Abstract
Prunus serotina is divided into five subspecies. Among these, P. serotina subsp. capuli, commonly known as capuli, is native to Central and South America. Its fruits are big, sweet, and consumed by locals in various forms, with the Ecuadorian Andes region providing the best fruit forms of capuli. The objective of this study was to characterize a collection of different genotypes of capuli’s fruit, and their endocarps, found growing in the wild in the Chimborazo, Tungurahua, and Cotopaxi provinces of Ecuador. The fruits were characterized for weight (11.7–50.3 g), diameter (12.4–21.7 mm), height (11.4–19.7 mm), and soluble solids content (SSC) (13.4–27.6 °Brix) across two years. Similarly, the endocarps’ diameters (7.5–12.2 mm) and heights (8.1–13.6 mm) were measured. Significant differences were found for all the fruit and endocarp variables studied. A multivariate analysis showed that all the fruit and endocarp size characteristics, except SSC, were positively correlated with each other within a season. No clear population differentiation was seen among the genotypes from different provinces, suggesting a lack of clear morphological differentiation. The future breeding and selection of a capuli with superior, commercial, large fruit and a high SSC shows great potential.
1. Introduction
Prunus serotina Ehrh. is commonly known as a black cherry [1]. It is commercially important due to its high-quality timber, which is used for making scientific and professional instrument boxes, furniture, and cabinets [2]. This species was introduced in central Europe in the 17th century as an ornamental species but now it is considered as an invasive species in western Europe [3,4,5]. Similarly, P. serotina has been evaluated as a rootstock for other Prunus species [6].
The Prunus serotina tree grows in cool, moist, temperate climates, with optimum and evenly dispersed rainfall [1]. Prunus serotina is further classified into the following five different subsp.: P. serotina subsp. serotina; P. serotina subsp. virens (Woot. and Standl.) McVaugh; P. serotina subsp. hirsuta (Elliot) McVaugh; P. serotina subsp. eximia (Small) McVaugh; and P. serotina subsp. capuli (Cav. Ex Spreng.) McVaugh [7]. These subspecies have a broad distribution range throughout North, Central, and South America, each occupying distinct geographical regions and each having different morphological characteristics. Among the five subspecies, P. serotina subsp. capuli, or capuli, is a unique subspecies, with a presence in Mexico, Ecuador, Colombia, and Guatemala, and with superior forms found in the Ecuadorian Andes [7].
The capuli’s fruit has a depressed globose shape, with a light maroon to deep purplish skin color. Its flesh is a pale brownish green, melting and juicy. The fruits have a sweet vinous flavor, with a taste of bitterness because of the skin [8]. Its flesh constitutes the mesocarp and its skin the exocarp of the fruit. The endocarp is the stone or pit. These fruits are abundantly available in Ecuadorian markets from December to February and are mostly eaten fresh. They can also be stewed, preserved, or made into wine or jam [9]. Tea and syrups made from capuli leaves are used in the treatment of hypertension, stomach problems, diarrhea, colds, malaria, and coughs [10]. Research has shown that capuli’s fruit has a high number of phenolic compounds, with antihypertensive and antioxidant effects [11,12]. Its consumption can be beneficial in preventing health issues such as hypertension and cardiovascular diseases [11].
The roasted seeds of capuli’s fruit are used as snacks in Mexico [13]. Capuli’s seeds have a protein content in their raw (37.95%) and toasted (36.55%) forms, which is higher than that in almonds and peanuts. The fat content in the raw (40.37%) and toasted seeds (39.97%) was found to be lower than that in almonds (49.64%), but not significantly different to that in peanuts (41.12%) [14]. Black cherry seed oil is rich in oleic (35%), linoleic (27%), and α-eleostearic acid (27%) [15]. Despite its significant health and nutritional benefits, there are no commercial capuli cultivars or breeding programs in the world.
Research regarding the capuli fruit’s morphological characteristics is fundamental for the establishment of future capuli breeding programs. Fruit size and firmness are among the most important morphological characteristics for evaluating a fruit’s economic value [16]. For example, fruit size and diameter are the main morphological characteristics on which sweet cherries (Prunus avium L.) are commercially graded in the United States [17]. Similar grading and commercialization exists for other fruits and in other countries in which fruit size is one of the most important characteristics. Christensen [16] studied sweet cherry cultivars from Denmark and found the average sweet cherry size to be 6.6 g per fruit. Khadivi et al. [18] studied the morphological characteristics of 45 sweet cherry, 62 sour cherry, and 39 duke cherry [Prunus × gondouinii (Poit. and Turpin.) Schneid.] cultivars from Iran. The fruits’ length varied from 18.9 to 28.5 mm for the sweet cherry cultivars, 11.2 mm to 14.6 mm for the sour cherry cultivars (Prunus cerasus L.), and 15.4 to 18.6 mm for the duke cherry cultivars. For fruit weight, the sweet cherry cultivars varied from 4.4 to 8.9 g, sour cherry cultivars from 1.4 to 2.7 g, and from 4.4 to 6.0 g for the duke cherry cultivars.
