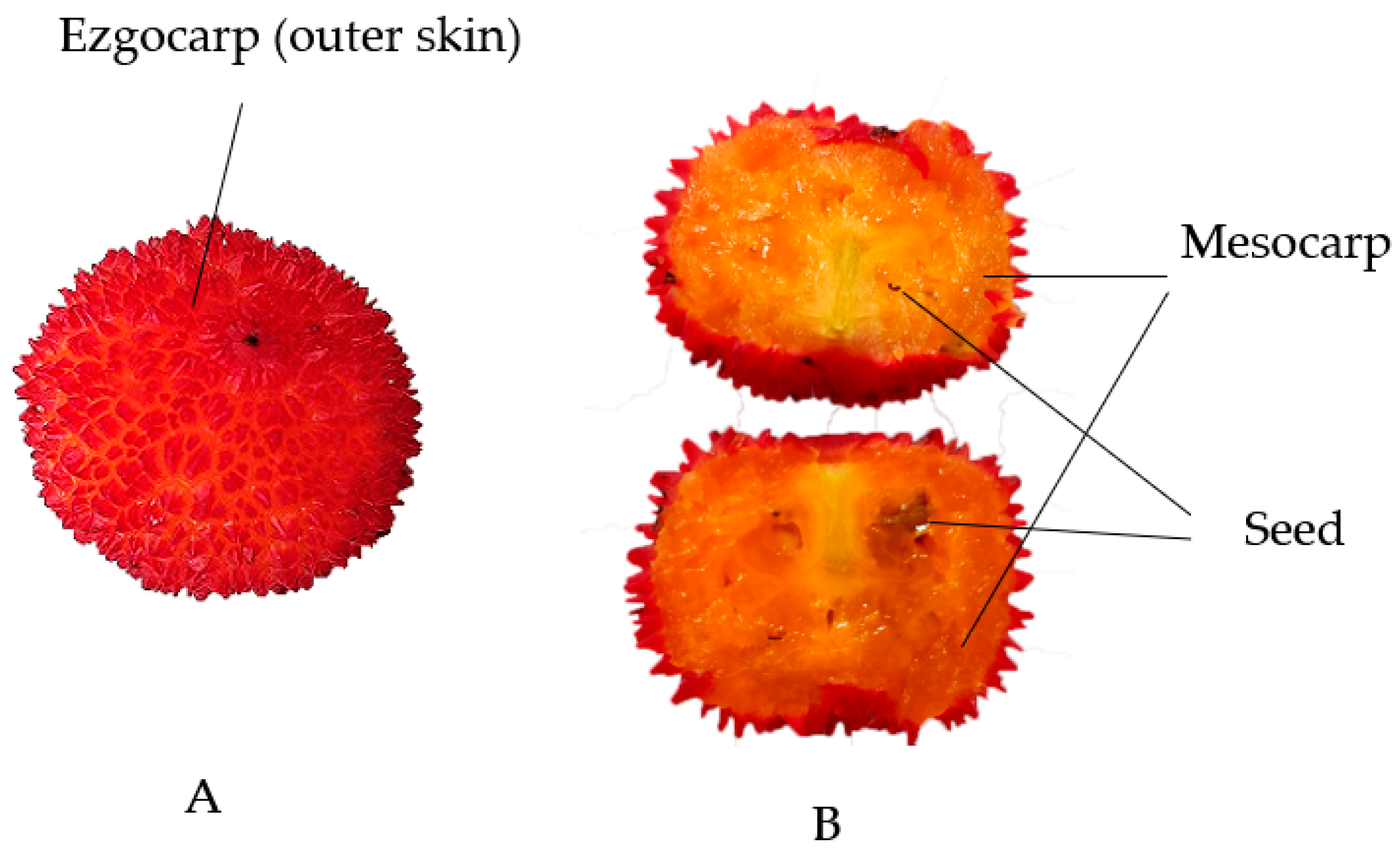
3.1. Fruits
3.2. Leaves
4. Biological Potential of Strawberry Tree
4.1. Fruits
4.2. Leaves
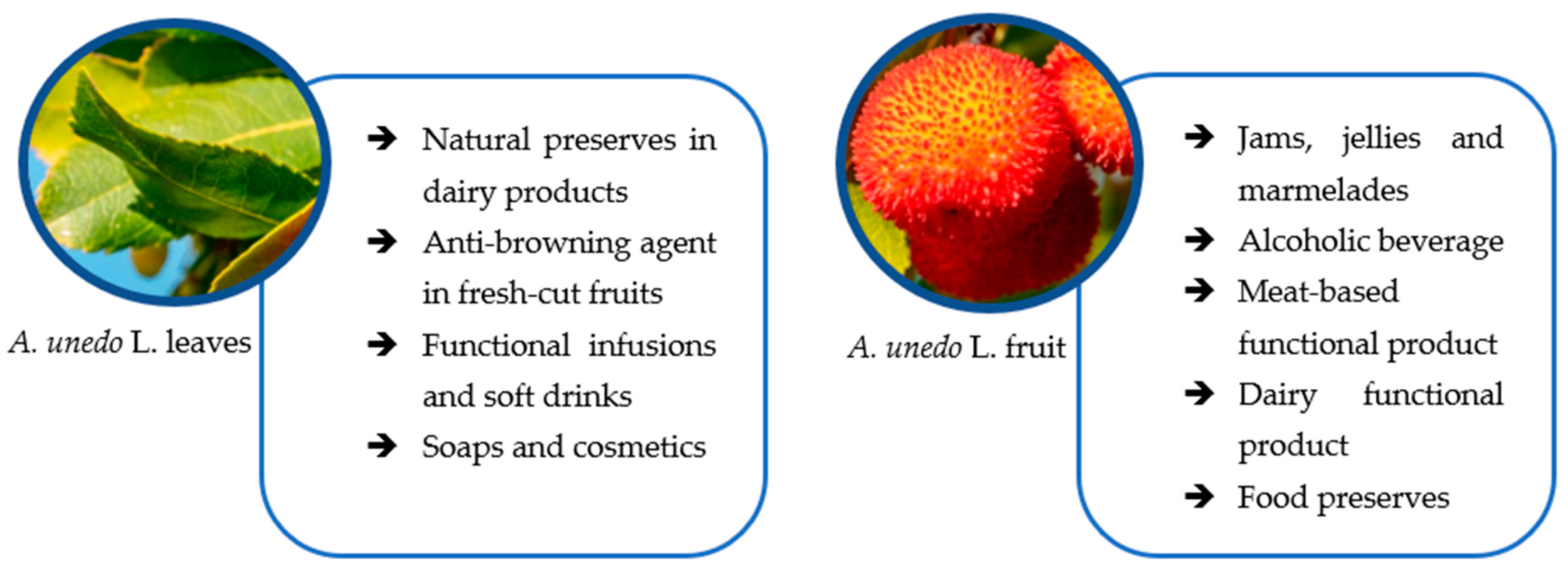
5. Economic Properties of Strawberry Tree
5.1. Fruits
5.2. Leaves
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jurica, K. Phenolic Compounds in Strawberry Tree (Arbutus unedo L.) and Their Biological Effects. Ph.D. Thesis, Department of Biology, Faculty of Science, University of Zagreb, Zagreb, Croatia, 2016. [Google Scholar]
- Linnæi, C. Species Plantarum; Laurentii Salvii: Stockholm, Sweden, 1753; p. 395. [Google Scholar]
- Sulusoglu Durul, M.; Memis, S. Optimization of conditions for in vitro culture of selected Arbutus unedo L. genotypes. Agronomy 2022, 12, 623. [Google Scholar] [CrossRef]
- Giovannetti, M.; Lioi, L. The mycorrhizal status of Arbutus unedo in relation to compatible and incompatible fungi. Can. J. Bot. 1990, 68, 1239–1244. [Google Scholar] [CrossRef]
- Brčić Karačonji, I.; Jurica, K.; Gašić, U.; Dramićanin, A.; Tešić, Ž.; Milojković Opsenica, D. Comparative study on the phenolic fingerprint and antioxidant activity of strawberry tree (Arbutus unedo L.) leaves and fruits. Plants 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Morgado, S.; Morgado, M.; Plácido, A.I.; Roque, F.; Duarte, A.P. Arbutus unedo L.: From traditional medicine to potential uses in modern pharmacotherapy. J. Ethnopharmacol. 2018, 225, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Gobin, I.; Kremer, D.; Čepo, D.V.; Grubešić, R.J.; Karačonji, I.B.; Kosalec, I. Arbutin and its metabolite hydroquinone as the main factors in the antimicrobial effect of strawberry tree (Arbutus unedo L.) leaves. J. Herb. Med. 2017, 8, 17–23. