Effect of Prolonged Cold Storage on the Dynamics of the Enzymatic and Non-Enzymatic Antioxidant System in the Mesocarp of Avocado (Persea americana) cv. Hass: Relationship with Oxidative Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Storage and Shelf-Life Conditions
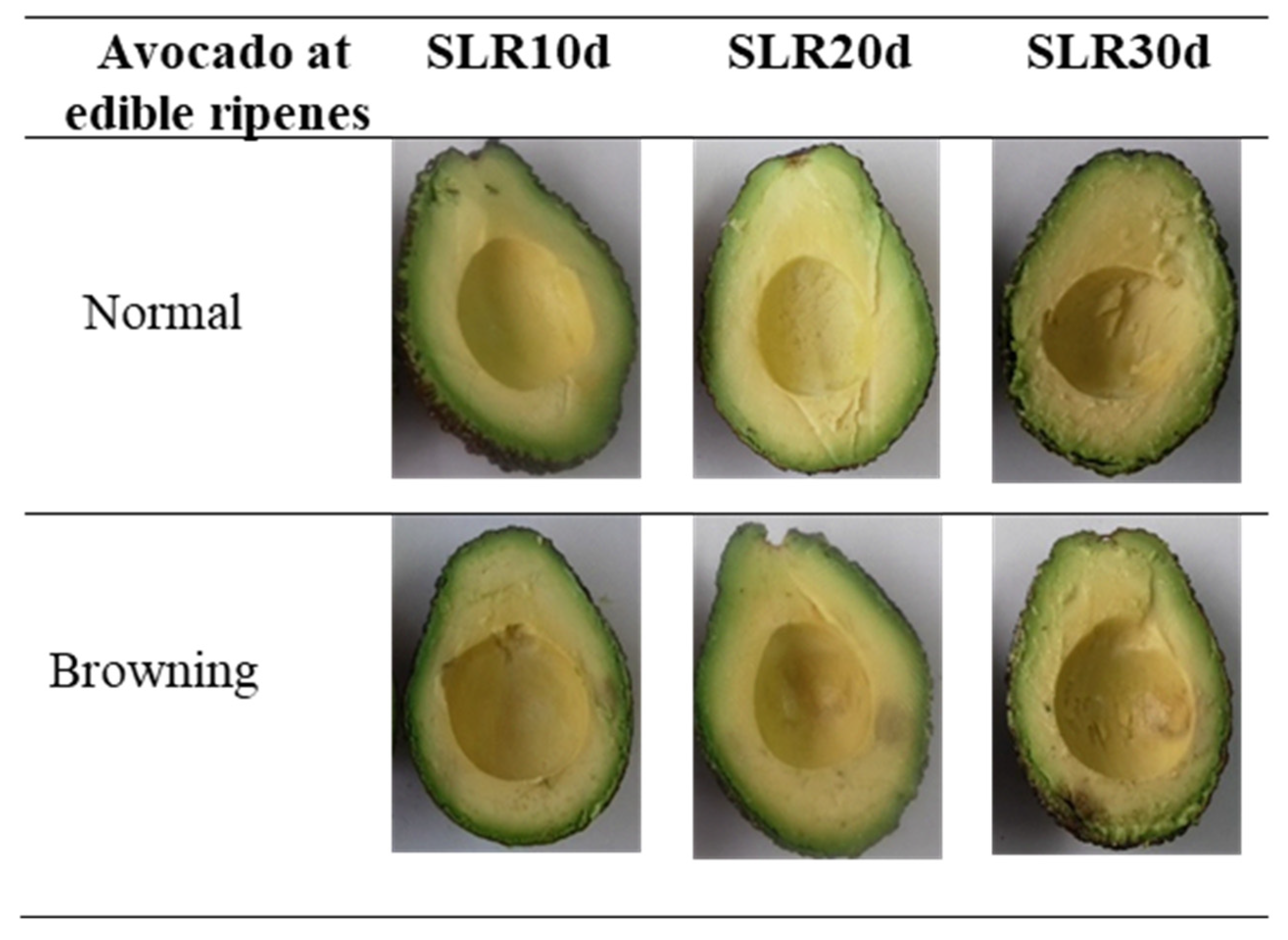
2.2. Pulp Browning Area
2.3. Soluble Protein Assay
2.4. Enzymatic Analysis
2.4.1. Catalase Activity (CAT)
2.4.2. Superoxide Dismutase Activity (SOD)
2.4.3. Peroxidase Activity (POD)
2.4.4. Polyphenol Oxidase Activity (PPO)
2.4.5. Lypoxigenase Activity (LOX)
2.5. Total Phenolic Compounds and Hydrophilic and Lipophilic Antioxidant Activities
2.6. Tocopherol Content
2.7. Total Carotenoids
2.8. TBARS Assay
2.9. Statistical Analysis
3. Results and Discussion
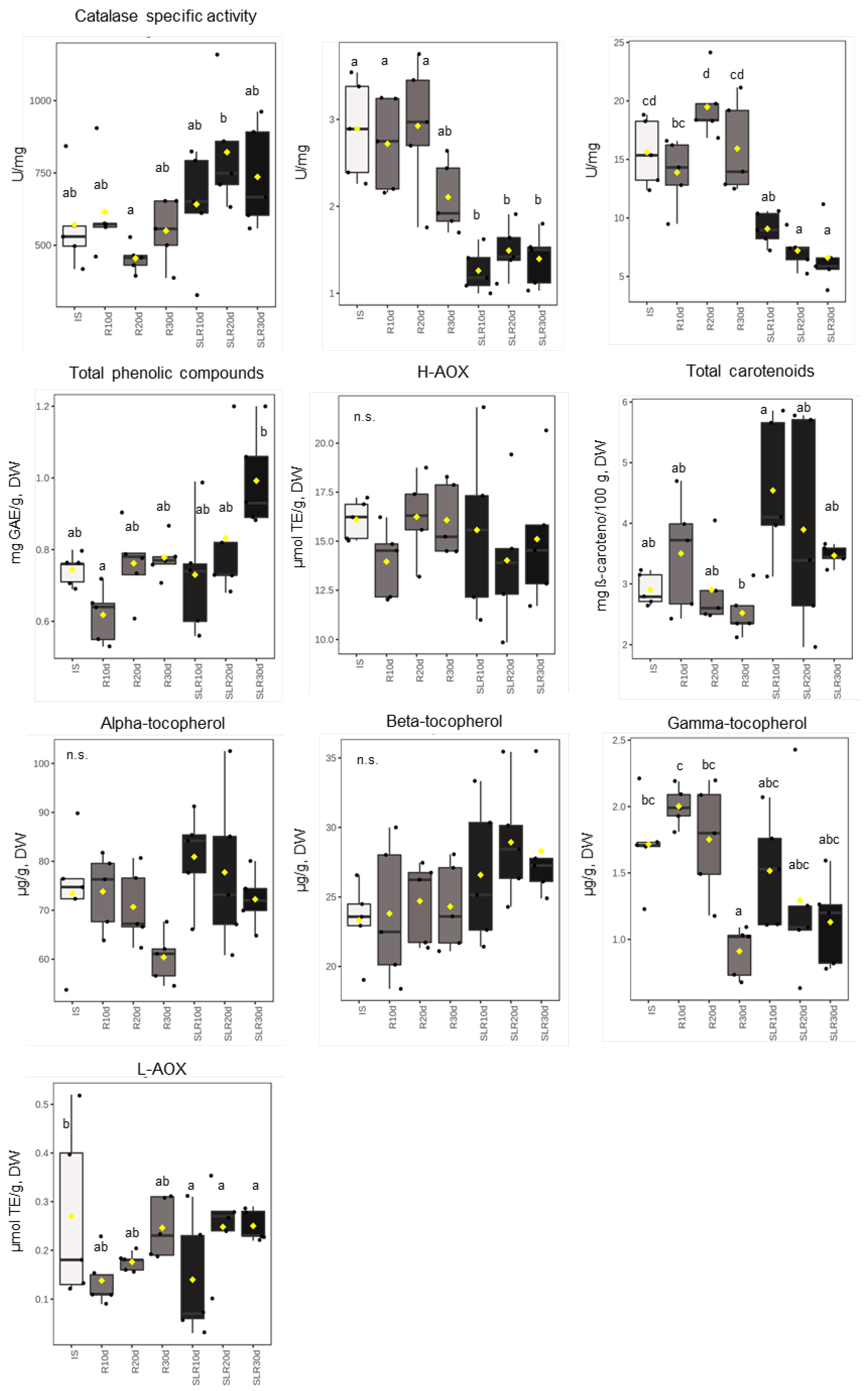
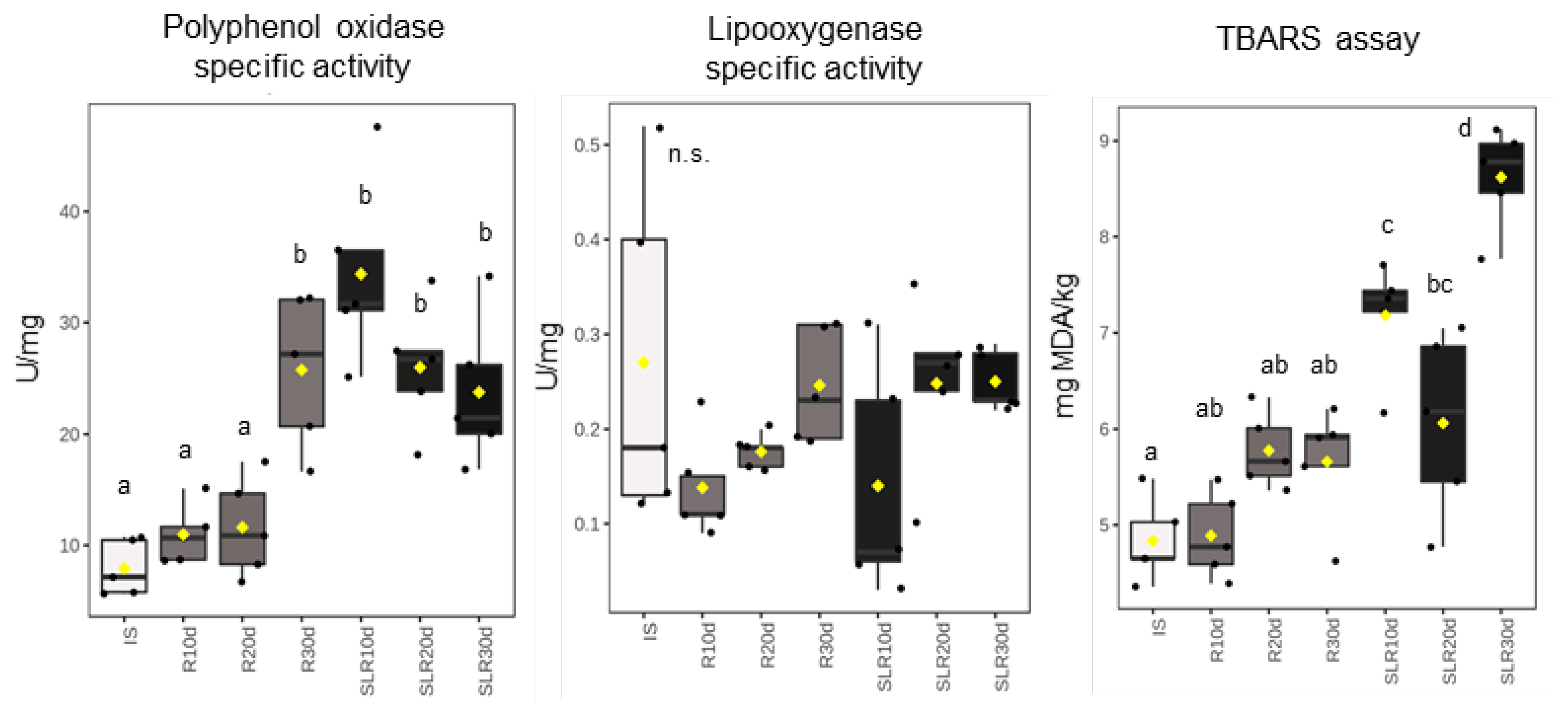
3.1. Enzymatic Antioxidant System
3.2. Non-Enzymatic Antioxidant System
3.3. Other Markers of Oxidative Stress
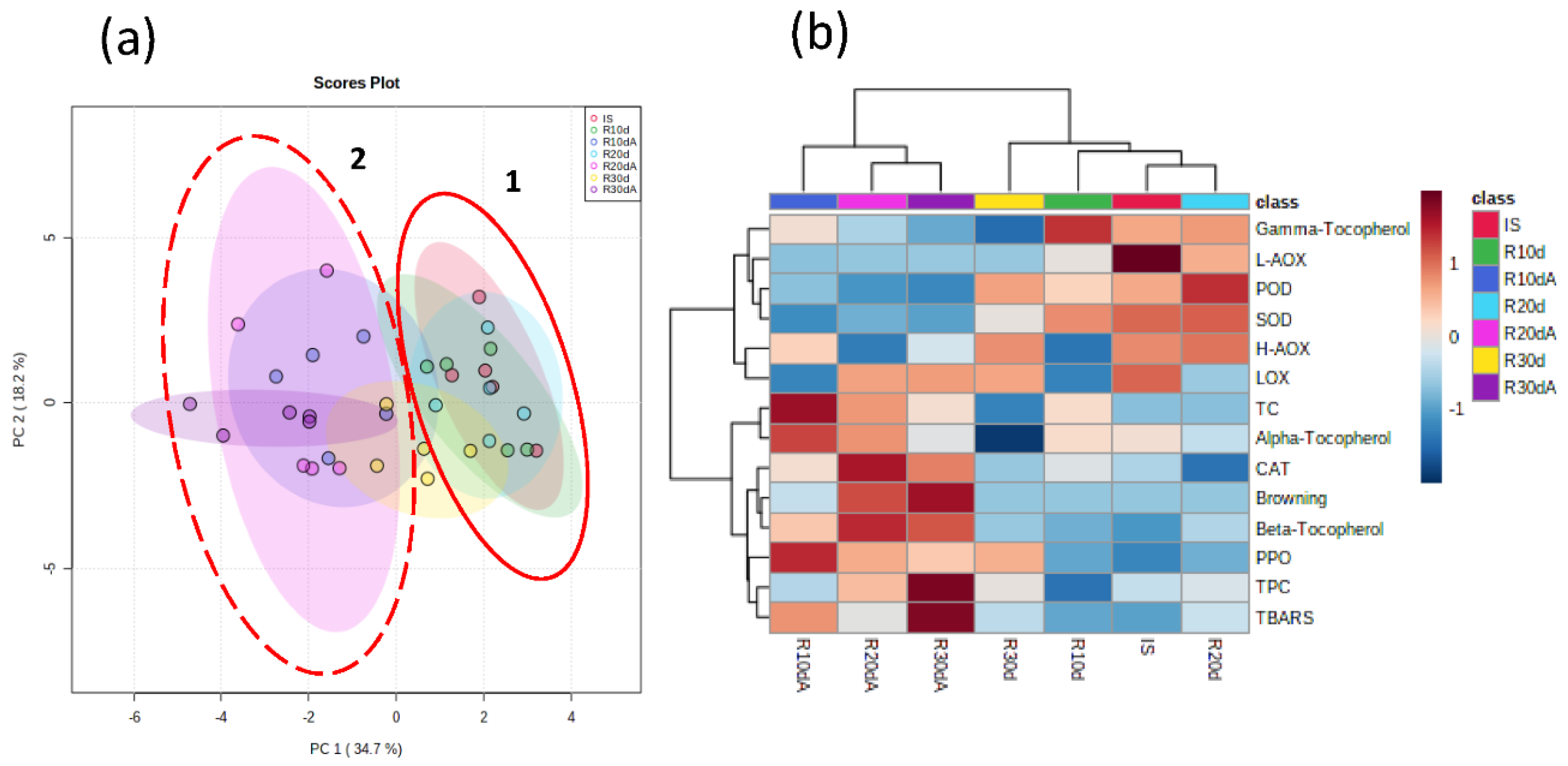
3.4. Multivariate and Correlation Analysis of Enzymatic and Non-Enzymatic Systems and Oxidative Stress Markers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado seed: A comparative study of antioxidant content and capacity in protecting oil models from oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Pedreschi, R.; Uarrota, V.; Fuentealba, C.; Martínez-Carrasco, J.; Olmedo, P.; Defilippi, B.G.; Campos-Vargas, R. Primary metabolism in avocado fruit. Front. Plant Sci. 