In North America, the average diameter of the P. serotina fruits growing in central Mississippi varied from 7–10 mm [19]. Marquis [20] also reported the diameter of the black cherry fruit to be approximately 10 mm. Limited studies have been published regarding the capuli fruit’s morphology. The presence of superior fruit genotypes of P. serotina subsp. capuli in the Ecuadorian Andes creates a unique opportunity for future breeding and selection. This study aims to characterize a collection of different genotypes of capuli’s fruit and their endocarps (stones, pits) found growing in the wild in the Chimborazo, Tungurahua, and Cotopaxi provinces of Ecuador. Capuli fruit’s characterization will help us to understand more about this species’ natural morphological variation, which will further aid in the establishment of future capuli breeding programs for superior fruit, with commercial attributes.
2. Materials and Methods
2.1. Plant Material
Fruit of 24 P. serotina subsp. capuli genotypes were collected in 2016 and 2019 from three Ecuadorian provinces (Chimborazo, Cotopaxi, and Tungurahua) in the Andes region. Plant material collection was performed in accordance with the plan for access to genetic resources, made by Ecuador’s Minister of Environment—collection permit MAE-DNB-CM-2019-0107P37-16-00098. Each genotype was given a unique ID, based on their collection site (Table 1). Genotypes were selected to represent a broad geographical range (~5–10 Km separation between collection sites) in the region (Figure 1). Generally, one individual tree/genotype was used per collection site, however, if more than two trees were observed per location, then fruit from one tree was randomly selected to represent that site.

Table 1.
List of Prunus serotina subsp. capuli genotypes, collected for fruit evaluation in the Andes region of Ecuador.
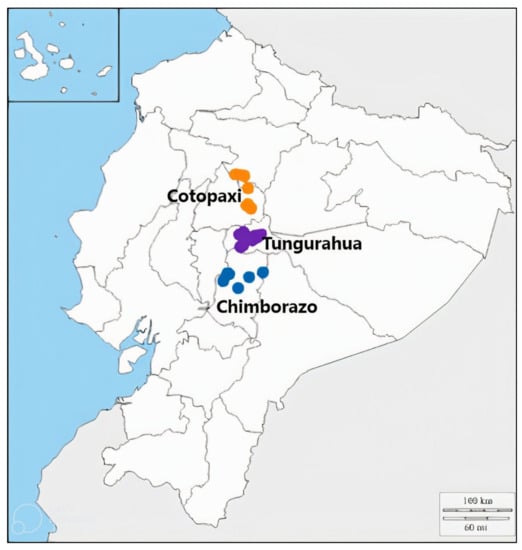
Figure 1.
Map representing collection sites of Prunus serotina subsp. capuli genotypes, collected from 3 different provinces of Ecuador in 2016 and 2019.
2.2. Fruit and Endocarp Measurements
Ripe fruits were collected in Ziploc bags and stored in a cooler with ice, after collection in February of 2016 and 2019 (Figure 2). Samples were then transported to the Escuela Politécnica del Chimborazo (ESPOCH) in Riobamba, Ecuador. Fruits were then evaluated for the following characteristics: weight of ten fruits (g), fruit diameter (mm) (measurement along the equatorial plane of the fruit at the widest point), fruit height (mm) (measurement from stem to the tip end of the fruit), and soluble solids content (SSC, °Brix). The flesh was separated from the endocarp after fruit measurements were taken. Endocarps were washed and left to dry for 48 h in paper towels. Endocarp diameter (mm) (measurement along the equatorial plane of the endocarp at the widest point) and height (mm) (measurement from stem to the tip end of the endocarp) were measured (Figure 3).

Figure 2.
Fruits of Prunus serotina subsp. capuli, genotype PserTU48, collected in the Andes region of Ecuador in 2016. A US one cent coin was used as a size reference (diameter 19.05 mm).

Figure 3.
Endocarps of Prunus serotina subsp. capuli genotype PserTU77, collected in the Andes region of Ecuador in 2016. A US one cent coin was used as a size reference (diameter 19.05 mm).
All measurements of size were completed using a digital caliper (Traceable® Carbon Fiber Calipers, Fisher Sci., Hampton, HN, USA). Weights were taken using a generic-brand digital balance. SSC was measured using a hand-held refractometer, from juice obtained from the flesh (Atago CO., LTD., Tokyo, Japan).
Three replicates of ten fruits per genotype were weighed (g). Five replicates of individual fruit and endocarp representatives of each genotype were measured for diameter (mm) and height (mm). SSC (°Brix) was measured for five individual fruit representatives of each genotype.
2.3. Statistical Analyses
Analyses of variance were performed on all fruit and endocarp parameters, with genotype, year, and genotype x year as main effects in SAS 9.4 (SAS Institute Inc.; Cary, NC, USA), using PROC GLIMMIX. Means were separated using LSD test, with a significance level of p ≤ 0.05. Multivariate analyses were performed for all parameters within the 2016 and 2019 seasons. PCA (Principal Component Analysis) was performed for both the years 2016 and 2019, with JMP® v.14 (SAS Institute Inc., Cary, NC, USA).
