Crossbreeding Rootstocks Improve Nitrogen Efficiency of Grafted Watermelon by Inducing Leaf Physiological and Root Morphological Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments and Experimental Layout
2.2. Harvest, Measurements of Shoot Dry and Root Dry Biomass
2.3. Calculation of N Efficiency Components
2.4. Leaf Physiological and Biochemical Measurements
2.5. Measurements of Root Morphology
2.6. Statistical Analysis
3. Results and Discussion
3.1. Biomass Accumulation and Partitioning
3.2. N Uptake, N Use and Biological Production Efficiency
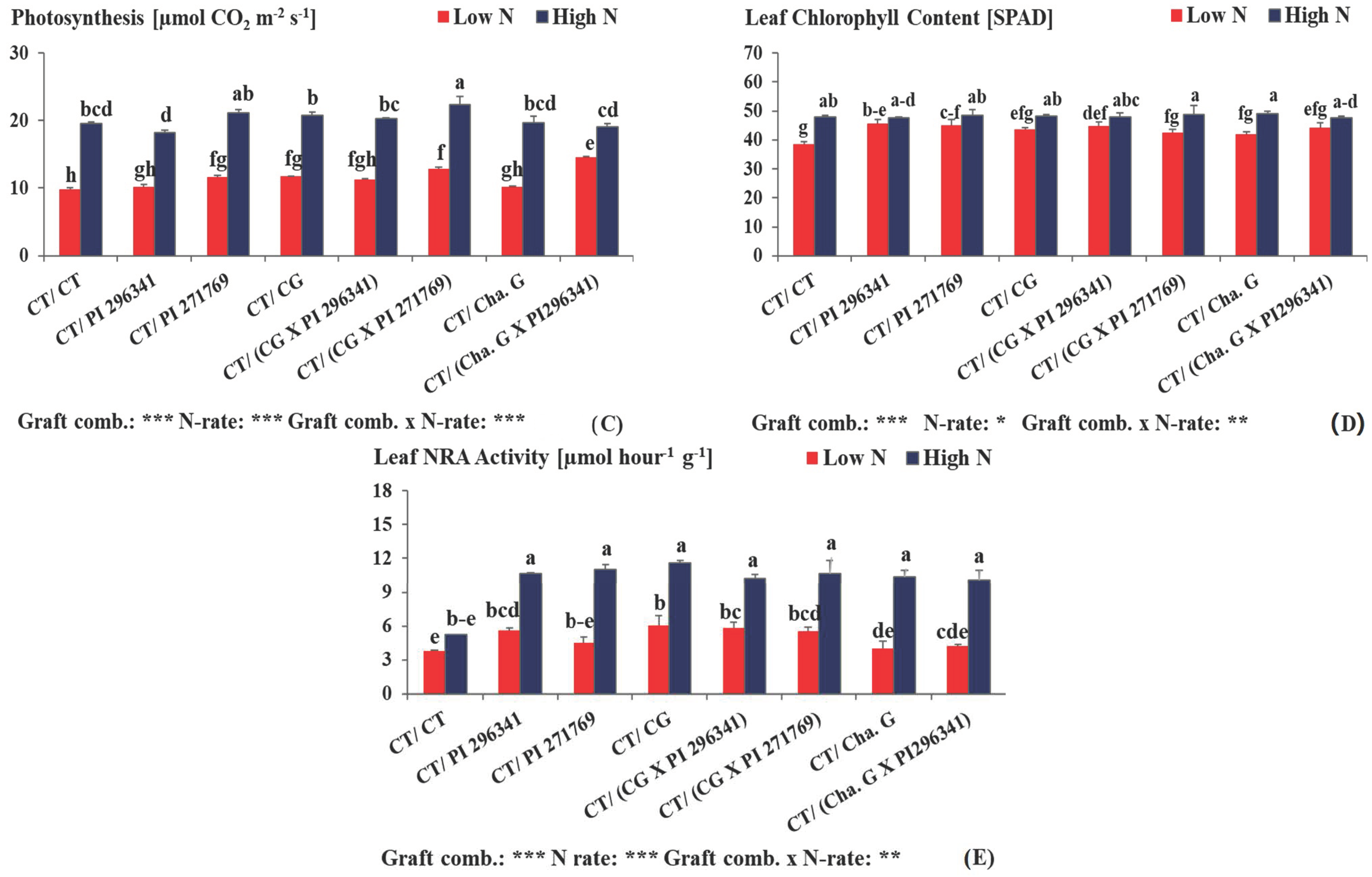
3.3. Total Leaf Area, Total Leaf Number, Intensity of Photosynthesis Measurements, Leaf Chlorophyll Content (SPAD) and Leaf NRA Activity
3.4. Total Root Length, Total Root Volume and Average Root Diameter
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects. The 2022 Revision of World Population Prospects. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 12 June 2022).