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Cappadone, C.; Mandrone, M.; Chiocchio, I.; Sanna, C.; Malucelli, E.; Bassi, V.; Picone, G.; Poli, F. Antitumor potential and phytochemical profile of plants from Sardinia (Italy), a hotspot for biodiversity in the Mediterranean basin. Plants 2019, 9, 26. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Tundis, R.; Xiao, J.; Loizzo, M.R.; Dugay, A.; Deguin, B. Arbutus species (Ericaceae) as source of valuable bioactive products. Crit. Rev. Food Sci. Nutr. 2018, 59, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; D’Urso, G.; Pagliuca, G.; Petretto, G.L.; Foddai, M.; Gallo, F.R.; Multari, G.; Caruso, D.; Montoro, P.; Pintore, G. HPTLC-PCA complementary to HRMS-PCA in the case study of Arbutus unedo antioxidant phenolic profiling. Foods 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Naceiri Mrabti, H.; Marmouzi, I.; Sayah, K.; Chemlal, L.; El Ouadi, Y.; Elmsellem, H.; Cherrah, Y.; Faouzi, M.A. Arbutus unedo L. aqueous extract is associated with in vitro and in vivo antioxidant activity. J. Mater. Environ. Sci. 2017, 8, 217–224. [Google Scholar]
- Juric, A.; Gasic, U.; Brcic-Karaconji, I.; Jurica, K.; Milojkovic-Opsenica, D. The phenolic profile of strawberry tree (Arbutus unedo L.) honey. J. Serb. Chem. Soc. 2020, 85, 1011–1019. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Miguel, M.; Faleiro, M.; Guerreiro, A.; Antunes, M. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Skendrović Babojelić, M.; Bogdanović, S.; Dlačić, I.; Duralija, B.; Prgomet, Ž.; Prgomet, I.; Šic Žlabur, J.; Voća, S. Strawberry Tree (Arbutus unedo L.) Biological, Chemical and Economic Properties; University of Zagreb Faculty of Agriculture: Zagreb, Croatia, 2020. [Google Scholar]
- Trinajstić, I. Plant Communities of the Republic of Croatia; Franjić, J., Ed.; Academy of Forestry Sciences: Zagreb, Croatia, 2008. [Google Scholar]
- Flora Croatica Database. Available online: http://hirc.botanic.hr/fcd (accessed on 5 May 2022).