3. Results and Discussion
Prunus serotina subsp. capuli is known in Ecuador as “capuli”. It plays an important part in the life of the Ecuadorian people and other Andean countries. There are no known commercial types available in Ecuador, with most of the fruit production obtained from individuals in the wild.
3.1. Fruit Measurements
The fruit and endocarps’ characteristics were evaluated for 24 capuli genotypes, collected in Ecuador in the 2016 and 2019 seasons. There were statistically significant differences (p ≤ 0.05) when comparing data across the seasons for fruit diameter, fruit height, ten fruit weight, the SSC, endocarp diameter, and endocarp height. The differences among the genotypes were identified for all the variables evaluated (p ≤ 0.05). No statistically significant differences were identified among the replicates for all variables (p > 0.05), except for endocarp height. Due to the variation present across the years, hereafter, all the analyses were performed in within-year comparisons.
The fruits’ diameters varied from 12.8 to 19.1 mm in 2016 and from 12.4 to 21.7 mm in 2019. The largest fruit diameter was observed for PserTU77 (19.1 mm) in 2016, and for PserTU53 (21.7 mm) in 2019. The smallest fruit diameter was reported for PserCH94 (12.8 mm) in 2016, and PserCO13 (12.4 mm) in 2019 (Table 2). For fruit height, the values varied from 11.4 to 17.7 mm (2016) and from 12.2 to 19.7 mm (2019). Fruit from PserTU43 had the highest height value for 2016 (17.7 mm), and PserTU41 and PserTU53 had the highest fruit height for 2019 (19.7 mm) (Table 2).
Limited studies have been published regarding capuli fruit’s morphology. McVaugh [7] characterized the fruit size of capuli. He reported a diameter approximately 20–25 mm. Vasco et al. [21] reported that the fruit of Ecuadorian capuli was 10–30 mm in diameter. Similarly, Avendaño-Gómez et al. [22] reported the average length of the capuli fruit found in Tlaxcala, Mexico, was 1.7 ± 0.2 cm, with a diameter of 1.5 ± 0.2 cm. The results from the research presented (Table 2) were consistent for P. serotina subsp. capuli. In the case of its sister species, Bonner [19] reported the average diameter of North American black cherry fruit to be 7–10 mm. Capuli fruit was generally larger than the fruit data reported for P. serotina subsp. serotina [19,22]. A variation in fruit size and morphology has also been observed in other Prunus species. Miloševic and Miloševic [23] reported the dimensions for sour cherry cultivars “Oblačinska” and “Cigančica” as follows: fruit length (between 14.85 ± 0.12 mm and 14.27 ± 0.19 mm, respectively) and fruit width (between 15.50 ± 0.09 mm and 15.23 ± 0.15 mm, respectively).
As previously described, fruit size is an important characteristic, which is used to evaluate a produce value for marketing and commercialization [16]. For example, this is an important standard used for the commercialization of sweet cherries. Different countries have different size standards. Spain has standards in which cherries with a 25 mm diameter are considered in the “extra category”, whereas, in Summerland (Canada) the ideal cherry diameter is 29–30 mm [24]. In the US, cherries with a diameter of 30 mm were preferred by consumers [25]. In addition, Kappel et al. [26] described an ideal sweet cherry for the North American market as 11–12 g in weight and 29–30 mm in diameter.
The ten fruit weight for P. serotina subsp. capuli varied from 11.7 to 43.7 g (2016) and from 12.0 to 50.3 g (2019). The highest value for the ten fruit weight in 2016 was found for PserTU48 (43.7g) and for PserTU53 (50.3g) in 2019. The smallest ten fruit weight was found consistently for PserCO13—15.4 g in 2016 and 12 g in 2019 (Table 2). The individual fruit weight was recorded for the largest and smallest fruit in each fruit lot (data not shown). The highest individual fruit weight was observed for PserTU48 and PserTU53: 5.82 g and 3.92 g (2016), and 4 g and 5 g (2019), respectively. These individual weights are consistent with the ten fruit weight average. Vasco et al. [21] studied the weight of capuli fruits from Ecuador and reported an individual fruit weight of between 2–8 g. Khadivi et al. [18] reported the average fruit weight of 45 sweet cherry cultivars (4.4 to 8.9 g), 62 sour cherry cultivars (1.4 to 2.7 g), and 39 duke cherry (4.4 to 6.0 g) cultivars from Iran. Similarly, the fruit weight of the sour cherry cultivars, “Oblačinska” and “Cigančica”, were reported as between 3.48 ± 0.11 g and 2.66 ± 0.09 g, respectively [23]. The capuli fruit weight reported in this study was below the average size of the commercial sweet cherry cultivars described above, but it was higher and comparable to the fruit data for the sour cherry and duke cherry cultivars, respectively.

Table 2.
Fruit characteristics of Prunus serotina subsp. capuli genotypes, collected in the Andes region of Ecuador in 2016 and 2019 seasons.