2019, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodríguez, J.; Yahia, E.; González-León, A.; Ifie, I.; Robles-Zepeda, R.; Domínguez-Ávila, J.; González-Aguilar, G. Ripening of ‘Hass’ avocado mesocarp alters its phytochemical profile and the in vitro cytotoxic activity of its methanolic extracts. S. Afr. J. Bot. 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and Their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef]
- Vicente, A.; Saladié, M.; Rose, J.; Labavitch, J. The linkage between cell wall metabolism and fruit softening: Looking to the future. J. Sci. Food Agric. 2007, 87, 1435–1448. [Google Scholar] [CrossRef]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Valenzuela, J.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative stress associated with chilling injury in immature fruit: Postharvest technological and biotechnological solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef]
- Toivonen, P. Postharvest storage procedures and oxidative stress. HortScience 2004, 39, 938–942. [Google Scholar] [CrossRef]
- Anwar, H.; Ghulam, H.; Imtiaz, M. Antioxidants from natural sources. In Antioxidants in Foods and Its Applications; Shalaby, E., Azzam, G.M., Eds.; IntechOpen: London, UK, 2018; pp. 3–28. [Google Scholar]
- Yahia, E.; Woolf, A. Avocado (Persea americana Mill). In Postharvest Biology & Technology of Tropical and Sub-Tropical Fruits, Volume 2; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 125–185. [Google Scholar]
- Woolf, A.B.; Cox, K.A.; White, A.; Ferguson, I.B. Low temperature conditioning treatments reduce external chilling injury of “Hass” avocados. Postharvest Biol. Technol. 2003, 28, 113–122. [Google Scholar] [CrossRef]