- Sagbas, H.I.; Ilhan, G.; Zitouni, H.; Anjum, M.A.; Hanine, H.; Necas, T.; Ondrasek, I.; Ercisli, S. Morphological and biochemical characterization of diverse strawberry tree (Arbutus unedo L.) genotypes from Northern Turkey. Agronomy 2020, 10, 1581. [Google Scholar] [CrossRef]
- Celikel, G.; Demirsoy, L.; Demirsoy, H. The strawberry tree (Arbutus unedo L.) selection in Turkey. Sci. Hortic. 2008, 118, 115–119. [Google Scholar] [CrossRef]
- Songlin, M.; Yuejian, Z.; Senmiao, L.; Huang, X.G.; Wang, S.F.; Miao, S.L.; Zhang, Y.J.; Liang, S.M. Zaose, a promising new Arbutus cultivar. China Fruits 1995, 4, 3–4. [Google Scholar]
- Jihua, H.; Zuyou, L.; Tıanrong, X.; Xıanjun, Z. Study on the characteristics of flower formation and fruit set of Dongkui arbutus variety in western part of Hubei. S. China Fruits 1997, 26, 33–34. [Google Scholar]
- Cai-Huang, C.H. The cultural practices for high and top quality production of Arbutus fruit trees. China Fruits 1997, 3, 48. [Google Scholar]
- Gilman, E.F.; Watson, D.G. Arbutus unedo, Strawberry-Tree. In A Series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences; University of Florida: Gainesville, FL, USA, 1993; Volume Fact Sheet ST-85. [Google Scholar]
- Gomes, F.; Simões, M.; Lopes, M.L.; Canhoto, J.M. Effect of plant growth regulators and genotype on the micropropagation of adult trees of Arbutus unedo L. (strawberry tree). New Biotechnol. 2010, 27, 882–892. [Google Scholar] [CrossRef]
- Pato, R.L.; Botelho, G.; Franco, J.; Santos, S.; Ressurreição, S.; Figueiredo, P.; Gama, J.; Gomes, F. Interaction between farming type, nutrient uptake and plant material in strawberry tree fruit production and quality. Acta Hortic. 2022, 275–284. [Google Scholar] [CrossRef]
- Mulas, M.; Deidda, P. Domestication of woody plants from Mediterranean maquis to promote new crops for mountain lands. Acta Hortic. 1998, 457, 295–302. [Google Scholar] [CrossRef]
- Karadeniz, T.; Kurt, H.; Kalkışım, Ö. Yomra (Trabzon) çevresinde yetişen kocayemiş (Arbutus unedo L.) tiplerinin meyve özellikleri üzerinde çalışmalar. YYÜZF Derg. 1996, 6, 65–70. [Google Scholar]
- Karadeniz, T.; Kalkışım, Ö.; Şişman, T. Trabzon çevresinde yetişen kocayemiş (Arbutus unedo L.) tiplerinin meyve özellikleri ve çelikle çoğaltılması. In Proceedings of the Ulusal Kivi ve Üzümsü Meyveler Sempozyumu, Ordu, Turkey, 23–25 October 2003; pp. 476–480. [Google Scholar]
- Gozlekci, Ş.; Alkaya, C.E.; Yaş, D. Antalya cevresinde dogal olarak yayılış gosteren çilek ağacı (Arbutus andrechne L.)’nin bazı fenolojik ve pomolojik ö zelliklerinin incelenmesi. In Proceedings of the Ü zümsü Kivi ve Ü zümsü Meyveler Sempozyumu, Ordu, Turkey, 23–25 October 2003; pp. 472–475. [Google Scholar]
- Sakar, M.K.; Berkman, M.Z.; Calis, I.; Ruedi, P. Constituents of Arbutus andrachne. Fitoterapia 1991, 62, 176–177. [Google Scholar]
- Duralija, B.; Putnik, P.; Brdar, D.; Bebek Markovinović, A.; Zavadlav, S.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Bursać Kovačević, D. The perspective of croatian old apple cultivars in extensive farming for the production of functional foods. Foods 2021, 10, 708. [Google Scholar] [CrossRef]
- Oliveira, I.; Baptista, P.; Malheiro, R.; Casal, S.; Bento, A.; Pereira, J.A. Influence of strawberry tree (Arbutus unedo L.) fruit ripening stage on chemical composition and antioxidant activity. Food Res. Int. 2011, 44, 1401–1407. [Google Scholar] [CrossRef]
- Torres, J.A.; Valle, F.; Pinto, C.; García-Fuentes, A.; Salazar, C.; Cano, E. Arbutus unedo L. communities in southern Iberian Peninsula mountains. Plant Ecol. 2002, 160, 207–223. [Google Scholar] [CrossRef]
- Molina, M.; Pardo-De-Santayana, M.; Aceituno, L.; Morales, R.; Tardio, J. Fruit production of strawberry tree (Arbutus unedo L.) in two Spanish forests. Forestry 2011, 84, 419–429. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Bogdanović, S.; Voća, S.; Skendrović Babojelić, M. Biological potential of fruit and leaves of strawberry tree (Arbutus unedo L.) from Croatia. Molecules 2020, 25, 5102. [Google Scholar] [CrossRef]
- Hart, H. Inviting All the World’s Crops to the Table Supporting Traditional Crops to Supply Future Needs; CGSpace: Boston, MA, USA, 2007; pp. 1–25. [Google Scholar]
- El Haouari, M.; Assem, N.; Changan, S.; Kumar, M.; Daştan, S.D.; Rajkovic, J.; Taheri, Y.; Sharifi-Rad, J.; Kabra, A. An insight into phytochemical, pharmacological, and nutritional properties of Arbutus unedo L. from Morocco. Evid. Based Complement. Altern. Med. 2021, 2021, 1794621. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Tariba, B.; Živković, T.; Brajenović, N.; Pizent, A. A multielement profile of Croatian strawberry tree (Arbutus unedo L.) fruit and leaves. In Proceedings of the ISTERH 2015 Conference “Recent Advances in Trace Element Research in Health and Disease”, Dubrovnik, Croatia, 18–22 October 2015. [Google Scholar]
- El Cadi, H.; El Cadi, A.; Kounnoun, A.; Oulad El Majdoub, Y.; Palma Lovillo, M.; Brigui, J.; Dugo, P.; Mondello, L.; Cacciola, F. Wild strawberry (Arbutus unedo): Phytochemical screening and antioxidant properties of fruits collected in northern Morocco. Arab. J. Chem. 2020, 13, 6299–6311. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.-M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.-C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Sá Morais, J.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Colak, A.M. Morphological and biochemical diversity in fruits of Arbutus unedo L. from east aegean region in Turkey. Erwerbs-Obstbau 2019, 61, 379–383. [Google Scholar] [CrossRef]