Table 2.
Fruit characteristics of Prunus serotina subsp. capuli genotypes, collected in the Andes region of Ecuador in 2016 and 2019 seasons.
| Genotype ID | Fruit Diameter (mm) | Fruit Height (mm) | Ten Fruit Weight (g) | SSC (°Brix) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2019 | 2016 | 2019 | 2016 | 2019 | 2016 | 2019 | |||||||||
| PserCH101 z | 13.0 | ij y | 14.4 | d–f | 12.3 | kj | 12.2 | h | 12.8 | kl | 17.7 | gh | 20.0 | b–f | 27.1 | a |
| PserCH108 | 16.0 | c–h | 15.5 | de | 14.6 | c–i | 14.4 | d–g | 28.5 | d–f | 21.3 | e–g | 25.3 | ab | 21.5 | b–f |
| PserCH110 | 15.1 | e–j | 14.5 | d–f | 13.7 | g–j | 12.5 | gh | 23.0 | g–i | 21.0 | e–g | 19.2 | d–g | 25.5 | ab |
| PserCH113 | 15.7 | c–i | 17.0 | cd | 14.5 | d–i | 13.5 | d–g | 24.1 | f–i | 29.0 | cd | 14.1 | g | 24.9 | a–c |
| PserCH132 | 16.1 | b–g | 14.4 | d–f | 14.1 | e–j | 12.7 | f–h | 21.8 | h–j | 21.3 | e–g | 16.2 | e–g | 21.6 | a–f |
| PserCH142 | 15.4 | d–j | 14.6 | d–f | 13.3 | h–k | 13.4 | d–h | 19.9 | h–j | 22.0 | e–g | 27.6 | a | 23.4 | a–d |
| PserCH94 | 12.8 | j | 16.4 | c–e | 11.4 | k | 14.7 | c–f | 11.7 | l | 22.3 | d–g | 18.5 | d–g | 21.8 | a–e |
| PserCO01 | 15.8 | c–h | 16.0 | c–e | 14.6 | c–i | 13.6 | d–h | 25.4 | e–i | 24.0 | d–g | 19.4 | d–g | 16.2 | f–h |
| PserCO13 | 14.0 | f–j | 12.4 | f | 13.8 | f–j | 12.4 | gh | 15.4 | j–l | 12.0 | h | 20.6 | b–f | 21.5 | b–f |
| PserCO14 | 15.9 | c–g | 16.2 | c–e | 15.5 | b–f | 13.7 | d–h | 24.3 | e–i | 19.0 | fg | 25.1 | a–c | 17.2 | e–h |
| PserCO16 | 13.3 | h–j | 19.8 | ab | 12.5 | i–k | 16.7 | bc | 19.2 | i–k | 49.3 | a | 23.5 | a–d | 14.3 | gh |
| PserCO21 | 18.3 | a–c | 15.1 | d–f | 15.9 | a–f | 13.4 | d–h | 24.7 | e–i | 27.0 | de | 17.2 | e–g | 20.6 | b–f |
| PserCO22 | 16.6 | a–g | 16.1 | c–e | 15.1 | c–g | 13.0 | e–h | 29.8 | c–f | 27.0 | de | 18.0 | d–g | 21.4 | b–f |
| PserCO26 | 14.9 | e–j | 15.0 | d–f | 14.7 | c–f | 14.1 | d–h | 26.1 | e–h | 29.0 | cd | 18.4 | d–g | 18.2 | d–h |
| PserCO31 | 16.8 | a–f | 18.7 | bc | 16.6 | a–d | 15.2 | b–d | 34.6 | cd | 37.3 | b | 17.7 | e–g | 19.5 | c–g |
| PserTU41 | 17.7 | a–e | 13.7 | ef | 16.7 | a–c | 19.7 | a | 30.3 | c–f | 21.0 | e–g | 19.4 | d–g | 20.8 | b–f |
| PserTU43 | 17.9 | a–d | 15.2 | de | 17.7 | a | 13.3 | d–h | 36.4 | bc | 20.3 | e–g | 17.3 | e–g | 20.0 | b–f |
| PserTU48 | 18.8 | ab | 16.4 | cd | 17.3 | ab | 17.0 | b | 43.7 | a | 33.8 | bc | 18.5 | d–g | 17.9 | e–h |
| PserTU53 | 16.3 | b–g | 21.7 | a | 16.2 | a–e | 19.7 | a | 30.9 | c–e | 50.3 | a | 15.2 | fg | 19.1 | d–g |
| PserTU57 | 14.5 | f–j | 16.3 | c–e | 13.5 | g–k | 14.9 | b–e | 20.4 | h–j | 26.3 | de | 21.0 | b–e | 21.6 | a–f |
| PserTU67 | 17.9 | a–d | 16.1 | c–e | 15.5 | b–g | 14.0 | d–h | 33.5 | cd | 25.3 | d–f | 23.4 | a–d | 18.2 | d–h |
| PserTU70 | 15.7 | d–i | 16.4 | cd | 14.6 | d–i | 14.0 | d–h | 23.0 | g–i | 24.3 | d–g | 18.5 | d–g | 14.2 | gh |
| PserTU71 | 14.0 | g–j | 15.9 | de | 14.0 | f–j | 13.5 | d–h | 19.8 | h–j | 20.7 | e–g | 20.1 | b–f | 19.2 | d–g |
| PserTU77 | 19.1 | a | 14.8 | d–f | 17.5 | ab | 13.0 | f–h | 41.4 | ab | 18.7 | f–h | 19.6 | d–g | 13.4 | h |
z Genotype: first letter represents the genus (P = Prunus), next three letters represent the species (ser = serotina), and the following letters represent the province of origin in Ecuador and collection number (CH101—Chimborazo collection 101). y Different letters within a column indicate significant difference between genotypes, using LSD test at p ≤ 0.05.