- George, H.L.; Christoffersen, R.E. Differential latency toward (–) epicatechin and catechol mediated by avocado mesocarp polyphenol oxidase (PPO). Postharvest Biol. Technol. 2016, 112, 31–38. [Google Scholar] [CrossRef]
- Uarrota, V.; Hernandez, I.; Ponce, E.; Vidal, J.; Fuentealba, C.; Defilippi, B.; Lindh, V.; Zulueta, C.; Chirinos, R.; Campos, D.; et al. Unravelling factors associated with ‘blackspot’ disorder in stored Hass avocado (Persea americana Mill) fruit. J. Hortic. Sci. 2020, 95, 804–815. [Google Scholar] [CrossRef]
- Fuentealba, C.; Vidal, J.; Zulueta, C.; Ponce, E.; Uarrota, V.; Defilippi, B.G.; Pedreschi, R. Controlled Atmosphere Storage Alleviates Hass Avocado Black Spot Disorder. Horticulturae 2022, 8, 369. [Google Scholar] [CrossRef]
- Woolf, A.; Wibisono, R.; Farr, J.; Hallett, I.; Richter, L.; Oey, I.; Wohlers, M.; Zhou, J.; Fletcher, C.; Requejo-Jackman, C. Effect of high pressure processing on avocado slices. Innov. Food Sci. Emerg. Technol. 2013, 18, 65–73. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Biochemical changes during the storage of high hydrostatic pressure processed avocado paste. J. Food Sci. 2010, 75, S264–S270. [Google Scholar] [CrossRef]
- Huamán-Alvino, C.; Chirinos, R.; Gonzales-Pariona, F.; Pedreschi, R.; Campos, D. Physicochemical and bioactive compounds at edible ripeness of eleven varieties of avocado (Persea americana) cultivated in the Andean Region of Peru. Int. J. Food Sci. Technol. 2021, 56, 5040–5049. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant capacity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef]