- Fortalezas, S.; Tavares, L.; Pimpão, R.; Tyagi, M.; Pontes, V.; Alves, P.; McDougall, G.; Stewart, D.; Ferreira, R.; Santos, C. Antioxidant properties and neuroprotective capacity of strawberry tree fruit (Arbutus unedo). Nutrients 2010, 2, 214–229. [Google Scholar] [CrossRef]
- Alarcão-E-Silva, M.L.C.M.M.; Leitão, A.E.B.; Azinheira, H.G.; Leitão, M.C.A. The Arbutus berry: Studies on its color and chemical characteristics at two mature stages. J. Food Compos. Anal. 2001, 14, 27–35. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; De Leo, M.; Braca, A. Phenolics of Arbutus unedo L. (Ericaceae) Fruits: Identification of anthocyanins and gallic acid derivatives. J. Agric. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; del Castillo, M.D.; Cano, M.P.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kucukislamoglu, M.; Reunanen, M. Sugar, non-volatile and phenolic acids composition of strawberry tree (Arbutus unedo L. var.ellipsoidea) fruits. J. Food Compos. Anal. 2000, 13, 171–177. [Google Scholar] [CrossRef]
- Oliveira, I.; Baptista, P.; Bento, A.; Pereira, J.A. Arbutus unedo L. and its benefits on human health. J. Food Nutr. Res. 2011, 50, 73–85. [Google Scholar]
- Ganhão, R.; Estévez, M.; Kylli, P.; Heinonen, M.; Morcuende, D. Characterization of selected wild mediterranean fruits and comparative efficacy as inhibitors of oxidative reactions in emulsified raw pork burger patties. J. Agric. Food Chem. 2010, 58, 8854–8861. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Coelho, V.; Baltasar, R.; Pereira, J.A.; Baptista, P. Scavenging capacity of strawberry tree (Arbutus unedo L.) leaves on free radicals. Food Chem. Toxicol. 2009, 47, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Moussaoui, N.; Abrini, J.; Bakri, Y.; Dakka, N. Determination of phenolic contents, antioxidant and antibacterial activities of strawberry tree (Arbutus unedo L.) leaf extracts. Br. Biotechnol. J. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Martins, J.; Batista, T.; Pinto, G.; Canhoto, J. Seasonal variation of phenolic compounds in Strawberry tree (Arbutus unedo L.) leaves and inhibitory potential on Phytophthora cinnamomi. Trees 2021, 35, 1571–1586. [Google Scholar] [CrossRef]
- Carcache-Blanco, E.J.; Cuendet, M.; Park, E.J.; Su, B.-N.; Rivero-Cruz, J.F.; Farnsworth, N.R.; Pezzuto, J.M.; Douglas Kinghorn, A. Potential cancer chemopreventive agents from Arbutus unedo. Nat. Prod. Res. 2006, 20, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Legssyer, A.; Ziyyat, A.; Mekh, H.; Bnouham, M.; Herrenknecht, C.; Roumy, V.; Fourneau, C.; Laurens, A.; Hoerter, J.; Fischmeister, R. Tannins and catechin gallate mediate the vasorelaxant effect of Arbutus unedo on the rat isolated aorta. Phytother. Res. 2004, 18, 889–894. [Google Scholar] [CrossRef]
- Males, Z.; Plazibat, M.; Vundac, V.B.; Zuntar, I. Qualitative and quantitative analysis of flavonoids of the strawberry tree–Arbutus unedo L. (Ericaceae). Acta Pharm. 2006, 56, 245–250. [Google Scholar]
- Sanjust, E.; Mocci, G.; Zucca, P.; Rescigno, A. Mediterranean shrubs as potential antioxidant sources. Nat. Prod. Res. 2008, 22, 689–708. [Google Scholar] [CrossRef]
- Fiorentino, A.; Castaldi, S.; D’Abrosca, B.; Natale, A.; Carfora, A.; Messere, A.; Monaco, P. Polyphenols from the hydroalcoholic extract of Arbutus unedo living in a monospecific Mediterranean woodland. Biochem. Syst. Ecol. 2007, 35, 809–811. [Google Scholar] [CrossRef]
- de Falco, B.; Grauso, L.; Fiore, A.; Bonanomi, G.; Lanzotti, V. Metabolomics and chemometrics of seven aromatic plants: Carob, eucalyptus, laurel, mint, myrtle, rosemary and strawberry tree. Phytochem. Anal. 2022, 33, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Asmaa, N.; Abdelaziz, G.; Boulanouar, B.; Carbonell-Barrachina, A.; Cano-Lamadrid, M.; Noguera-Artiaga, L. Chemical composition, antioxidant activity and mineral content of Arbutus unedo (leaves and fruits). J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1335–1339. [Google Scholar] [CrossRef]
- Kivcak, B.; Mert, T.; Demirci, B.; Baser, K.H.C. Composition of the essential oil of Arbutus unedo L. Chem. Nat. Compd. 2001, 37, 445–446. [Google Scholar] [CrossRef]
- Berka-Zougali, B.; Hassani, A.; Besombes, C.; Allaf, K. Extraction of essential oils from Algerian myrtle leaves using instant controlled pressure drop technology. J. Chromatogr. A 2010, 1217, 6134–6142. [Google Scholar] [CrossRef] [PubMed]
- Koukos, D.; Meletiou-Christou, M.-S.; Rhizopoulou, S. Leaf surface wettability and fatty acid composition of Arbutus unedo and Arbutus andrachne grown under ambient conditions in a natural macchia. Acta Bot. Gall. 2015, 162, 225–232. [Google Scholar] [CrossRef][Green Version]
- Dib, M.A.; Paolini, J.; Bendahou, M.; Varesi, L.; Allali, H.; Desjobert, J.-M.; Tabti, B.; Costa, J. Chemical composition of fatty acid and unsaponifiable fractions of leaves, stems and roots of Arbutus unedo and in vitro antimicrobial activity of unsaponifiable extracts. Nat. Prod. Commun. 2010, 5, 721. [Google Scholar] [CrossRef]
- Kachkoul, R.; Squalli Housseini, T.; Mohim, M.; El Habbani, R.; Miyah, Y.; Lahrichi, A. Chemical compounds as well as antioxidant and litholytic activities of Arbutus unedo L. leaves against calcium oxalate stones. J. Integr. Med. 2019, 17, 430–437. [Google Scholar] [CrossRef]