The Chimborazo genotypes had the highest average SSC values, with genotypes PserCH142 (27.6 °Brix) in 2016, and PserCH101 (27.1 °Brix) in 2019 (Table 2). The lowest SSC values were 14.1 °Brix (2016) and 13.4 °Brix (2019) for PserCH113 and PserTU77, respectively (Table 2). Vasco et al. [21] studied the average SSC of capuli fruits from Ecuador and reported values between 16.3–22.2 °Brix. Our results are consistent with the research of Vasco et al. [21]. Kappel et al. [26] reported a minimum SSC value of 15 °Brix for sweet cherry cultivars. Khadivi et al. [18] reported the range of TSS (total soluble solids) values of sweet cherries as from 15.6% to 20.88%, sour cherries from 15 to 28%, and duke cherries from 17.13% to 22.53% in Iran. Crisosto et al. [27] studied the importance of TSS, TSS: TA (titratable acidity), and skin color with regard to consumer acceptance for “Bing” and “Brooks” cherry cultivars. The authors pointed out that TSS plays an important role in consumer acceptance. Consumer acceptability is increased with high TSS levels and a minimum 16% was proposed for cherries in the American market. Our study reported average SSC values higher than those reported for sweet cherry, sour cherry, and duke cherry cultivars.
3.2. Endocarp Measurements
In the case of the endocarps’ morphological characteristics, the largest endocarp diameter was found in the genotypes PserTU41 and PserTU77 (10.4 mm) in 2016, and PserTU53 (12.2 mm) in 2019 (Table 3) (Figure 2 and Figure 3). The highest endocarp height was reported for PserTU43 (13.6 mm) in 2016, and for PserTU53 (12.2 mm) in 2019 (Table 3). Avendaño-Gómez et al. [22] studied the endocarps of capuli fruits from Tlaxcala, Mexico, under a cultivated management system. The authors observed an endocarp thickness of 0.14 ± 0.04 mm, a seed length of 0.94 ± 0.08 cm, and a seed diameter of 0.78 ± 0.13 cm.

Table 3.
Endocarp characteristics of Prunus serotina subsp. capuli genotypes, collected in the Andes region of Ecuador in 2016 and 2019 seasons.
In other Prunus species, Khadivi et al. [18] studied Iranian sweet, sour, and duke cherries. The stone lengths ranged from 10.57 to 12.40 mm (sweet cherries), 7.73 to 10.18 mm (sour cherries), and 9.26 to 11.78 mm (duke cherries); whereas the stone widths ranged from 8.50 to 10.35 mm (sweet cherries), 8.94 to 10.51 mm (duke cherries), and 7.17 to 10.30 mm (sour cherries). The endocarp data collected in our research are comparable to the endocarp data collected for capuli from Mexico and other cherries.
The overall means for all the variables within a year were calculated separately, according to their province of origin (Table 4). Significant differences were present across the different provinces, while comparing the overall means for the variable fruit height and endocarp diameter for both years. The SSC values were the highest for Chimborazo province genotypes for the year 2019 but no significant differences among the provinces were observed for the year 2016. The ten fruit weight was significantly higher for the Tungurahua genotypes for the year 2016. No significant differences were present among the provinces for the ten fruit weight for the year 2019. The fruit diameter and endocarp height were significantly higher for Tungurahua, in comparison to the other provinces in 2016, but no significant differences were present across provinces for the year 2019.

Table 4.
Fruit and endocarp characteristics of P. serotina subsp. capuli, according to its provinces of origin in Ecuador.
In the current study, fruit collected from the wild in Ecuador comprised a large variation in fruit diameter, fruit height, fruit weight, SSC, endocarp diameter, and endocarp height (Table 2, Table 3 and Table 4). This phenotypic variation present in the wild Ecuadorian capuli fruit can be used as a foundation for the future capuli breeding programs. Prunus serotina, in North America, is mostly used for furniture purposes, but its fruit lacks flavor and size, which are important commercial fruit attributes. The best forms of capuli fruits are found in Ecuador [8]. Although capuli fruit is important to the Ecuadorian people, no commercial varieties of capuli fruits are available in the Ecuadorian market. In Mexico, the seeds of the capuli fruit are also used as snacks, in addition to eating fresh or dried fruit, or making other products [28]. Capuli fruit holds the potential for being a multipurpose commercial product, where both the fruit and seeds can be utilized for different purposes.