- Bailly, C.; Kranner, I. Analyses of reactive oxygen species and antioxidants in relation to seed longevity and germination. Methods Mol. Biol. 2011, 773, 343–367. [Google Scholar] [CrossRef]
- Balois-Morales, R.; Colinas-León, M.T.; Peña-Valdivia, C.B.; Chávez-Franco, S.H.; Alia-Tejacal, I. Sistema enzimático antisenescencia, catalasa-superóxido dismutasa, de frutos de pitahaya (Hylocereus undatus) almacenados con frío. (text in Spanish). Rev Chapingo Ser. Hortic. 2008, 14, 295–299. [Google Scholar]
- Lafuente, M.; Sala, J.M.; Zacarias, L. Active Oxygen Detoxifying Enzymes and Phenylalanine Ammonia-lyase in the Ethylene-Induced Chilling Tolerance in Citrus Fruit. J. Agric. Food Chem. 2004, 52, 3606–3611. [Google Scholar] [CrossRef] [PubMed]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P. Anti-oxidant levels in various tissues during the maturation of ‘Hass’ avocado (Persea americana Mill.). J. Hortic. Sci. Biotechnol. 2010, 85, 106–112. [Google Scholar] [CrossRef]
- Imahori, Y.; Takemura, M.; Bai, J. Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperatura storage. Postharvest Biol. Technol. 2008, 49, 54–60. [Google Scholar] [CrossRef]
- Bukowska, B.; Michałowicz, J.; Pieniążek, D.; Sicińska, P.; Duda, W. Superoxide Dismutases and Their Inhibitors–the Role in Some Diseases. Curr. Enzyme Inhib. 2006, 2, 379–397. [Google Scholar] [CrossRef]