- Zitouni, H.; Hssaini, L.H.; Ouaabou, R.; Viuda-Martos, M.; Hernandez, F.; Ercisli, S.; Hachimi, H.; Zerhoune, M.; Hanine, H. Functionnal and technological properties of five strawberry (Arbutus unedo L.) fruit as bioactive ingredients in functional foods. Int. J. Food Prop. 2021, 24, 380–399. [Google Scholar] [CrossRef]
- Izcara, S.; Morante-Zarcero, S.; Casado, N.; Sierra, I. Study of the phenolic compound profile of Arbutus unedo L. fruits at different ripening stages by HPLC-TQ-MS/MS. Appl. Sci. 2021, 11, 11616. [Google Scholar] [CrossRef]
- Gündoğdu, M.; Ercisli, S.; Canan, I.; Orman, E.; Sameeullah, M.; Naeem, M.; Ben Ayed, R. Diversity in phenolic compounds, biochemical and pomological characteristics of Arbutus unedo fruits. Folia Hortic. 2018, 30, 139–146. [Google Scholar] [CrossRef]
- Maleš, Ž.; Šarić, D.; Bojić, M. Quantitative determination of flavonoids and chlorogenic acid in the leaves of Arbutus unedo L. using thin layer chromatography. J. Anal. Methods Chem. 2013, 2013, 385473. [Google Scholar] [CrossRef]
- Maleš, Ž.; Fabijančić, P.; Barman, A.; Gregov, I.; Bojić, M. Antioksidacijski učinak i HPLC analiza listova planike-Arbutus unedo L. Farm. Glas. 2015, 71, 523–528. [Google Scholar]
- Jurica, K.; Karačonji, I.B.; Šegan, S.; Opsenica, D.M.; Kremer, D. Quantitative analysis of arbutin and hydroquinone in strawberry tree (Arbutus unedo L., Ericaceae) leaves by gas chromatography-mass spectrometry/Kvantitativna analiza arbutina i hidrokinona u listovima obične planike (Arbutus unedo L., Ericaceae) plinskokromatografskom metodom uz detekciju masenim spektrometrom. Arch. Ind. Hyg. Toxicol. 2015, 66, 197–202. [Google Scholar] [CrossRef]
- Pavlović, R.D.; Lakušić, B.; Došlov-Kokoruš, Z.; Kovačević, N. Arbutin content and antioxidant activity of some Ericaceae species. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 656–659. [Google Scholar]
- Vidrih, R.; Hribar, J.; Prgomet, Ž.; Poklar Ulrih, N. The physico-chemical properties of strawberry tree (Arbutus unedo L.) fruits. Croat. J. Food Sci. Technol. 2013, 5, 29–33. [Google Scholar]
- Camejo-Rodrigues, J.S. Recolha dos ‘Saber-Fazer’ Tradicionais das Plantas Aromáticas e Medicinais; Bordeira Concelhos de Aljezur: Bordeira, Portugal, 2006. [Google Scholar]
- Carvalho, A.M. Etnobotánica del Parque Natural de Montesinho Plantas, Tradición y Saber Popular en un Territorio del Nordeste de Portugal. Ph.D. Thesis, Universidad Autónoma, Madrid, Spain, 2005. [Google Scholar]
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.B.; Ouesleti, S.; Mabrouk, Y.; Landolsi, A.; Saidi, M.; Boulilla, A. Exploring the nutraceutical potential and biological activities of Arbutus unedo L. (Ericaceae) fruits. Ind. Crops Prod. 2018, 122, 726–731. [Google Scholar] [CrossRef]
- Dib, M.E.A.; Allali, H.; Bendiabdellah, A.; Meliani, N.; Tabti, B. Antimicrobial activity and phytochemical screening of Arbutus unedo L. J. Saudi Chem. Soc. 2013, 17, 381–385. [Google Scholar] [CrossRef]
- Kivçak, B.; Mert, T.; Ertabaklar, H.; Balcioğlu, I.C.; Ozensoy Töz, S. In vitro activity of Arbutus unedo against Leishmania tropica promastigotes. Turk. J. Parasitol. 2009, 33, 114–115. [Google Scholar]
- Zitouni, H.; Hssaini, L.; Messaoudi, Z.; Ourradi, H.; Viuda-Martos, M.; Hernández, F.; Ercisli, S.; Hanine, H. Phytochemical components and bioactivity assessment among twelve strawberry (Arbutus unedo L.) genotypes growing in Morocco using chemometrics. Foods 2020, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M. Understanding local Mediterranean diets: A multidisciplinary pharmacological and ethnobotanical approach. Pharmacol. Res. 2005, 52, 353–366. [Google Scholar]
- Afkir, S.; Nguelefack, T.B.; Aziz, M.; Zoheir, J.; Cuisinaud, G.; Bnouham, M.; Mekhfi, H.; Legssyer, A.; Lahlou, S.; Ziyyat, A. Arbutus unedo prevents cardiovascular and morphological alterations in L-NAME-induced hypertensive rats. J. Ethnopharmacol. 2008, 116, 288–295. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of different enriched phenolic extracts of wild fruits from Northeastern Portugal: A comparative study. Plant Foods Hum. Nutr. 2013, 69, 37–42. [Google Scholar] [CrossRef]
- Locatelli, C.; Leal, P.C.; Yunes, R.A.; Nunes, R.J.; Creczynski-Pasa, T.B. Gallic acid ester derivatives induce apoptosis and cell adhesion inhibition in melanoma cells: The relationship between free radical generation, glutathione depletion and cell death. Chem. Biol. Interact. 2009, 181, 175–184. [Google Scholar] [CrossRef]
- Le Marchand, L. Cancer preventive effects of flavonoids—A review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef]
- El-Hilaly, J.; Hmammouchi, M.; Lyoussi, B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). J. Ethnopharmacol. 2003, 86, 149–158. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, P.; Pabón, B.; Rodríguez, Á. Chemical and biological properties of Arbutus unedo, a potential medicinal plant. Rev. Cubana Farm. 2015, 49, 144–155. [Google Scholar]
- Ait lhaj, Z.; Bchitou, R.; Gaboun, F.; Abdelwahd, R.; Benabdelouahab, T.; Kabbour, M.R.; Pare, P.; Diria, G.; Bakhy, K. Moroccan strawberry tree (Arbutus unedo L.) fruits: Nutritional value and mineral composition. Foods 2021, 10, 2263. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Assemian, I.C.C.; Abrini, J.; Dakka, N. In vitro antiproliferative activity of selected medicinal plants from the North-West of Morocco on several cancer cell lines. Eur. J. Integr. Med. 2018, 18, 23–29. [Google Scholar] [CrossRef]