3.3. Multivariate Analyses for 2016 and 2019
The edible portions in cherries are the epicarp and mesocarp, i.e., the skin and flesh of the fruit. Beneath these, there is the stony endocarp, which is inedible [29]. In the case of sweet cherries, consumers prefer cherries with a small endocarp and a large quantity of fruit pulp [24]. This study focused on characterizing the fruit and endocarp variables and their relationships.
The results of the multivariate analyses are shown in Table 5 and Table 6, for the years 2016 and 2019, respectively. A strong positive correlation was found between the fruit weight and diameter within both years (rfruitwt vs fruitdia 2016,2019 = 0.89, p ≤ 0.0001). Likewise, the fruit diameter and endocarp diameter were positively correlated for both years (rfruitdia vs endocarpdia 2016 = 0.78, rfruitdia vs endocarpdia 2019 = 0.79; p ≤ 0.0001). The endocarp height and fruit height were positively correlated (rfruitht vs endocarpht 2016 = 0.78, rfruitht vs endocarpht 2019 = 0.61; p ≤ 0.05). All fruit and endocarp size characteristics were positively correlated with each other within a season. SSC was not correlated with any of the size variables for the fruits or endocarps in both years (Table 5 and Table 6). Large-fruited capuli genotypes were consistently characterized by a large endocarp. This will make the selection for a large-fruited and small endocarp genotype difficult. On the other hand, the selection for a high SSC can be made independently of other variables.

Table 5.
Multivariate analysis of fruit and endocarp characteristics of Prunus serotina subsp. capuli genotypes, collected in the Andes region of Ecuador in 2016.

Table 6.
Multivariate analysis of fruit and endocarp characteristics of Prunus serotina subsp. capuli genotypes, collected in the Andes region of Ecuador in 2019.
Other studies have reported the presence of a positive correlation between the fruit and endocarp size variables. Rakonjac et al. [30] reported that all the fruit size variables in “Oblačinska” sour cherry accessions were positively related to each other. Demirsoy and Demirsoy [31] also found a positive polynomial relationship between fruit weight and fruit diameter in sweet cherries. Khadivi-Khub [32] found a positive correlation between fruit weight vs. stone weight, fruit weight vs. fruit length, and fruit width vs. fruit diameter when evaluating 70 cherry genotypes. In addition, significant negative correlations between the TSS vs. fruit were reported in cherry genotypes by Khadivi et al. [18].
3.4. Principal Component Analysis (PCA)
A principal component analysis aims to reduce the number of parameters to differentiate the relationships between variables and genotypes. This technique helps in dividing the original variables in the dataset into smaller groups. The groups in a PCA are not related to each other, except the variables within each group [33]. The results from the PCA conducted in this study identified that PC1 and PC2 components accounted for 83.7% of the total variation in the studied variables for P. serotina subsp. capuli (Table 7).

Table 7.
Eigenvectors and proportion of the total variability for each principal component axes for fruit and endocarp characteristics of Prunus serotina subsp. capuli genotypes, collected in the Andes region of Ecuador in 2016 and 2019.
In PC1, the variables with the highest factor loadings were the ten fruit weight, the fruit diameter, the fruit height, and the endocarp diameter and height. In the SSC, °Brix had the highest factor loadings. These results are confirmed in Figure 4, where the SSC variable is observed on a different quadrant from all the other variables.
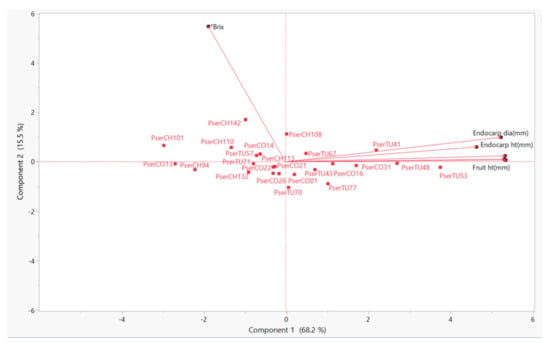
Figure 4.
Principal component analysis (PCA) based on the fruit and endocarp characteristics of Prunus serotina subsp. capuli genotypes, collected in the Andes region of Ecuador in 2016 and 2019.
The genotypes with the highest PC1 scores were those with overall high values for fruit and endocarp variables, such as PserTU48 and PserTU53. Whereas, the genotypes with high PC2 scores are the ones with high SSC values, such as the genotypes PserCH142, PserCH110, and PserCH108. No clear-cut groups were observed according to their province of origin in the PCA, based on the morphological characteristics studied (Figure 4).