- Kolodyaznaya, V.S.; Evgenievna, V.T.; Kiprushkina, E.I.; Shestopalova, I.A.; Broyko, Y.V.; Filippov, V.I.; Klementiev, D.A.; Kostyuk, V.A. Dynamics of physiological and biochemical processes in avocado fruit treated with preparations during storage. Prog. Chem. Appl. Chitin Deriv. 2020, 25, 111–123. [Google Scholar] [CrossRef]
- Zhang, Z.; Donald, J.; Huber, D.J.; Rao, J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- Switala, J.; Loewen, P.C. Diversity of Properties Among Catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.; Gonzáles-Aguilar, G. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Khuong, T.; Lovatt, C. Effect of harvest date on the nutritional quality and antioxidant capacity in Hass avocado during storage. Food Chem. 2012, 135, 694–698. [Google Scholar] [CrossRef]
- Campos, D.; Terán-Hilares, F.; Chirinos, R.; Aguilar, A.; García, D.; Pacheco-Avalos, A.; Pedreschi, R. Bioactive compounds and antioxidant activity from harvest to edible ripeness of avocado cv. Hass (Persea americana) throughout the harvest seasons. Int. J. Food Sci. Technol. 2020, 55, 2208–2218. [Google Scholar] [CrossRef]
- Fox, A.; Del Pozo-Insfran, D.; Lee, J.; Sargent, S.; Talcott, S. Ripening-induced chemical and antioxidant changes in bell peppers as affected by harvest maturity and postharvest ethylene exposure. HortScience 2005, 40, 732–736. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Schmidt, Š.; Pokorný, J. Potential application of oilseeds as sources of antioxidants for food lipids—A review. Czech J. Food Sci. 2005, 23, 93–102. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C.; Cisneros-Zevallos, L.; Benavides, J. Partial purification and enzymatic characterization of avocado (Persea americana Mill, cv. Hass) lipoxygenase. Food Res. Int. 2010, 43, 1079–1085. [Google Scholar] [CrossRef]
- Queiroz, C.; Mendes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase: Characteristics and mechanisms of browning control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Whitaker, J.R. Lipoxygenases. In Oxidative enzymes in foods; Robinson, D.S., Eskin, A.M., Eds.; Elsevier Science: New York, NY, USA, 1991; pp. 175–215. [Google Scholar]
- Rosahl, S. Lipoxygenases in Plants—Their Role in Development and Stress Response. Z. Naturforsch. 1996, 51, 123–138. [Google Scholar] [CrossRef]
- Thompson, J.E.; Legge, R.L.; Barber, R.F. The role of free radicals in senescence and wounding. New Phytol. 1987, 105, 317–344. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Thompson, A.; Korsten, L. Avocado fruit quality management during the postharvest supply chain. Food Rev. Int. 2014, 30, 169–202. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Mpai, S.; Sivakumar, D. Extension of avocado fruit postharvest quality using non-chemical treatments. Agronomy 2020, 10, 212. [Google Scholar] [CrossRef]
- Thorp, T.; Hutching, D.; Lowe, T.; Marsh, K. Survey of fruit mineral concentrations and postharvest quality of New Zealand-grown ‘Hass’ avocado (Persea americana Mill.). N. Z. J. Crop Hortic. Sci. 2010, 25, 251–260. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirinos, R.; Ramon, K.; Mendoza, M.; Figueroa-Merma, A.; Pacheco-Ávalos, A.; Campos, D.; Pedreschi, R. Effect of Prolonged Cold Storage on the Dynamics of the Enzymatic and Non-Enzymatic Antioxidant System in the Mesocarp of Avocado (Persea americana) cv. Hass: Relationship with Oxidative Processes. Horticulturae 2022, 8, 880. https://doi.org/10.3390/horticulturae8100880
Chirinos R, Ramon K, Mendoza M, Figueroa-Merma A, Pacheco-Ávalos A, Campos D, Pedreschi R. Effect of Prolonged Cold Storage on the Dynamics of the Enzymatic and Non-Enzymatic Antioxidant System in the Mesocarp of Avocado (Persea americana) cv. Hass: Relationship with Oxidative Processes. Horticulturae. 2022; 8(10):880. https://doi.org/10.3390/horticulturae8100880
Chicago/Turabian StyleChirinos, Rosana, Karolina Ramon, Mirtha Mendoza, Andrés Figueroa-Merma, Alejandro Pacheco-Ávalos, David Campos, and Romina Pedreschi. 2022. "Effect of Prolonged Cold Storage on the Dynamics of the Enzymatic and Non-Enzymatic Antioxidant System in the Mesocarp of Avocado (Persea americana) cv. Hass: Relationship with Oxidative Processes" Horticulturae 8, no. 10: 880. https://doi.org/10.3390/horticulturae8100880
APA StyleChirinos, R., Ramon, K., Mendoza, M., Figueroa-Merma, A., Pacheco-Ávalos, A., Campos, D., & Pedreschi, R. (2022). Effect of Prolonged Cold Storage on the Dynamics of the Enzymatic and Non-Enzymatic Antioxidant System in the Mesocarp of Avocado (Persea americana) cv. Hass: Relationship with Oxidative Processes. Horticulturae, 8(10), 880. https://doi.org/10.3390/horticulturae8100880