- Bnouham, M.; Merhfour, F.Z.; Legssyer, A.; Mekhfi, H.; Maal-lem, S.; Ziyyat, A. Antihyperglycemic activity of Arbutus unedo, Ammoides pusilla and Thymelaea hirsuta. Pharm. Int. J. Pharm. Sci. 2007, 62, 630–632. [Google Scholar]
- Mekhfi, H.; Haouari, M.E.; Legssyer, A.; Bnouham, M.; Aziz, M.; Atmani, F.; Remmal, A.; Ziyyat, A. Platelet anti-aggregant property of some Moroccan medicinal plants. J. Ethnopharmacol. 2004, 94, 317–322. [Google Scholar] [CrossRef]
- Malheiro, R.; Sá, O.; Pereira, E.; Aguiar, C.; Baptista, P.; Pereira, J.A. Arbutus unedo L. leaves as source of phytochemicals with bioactive properties. Ind. Crops Prod. 2012, 37, 473–478. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef]
- El Haouari, M.; López, J.J.; Mekhfi, H.; Rosado, J.A.; Salido, G.M. Antiaggregant effects of Arbutus unedo extracts in human platelets. J. Ethnopharmacol. 2007, 113, 325–331. [Google Scholar] [CrossRef]
- Tavares, L.; Fortalezas, S.; Carrilho, C.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C.N. Antioxidant and antiproliferative properties of strawberry tree tissues. J. Berry Res. 2010, 1, 3–12. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Gobin, I. Medicinal herbs and herbal preparations for the treatment of urinary infections. Med. Flum. 2018, 54, 262–267. [Google Scholar] [CrossRef][Green Version]
- Coimbra, A.T.; Luís, Â.F.S.; Batista, M.T.; Ferreira, S.M.P.; Duarte, A.P.C. Phytochemical characterization, bioactivities evaluation and synergistic effect of Arbutus unedo and crataegus monogyna extracts with amphotericin B. Curr. Microbiol. 2020, 77, 2143–2154. [Google Scholar] [CrossRef]
- Oliveira, I.; Nunes, A.; Lima, A.; Borralho, P.; Rodrigues, C.; Ferreira, R.; Ribeiro, A. New lectins from Mediterranean flora. Activity against HT29 colon cancer cells. Int. J. Mol. Sci. 2019, 20, 3059. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Mikolić, A.; Milojković-Opsenica, D.; Benković, V.; Kopjar, N. In vitro safety assessment of the strawberry tree (Arbutus unedo L.) water leaf extract and arbutin in human peripheral blood lymphocytes. Cytotechnology 2018, 70, 1261–1278. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Kopjar, N.; Shek-Vugrovečki, A.; Cikač, T.; Benković, V. The effects of strawberry tree water leaf extract, arbutin and hydroquinone on haematological parameters and levels of primary DNA damage in white blood cells of rats. J. Ethnopharmacol. 2018, 215, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Benković, V.; Sikirić, S.; Kopjar, N.; Brčić Karačonji, I. Liver function and DNA integrity in hepatocytes of rats evaluated after treatments with strawberry tree (Arbutus unedo L.) water leaf extract and arbutin. Drug Chem. Toxicol. 2018, 43, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Benković, V.; Sikirić, S.; Brčić Karačonji, I.; Kopjar, N. The effects of strawberry tree (Arbutus unedo L.) water leaf extract and arbutin upon kidney function and primary DNA damage in renal cells of rats. Nat. Prod. Res. 2018, 34, 2354–2357. [Google Scholar] [CrossRef] [PubMed]
- Orak, H.H.; Yagar, H.; Isbilir, S.S.; Demirci, A.Ş.; Gümüş, T.; Ekinci, N. Evaluation of antioxidant and antimicrobial potential of strawberry tree (Arbutus unedo L.) leaf. Food Sci. Biotechnol. 2011, 20, 1249–1256. [Google Scholar] [CrossRef]
- Kahriman, N.; Albay, C.G.; Dogan, N.; Usta, A.; Karaoglu, S.A.; Yayli, N. Volatile constituents and antimicrobial activities from flower. Asian J. Chem. 2010, 22, 6422–6437. [Google Scholar]
- Mekhfi, H.; ElHaouari, M.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A. Effects of extracts and tannins from Arbutus unedo leaves on rat platelet aggregation. Phytother. Res. 2006, 20, 135–139. [Google Scholar] [CrossRef]
- Orak, H.H.; Aktas, T.; Yagar, H.; İsbilir, S.S.; Ekinci, N.; Sahin, F.H. Effects of hot air and freeze drying methods on antioxidant activity, colour and some nutritional characteristics of strawberry tree (Arbutus unedo L.) fruit. Food Sci. Technol. Int. 2012, 18, 391–402. [Google Scholar] [CrossRef]
- Pavlović, D.R.; Branković, S.; Kovačević, N.; Kitić, D.; Veljković, S. Comparative study of spasmolytic properties, antioxidant activity and phenolic content of Arbutus unedo from Montenegro and Greece. Phytother. Res. 2011, 25, 749–754. [Google Scholar] [CrossRef]
- Mariotto, S.; Ciampa, A.; de Prati, A.; Darra, E.; Vincenzi, S.; Sega, M.; Cavalieri, E.; Shoji, K.; Suzuki, H. Aqueous extract of Arbutus unedo inhibits STAT1 activation in human breast cancer cell line MDA-MB-231 and human fibroblasts through SHP2 activation. Med. Chem. 2008, 4, 219–228. [Google Scholar] [CrossRef]
- Mariotto, S.; Esposito, E.; Di Paola, R.; Ciampa, A.; Mazzon, E.; de Prati, A.C.; Darra, E.; Vincenzi, S.; Cucinotta, G.; Caminiti, R. Protective effect of Arbutus unedo aqueous extract in carrageenan-induced lung inflammation in mice. Pharmacol. Res. 2008, 57, 110–124. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.; Barba, F.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Bifulco, E.; Caboni, P.; Cottiglia, F.; Cabras, P.; Floris, I. Floral markers of strawberry tree (Arbutus unedo L.) honey. J. Agric. Food Chem. 2009, 58, 384–389. [Google Scholar] [CrossRef]
- Osés, S.M.; Nieto, S.; Rodrigo, S.; Pérez, S.; Rojo, S.; Sancho, M.T.; Fernández-Muiño, M.Á. Authentication of strawberry tree (Arbutus unedo L.) honeys from southern Europe based on compositional parameters and biological activities. Food Biosci. 2020, 38, 100768. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Bouyahya, A.; Ed-Dra, A.; Kachmar, M.R.; Mrabti, N.N.; Benali, T.; Shariati, M.A.; Ouahbi, A.; Doudach, L.; Faouzi, M.E.A. Polyphenolic profile and biological properties of Arbutus unedo root extracts. Eur. J. Integr. Med. 2021, 42, 101266. [Google Scholar] [CrossRef]