The results suggest that there is morphological variation in the genotypes from Chimborazo, Cotopaxi, and Tungurahua. However, these differences did not result in separate groupings, as related to their province of origin. Guadalupe et al. [34], while studying the genetic diversity and population structure of capuli from eight Ecuadorian provinces, reported the lack of clear population differentiation among the capuli from the different provinces. They proposed the reason for the diversification of Ecuadorian capuli could be outcrossing and self-incompatibility.
4. Conclusions
The results of this study can be used as a reference for P. serotina subsp. capuli. In addition, the information presented is important for future capuli breeding and management programs in Ecuador and the US. The present study shows the feasibility and the great potential in selecting and further breeding capuli fruit with superior commercial characteristics. The breeding and selection of a large-fruited capuli with a high °Brix should be attainable, based on our study. Superior genotypes for fruit size and weight, such as PserTU48 and PserTu53, could be used for breeding and selection to obtain a large- fruited capuli. On the other hand, the genotypes PserCH142, PserCH110, and PserCH108 could be used to obtain a high SSC-fruited capuli. The material studied in this research was collected in the wild, without any advanced breeding, selection, or commercial management. Capuli fruit holds plenty of commercial potential in Ecuador and could be an interesting species to be evaluated in the US. Capuli fruit has a promising future, similar to other stone fruits. Future research will include adaptation to the south-eastern US. Open-pollinated seeds from a wide collection of individual fruits will be germinated and grown for further evaluation and selection.
Author Contributions
Conceptualization, D.J.C.; formal analysis, S.P. and D.J.C.; investigation, S.P., D.J.C., R.A.I., L.F.L., C.R.C., V.C.-S. and J.C.C.; resources, D.J.C. and R.A.I.; writing—original draft preparation, S.P. and D.J.C.; writing—review and editing, S.P., R.A.I. and D.J.C.; supervision, D.J.C.; funding acquisition, D.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
The research in US was funded by the Georgia Research Foundation, Hatch Project GEO00766, Department of Horticulture, and the University of Georgia. The research in Ecuador was funded by ESPOCH in accordance to our collaborative research agreement between institutions.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank our collaborators at ESPOCH, as well as the technical help of the Department of Horticulture.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hough, A.F. Silvical characteristics of black cherry (Prunus serotina). In Station Paper NE-139; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1960; Volume 139, 26p. [Google Scholar]
- Cassens, D.L. Hardwood Lumber and Veneer Series, Black Cherry; Purdue University, Purdue Extension: West Lafayette, IN, USA, 2007. [Google Scholar]
- Camenen, E.; Porte, A.J.; Garzon, M.B. American trees shift their niches when invading Western Europe: Evaluating invasion risks in a changing climate. Ecol. Evol. 2016, 6, 7263–7275. [Google Scholar] [CrossRef] [PubMed]
- Segura, S.; Guzmán-Díaz, F.; López-Upton, J.; Mathuriau, C.; López-Medina, J. Distribution of Prunus serotina Ehrh. in North America and its invasion in Europe. J. Geosci. Environ. Prot. 2018, 6, 111–124. [Google Scholar] [CrossRef]
- Starfinger, U.; Kowarik, I.; Rode, M.; Schepker, H. From desirable ornamental plant to pest to accepted addition to the flora?—The perception of an alien tree species through the centuries. Biol. Invasions 2003, 5, 323–335. [Google Scholar] [CrossRef]
- Guzmán, F.; Torres, M.; Herrera, M.D.C.; Nieto, R.; Almaguer, G.; López, J.; Segura, S. Incompatibility of the capulín (Prunus serotina ssp. capuli (Cav.) McVaugh) as rootstock of the sweet cherry tree (Prunus avium L.). Rev. Mexicana Cienc. Agric. 2018, 9, 1035–1044. [Google Scholar]
- McVaugh, R. A revision of the North American black cherries (Prunus serotina ehrh., and relatives). Brittonia 1951, 7, 279–315. [Google Scholar] [CrossRef]
- Popenoe, W.; Pachano, A. The Capulin Cherry. Bull. Pan Am. Union 1923, 56, 152–168. [Google Scholar]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Phenolic Compounds in Rosaceae Fruits from Ecuador. J. Agric. Food Chem. 2009, 57, 1204–1212. [Google Scholar] [CrossRef]
- Martínez, M. Plantas útiles de la Flora Mexicana; Ediciones Botas: Ciudad de México, México, 1959; p. 621. [Google Scholar]