- Tomašević, I.; Putnik, P.; Valjak, F.; Pavlić, B.; Šojić, B.; Bebek Markovinović, A.; Bursać Kovačević, D. 3D printing as novel tool for fruit-based functional food production. Curr. Opin. Food Sci. 2021, 41, 138–145. [Google Scholar] [CrossRef]
- Seidemann, J. Zur Kenntnis von wenig bekannten exotischen Früchten. 5. (Mitt.): Baumerdbeere (Arbutus unedo L.). Dtsch. Lebensm. Rundsch. 1995, 91, 110–113. [Google Scholar]
- TardÍO, J.; Pardo-De-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Soufleros, E.H.; Mygdalia, S.A.; Natskoulis, P. Production process and characterization of the traditional Greek fruit distillate “Koumaro” by aromatic and mineral composition. J. Food Compos. Anal. 2005, 18, 699–716. [Google Scholar] [CrossRef]
- Wormit, A.; Usadel, B. The multifaceted role of pectin methylesterase inhibitors (PMEIs). Int. J. Mol. Sci. 2018, 19, 2878. [Google Scholar] [CrossRef]
- European Union. Regulation EU n° 2019/787 of the European Parliament and of the Council of 17 April 2019 on the Definition, d., Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No110/2008. O. J. Eur. Union. O. J.; European Union: Maastricht, The Netherlands, 2019; Volume L130, pp. 1–54. [Google Scholar]
- Botelho, G.; Gomes, F.; Ferreira, F.M.; Caldeira, I. Influence of maturation degree of Arbutus (Arbutus unedo L.) fruits in spirit composition and quality. Int. J. Agric. Biol. Eng. 2015, 9, 615–620. [Google Scholar]
- Santo, D.E.; Galego, L.; Gonçalves, T.; Quintas, C. Yeast diversity in the Mediterranean strawberry tree (Arbutus unedo L.) fruits’ fermentations. Food Res. Int. 2012, 47, 45–50. [Google Scholar] [CrossRef]
- Caldeira, I.; Gomes, F.; Botelho, G. Arbutus unedo L. spirit: Does the water addition before fermentation matters? In Proceedings of the 1st International Congress on Engineering and Sustainability in the XXI Century—INCREaSE, Faro, Portugal, 11–13 October; Mortal, A., Aníbal, J., Monteiro, J., Sequeira, C., Semião, J., Silva, M.M., Oliveira, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 206–215. [Google Scholar]
- Cavaco, T.; Longuinho, C.; Quintas, C.; Saraiva De Carvalho, I. Chemical and microbial changes during the natural fermentation of strawberry tree (Arbutus unedo L.) fruits. J. Food Biochem. 2007, 31, 715–725. [Google Scholar] [CrossRef]
- Anjos, O.; Canas, S.; Gonçalves, J.C.; Caldeira, I. Development of a spirit drink produced with strawberry tree (Arbutus unedo L.) fruit and honey. Beverages 2020, 6, 38. [Google Scholar] [CrossRef]
- Masmoudi, M.; Ammar, I.; Ghribi, H.; Attia, H. Physicochemical, radical scavenging activity and sensory properties of a soft cheese fortified with Arbutus unedo L. extract. Food Biosci. 2020, 35, 100579. [Google Scholar] [CrossRef]
- Cossu, M.; Juliano, C.; Pisu, R.; Alamanni, M.C. Effects of enrichment with polyphenolic extracts from sardinian plants on physico-chemical, antioxidant and microbiological properties of yogurt. Ital. J. Food Sci. 2009, 21, 447–459. [Google Scholar]
- Takwa, S.; Caleja, C.; Barreira, J.C.M.; Soković, M.; Achour, L.; Barros, L.; Ferreira, I.C.F.R. Arbutus unedo L. and Ocimum basilicum L. as sources of natural preservatives for food industry: A case study using loaf bread. LWT 2018, 88, 47–55. [Google Scholar] [CrossRef]
- Derbassi, N.; Pedrosa, M.C.; Heleno, S.; Fernandes, F.; Dias, M.I.; Calhelha, R.C.; Rodrigues, P.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Arbutus unedo leaf extracts as potential dairy preservatives: Case study on quark cheese. Food Funct. 2022, 13, 5442–5454. [Google Scholar] [CrossRef]
- Dias, C.; Fonseca, A.M.A.; Amaro, A.L.; Vilas-Boas, A.A.; Oliveira, A.; Santos, S.A.O.; Silvestre, A.J.D.; Rocha, S.M.; Isidoro, N.; Pintado, M. Natural-based antioxidant extracts as potential mitigators of fruit browning. Antioxidants 2020, 9, 715. [Google Scholar] [CrossRef]
- Erkekoglou, I.; Nenadis, N.; Samara, E.; Mantzouridou, F.T. Functional teas from the leaves of Arbutus unedo: Phenolic content, antioxidant activity, and detection of efficient radical scavengers. Plant Foods Hum. Nutr. 2017, 72, 176–183. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Himejima, M.; Nihei, K.I.; Kubo, I. Hydroquinone, a control agent of agglutination and adherence of Streptococcus mutans induced by sucrose. Bioorganic Med. Chem. 2004, 12, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Sato, N. Benzoquinone, the substance essential for antibacterial activity in aqueous extracts from succulent young shoots of the pear Pyrus spp. Phytochemistry 2003, 62, 101–107. [Google Scholar] [CrossRef]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Pixabay. Available online: https://pixabay.com/photos/fruits-strawberry-tree-sheets-5829002/ (accessed on 7 September 2020).

| Plant Source | Analytics | Bioactive Compound | Concentration | References |
|---|---|---|---|---|
| Fruit | HPLC | Gallic acid | 4.56–36.93 mg 100 g−1 DW | [69] |
| Protocatechuic | 1.84–5.90 mg 100 g−1 DW | |||
| Gallocatechin | 16.15–65.31 mg 100 g−1 DW | |||
| Catechin | 22.09–49.36 mg 100 g−1 DW | |||
| Chlorogenic acid | 5.55–27.42 mg 100 g−1 DW | |||
| Syringic acid | 4.27–7.94 mg 100 g−1 DW | |||