- Luna-Vazquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, J.I.; Yahia, E.M.; Rivera-Pastrana, D.M.; Rojas-Molina, A.; Zavala-Sanchez, M.A. Nutraceutical value of black cherry Prunus serotina Ehrh. fruits: Antioxidant and antihypertensive properties. Molecules 2013, 18, 14597–14612. [Google Scholar] [CrossRef]
- Guerra-Ramìrez, D.; Rodríguez, G.H.; Espinosa-Solares, T.; Perez-Lopez, A.; Salgado-Escobar, I. Antioxidant capacity of capulin (Prunus serotina subsp. capuli (Cav). McVaugh) fruit at different stages of ripening. Ecosist. Recur. Agropec. 2019, 6, 35–44. [Google Scholar] [CrossRef]
- Ordaz-Galindo, A.; Wesche-Ebeling, P.; Wrolstad, R.E.; Rodriguez-Saona, L.; Argaiz-Jamet, A. Purification and identication of capulin (Prunus serotina Ehrh) anthocyanins. Food Chem. 1999, 65, 201–206. [Google Scholar] [CrossRef]
- Garcia-Aguilar, L.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Rojas-Molina, J.I.; Vazquez-Landaverde, P.A.; Luna-Vazquez, F.J.; Zavala-Sanchez, M.A. Nutritional value and volatile compounds of black cherry (Prunus serotina) seeds. Molecules 2015, 20, 3479–3495. [Google Scholar] [CrossRef]
- Aguerrebere, I.A.; Molina, A.R.; Oomah, B.D.; Drover, J.C.G. Characteristics of Prunus serotina seed oil. Food Chem. 2011, 124, 983–990. [Google Scholar] [CrossRef]
- Christensen, J.V. Numerical studies of qualitative and morphological characteristics of 41 sweet cherry cultivars II. Tidsskr. Planteavl 1974, 78, 303–312. [Google Scholar]
- Whiting, M.D.; Lang, G.; Ophardt, D. Rootstock and training system affect sweet cherry growth, yield, and fruit quality. HortScience 2005, 40, 582–586. [Google Scholar] [CrossRef]
- Khadivi, A.; Mohammadi, M.; Asgari, K. Morphological and pomological characterizations of sweet cherry (Prunus avium L.), sour cherry (Prunus cerasus L.) and duke cherry (Prunus × gondouinii Rehd.) to choose the promising selections. Sci. Hortic. 2019, 257, 108719. [Google Scholar] [CrossRef]
- Bonner, F.T. Maturation of Black Cherry Fruits in Central Mississippi; USDA, Forest Service, Southern Forest Experiment Station: New Orleans, LA, USA, 1975; Volume 205. [Google Scholar]
- Marquis, D.A. Prunus serotina Ehrh. Black Cherry. Silv. N. Am. 1990, 2, 594–604. [Google Scholar]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Avendaño-Gómez, A.; Lira-Saade, R.; Madrigal-Calle, B.; García-Moya, E.; Soto-Hernández, M.; Romo de Vivar-Romo, A. Management and domestication syndromes of capulin (Prunus serotina Ehrh ssp. capuli (Cav.) McVaugh) in communities of the state of Tlaxcala. Agrociencia 2015, 49, 189–204. [Google Scholar]
- Miloševic, T.; Miloševic, N. Fruit quality attributes of sour cherry cultivars. ISRN Agron. 2012, 2012, 593981. [Google Scholar] [CrossRef][Green Version]
- Pérez-Sánchez, R.; Gómez-Sánchez, M.Á.; Morales-Corts, M.R. Description and quality evaluation of sweet cherries cultured in Spain. J. Food Qual. 2010, 33, 490–506. [Google Scholar] [CrossRef]
- Turner, J.; Seavert, C.; Colonna, A.; Long, L.E. Consumer sensory evaluation of sweet cherry cultivars in Oregon, USA. Acta Hortic. 2008, 795, 781–786. [Google Scholar] [CrossRef]
- Kappel, F.; Fisher Fleming, B.; Hogue, E. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience 1996, 31, 443–446. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Raya-Pérez, J.C.; Aguirre-Mancilla, C.L.; Ramírez-Pimentel, J.G.; Tapia-Aparicio, R.; Covarrubias-Prieto, J. Characterization of the reserve proteins and mineral composition of the capulin seed (Prunus serotina). Polybotany 2012, 34, 223–235. [Google Scholar]
- Tukey, H.B.; Young, J.O. Histological study of the developing fruit of the sour cherry. Bot. Gaz. 1939, 100, 723–749. [Google Scholar] [CrossRef]
- Rakonjac, V.; Akšić, M.F.; Nikolić, D.; Milatović, D.; Čolić, S. Morphological characterization of ‘Oblačinska’ sour cherry by multivariate analysis. Sci. Hortic. 2010, 125, 679–684. [Google Scholar] [CrossRef]
- Demirsoy, H.; Demirsoy, L. A study on the relationships between some fruit characteristics in cherries. Fruits 2004, 59, 219–223. [Google Scholar] [CrossRef]
- Khadivi-Khub, A. Assessment of cultivated cherry germplasm in Iran by multivariate analysis. Trees 2014, 28, 669–685. [Google Scholar] [CrossRef]
- Iezzoni, A.F.; Pritts, M.P. Applications of Principal Component Analysis to Horticultural Research. HortScience 1991, 26, 334–338. [Google Scholar] [CrossRef]
- Guadalupe, J.J.; Gutiérrez, B.; Intriago-Baldeón, D.P.; Arahana, V.; Tobar, J.; Torres, A.F.; Torres, M.L. Genetic diversity and distribution patterns of Ecuadorian capuli (Prunus serotina). Biochem. Syst. Ecol. 2015, 60, 67–73. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).