| Ellagic acid | 8.42–33.73 mg 100 g−1 DW | |||
| Quercetin-3-xyloside | 1.43–4.09 mg 100 g−1 DW | |||
| Rutin | 0.90–1.26 mg 100 g−1 DW | |||
| Quercetin-3-galactoside | 1.66–3.46 mg 100 g−1 DW | |||
| Quercetin-3-glucoside | 2.11–2.89 mg 100 g−1 DW | |||
| Cyanidin-3-glucoside | 0.43–7.21 mg 100 g−1 DW | |||
| Cyanidin-3-arabinoside | 0.36–1.64 mg 100 g−1 DW | |||
| Fruit | HPLC-TQ-MS/MS | 4-hydroxybenzoic acid | 0.34–0.50 mg 100 g−1 DW | [70] |
| Gallic acid | 1.4–4.7 mg 100 g−1 DW | |||
| Syringic acid | 0.63 mg 100 g−1 DW | |||
| Chlorogenic acid | 0.676 mg 100 g−1 DW | |||
| Quercetin | 0.79–0.84 mg 100 g−1 DW | |||
| Quercetin 3-β-glucoside | 1.7–2.6 mg 100 g−1 DW | |||
| Rutin | 0.43–0.57 mg 100 g−1 DW | |||
| Kaempherol | 0.39–0.74 mg 100 g−1 DW | |||
| Catequin | 28–149 mg 100 g−1 DW | |||
| Epigallocatechin | 10–26 mg 100 g−1 DW | |||
| Naringin | 0.35 mg 100 g−1 DW | |||
| Fruit | MS/MS | Arbutin | NQ | [11] |
| Myricetin pentoside | NQ | |||
| Myricetin rhamnoside | NQ | |||
| Kaempferol-rhamnoside (afzelin) | NQ | |||
| Fruit | HPLC | Protocatechuic acid | 0.11–0.61 mg 100 g−1 FW | [71] |
| Vanillic acid | 0.10–1.17 mg 100 g−1 FW | |||
| Ellagic acid | 1.11–2.13 mg 100 g−1 FW | |||
| Rutin | 0.15–0.95 mg 100 g−1 FW | |||
| Quercetin | 0.12–0.31 mg 100 g−1 FW | |||
| Gallic acid | 1.62–7.29 mg 100 g−1 FW | |||
| Catechin | 1.16–5.75 mg 100 g−1 FW | |||
| Leaves | UHPLC-LTQ Orbitrap MS | Gallocatechin | 64.21–211.60 mg kg−1 DW | [5] |
| Protocatechuic acid | 1.27–2.47 mg kg−1 DW | |||
| Aesculin | 1.95–5.88 mg kg−1 DW | |||
| Chlorogenic acid | ND–1.95 mg kg−1 DW | |||
| Catechin | 47.73–102.95 mg kg−1 DW | |||
| p-Hydroxybenzoic acid | 16.21–27.08 mg kg−1 DW | |||
| Caffeic acid | 2.61–5.75 mg kg−1 DW | |||
| Syringic acid | 0.66–2.67 mg kg−1 DW | |||
| Vanillic acid | 3.71–7.96 mg kg−1 DW | |||
| Rutin | 29.93–106.03 mg kg−1 DW | |||
| p-Hydroxyphenylacetic acid | 4.35–6.57 mg kg−1 DW | |||
| Hyperoside | 635.10–1512.94 mg kg−1 DW | |||
| p-Coumaric acid | 10.11–32.83 mg kg−1 DW | |||
| Catechin gallate | 34.48–73.70 mg kg−1 DW | |||
| Ferulic acid | 2.55–4.85 mg kg−1 DW | |||
| Myricetin | ND–1.78 mg kg−1 DW | |||
| Quercetin | 41.28–124.91 mg kg−1 DW | |||
| Naringenin | ND–4.39 mg kg−1 DW | |||
| Kaempferol | 10.63–35.50 mg kg−1 DW | |||
| Leaves | HPTLC | Quercitrin | 1.21–2.20 mg g−1 DW | [72] |
| Isoquercitrin | ND–0.33 mg g−1 DW | |||
| Hyperoside | ND–0.35 mg g−1 DW | |||
| Chlorogenic acid | 0.61–1.46 mg g−1 DW | |||
| Leaves | HPLC-PDA | Chlorogenic acid | 0.8–6.5 mg g−1 DW | [73] |
| Caffeic acid | 0.6–1.0 mg g−1 DW | |||
| p-Coumaric acid | 0.2–6.6 mg g−1 DW | |||
| Quercetin | 0.5–10.7 mg g−1 DW | |||
| Leaves | GC-MS | Arbutin | 2.75–6.82 mg g−1 DW | [74] |
| Leaves | HPLC-PDA | Arbutin | 12.1 mg g−1 DW | [75] |
| Part of Plant | Type of Study | Biological Potential | References |
|---|---|---|---|
| Leaves | Determination of growth inhibition zones by radial diffusion | Antibacterial and antifungal potential | [95] |
| Leaves | Determination of growth inhibition zones by disc diffusion | Antibacterial and antifungal potential | [106] |
| Fruits | Determination of MIC by dilution on broth media | Antibacterial potential | [107] |
| Leaves | In vitro platelet aggregation | Antiaggregant potential | [97] |
| Leaves | In vitro platelet aggregation | Antiaggregant potential | [108] |
| Fruits | In vitro, BrdU assay | Antitumoral potential | [84] |
| Fruits | DPPH assay, scavenging activity, β-carotene bleaching activity | Antioxidant potential | [109] |
| Leaves | DPPH assay | Antioxidant potential | [110] |
| Leaves and fruits | ORAC assay, MMP-9 inhibitory activity assay | Antioxidant potential | [98] |
| Leaves | FRAP, Lipid peroxidation, DPPH assay | Antioxidant potential | [75] |
| Fruits | DPPH assay, DNA damage | Antioxidant potential | [84] |
| Leaves | Inflammatory activation, In vitro inhibition of STAT1 activation | Anti-inflammatory potential | [111,112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebek Markovinović, A.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Skendrović Babojelić, M.; Duralija, B.; Šic Žlabur, J.; Putnik, P.; Bursać Kovačević, D. Strawberry Tree Fruits and Leaves (Arbutus unedo L.) as Raw Material for Sustainable Functional Food Processing: A Review. Horticulturae 2022, 8, 881. https://doi.org/10.3390/horticulturae8100881
Bebek Markovinović A, Brčić Karačonji I, Jurica K, Lasić D, Skendrović Babojelić M, Duralija B, Šic Žlabur J, Putnik P, Bursać Kovačević D. Strawberry Tree Fruits and Leaves (Arbutus unedo L.) as Raw Material for Sustainable Functional Food Processing: A Review. Horticulturae. 2022; 8(10):881. https://doi.org/10.3390/horticulturae8100881
Chicago/Turabian StyleBebek Markovinović, Anica, Irena Brčić Karačonji, Karlo Jurica, Dario Lasić, Martina Skendrović Babojelić, Boris Duralija, Jana Šic Žlabur, Predrag Putnik, and Danijela Bursać Kovačević. 2022. "Strawberry Tree Fruits and Leaves (Arbutus unedo L.) as Raw Material for Sustainable Functional Food Processing: A Review" Horticulturae 8, no. 10: 881. https://doi.org/10.3390/horticulturae8100881
APA StyleBebek Markovinović, A., Brčić Karačonji, I., Jurica, K., Lasić, D., Skendrović Babojelić, M., Duralija, B., Šic Žlabur, J., Putnik, P., & Bursać Kovačević, D. (2022). Strawberry Tree Fruits and Leaves (Arbutus unedo L.) as Raw Material for Sustainable Functional Food Processing: A Review. Horticulturae, 8(10), 881. https://doi.org/10.3390/horticulturae8100881