- Byrnes, B.H.; Bumb, B.L. Population growth, food production and nutrient requirements. In Journal of Crop Production; Rengel, Z., Ed.; The Haworth Press: New York, NY, USA, 1998; pp. 1–27. [Google Scholar]
- Food and Agriculture Organization. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 June 2022).
- Amalfitano, C.; Del Vacchio, L.; Somma, S.; Cuciniello, A.; Caruso, G. Effects of cultural cycle and nutrient solution electrical conductivity on plant growth, yield and fruit quality of “Friariello” pepper grown in hydroponics. Hortic. Sci. 2017, 44, 91–98. [Google Scholar]
- Grindlay, D.J.C. Towards an explanation of crop nitrogen demand based on the optimization of leaf nitrogen per unit leaf area. J. Agric. Sci. 1997, 128, 377–396. [Google Scholar] [CrossRef]
- Brégard, A.; Bélanger, G.; Michaud, R. Nitrogen use efficiency and morphological characteristics of timothy populations selected for low and high forage nitrogen concentrations. Crop Sci. 2000, 40, 422–429. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Laegreid, M.; Bockman, O.C.; Kaarstad, O. Agriculture, Fertilisers and the Environment; CABI Publishing: Wallingford, UK, 1999; p. 294. [Google Scholar]
- Wiesler, F.; Behrens, T.; Horst, W.J. The role of nitrogen-efficient cultivars in sustainable agriculture. Sci. World J. 2001, 1, 61–69. [Google Scholar] [CrossRef]
- Ulas, A.; Doganci, E.; Ulas, F.; Yetisir, H. Root-growth characteristics contributing to genotypic variation in nitrogen efficiency of bottle gourd and rootstock potential for watermelon. Plants 2019, 8, 77. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubotab, C.; Tsaoc, S.J.; Bied, Z.; Hoyos Echevarriae, P.; Morraf, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Ulas, F.; Aydın, A.; Ulas, A.; Yetisir, H. Grafting for sustainable growth performance of melon (Cucumis melo) under salt stressed hydroponic condition. Eur. J. Sustain. Dev. 2019, 8, 201–210. [Google Scholar] [CrossRef]
- Ulas, F.; Fricke, A.; Stützel, H. Leaf physiological and root morphological parameters of grafted tomato plants drought stress conditions. Fresenius Environ. Bull. 2019, 28, 3423–3434. [Google Scholar]
- Ulas, A.; Aydin, A.; Ulas, F.; Yetisir, H.; Miano, T.F. Cucurbita rootstocks improve salt tolerance of melon scions by inducing physiological, biochemical and nutritional responses. Horticulturae 2020, 6, 66. [Google Scholar] [CrossRef]
- Ulas, F.; Yetisir, H.; Ulas, A. Effects of grafting on fruit yield and leaf nutrient contents of pepper (Capsicum annuum L.) inbred lines. Genetika-Belgrade 2020, 52, 1041–1053. [Google Scholar] [CrossRef]
- Ulas, F. Response of different rootstocks on vegetative growth, fruit and seed yield of eggplant (Solanum melongena L.). Genetika-Belgrade 2021, 53, 593–608. [Google Scholar] [CrossRef]
- Ulas, F. Effects of grafting on growth, root morphology and leaf physiology of pepino (Solanum muricatum Ait.) as affected by salt stress under hydroponic conditions. Int. J. Agric. Environ. Food Sci. 2021, 5, 203–212. [Google Scholar] [CrossRef]
- Ulas, F.; Aydin, A.; Ulas, A.; Yetisir, H. The efficacy of grafting on alkali stressed watermelon cultivars under hydroponic conditions. Gesunde Pflanz. 2021, 73, 345–357. [Google Scholar] [CrossRef]
- Ulas, F.; Erdogdu, S.; Yetisir, H.; Ulas, A. Investigation on morphology and physiology of Nitrogen efficiency in different pepper (Capsicum annuum L.) inbred lines. Genetika 2021, 53, 3. [Google Scholar] [CrossRef]
- Ulas, F.; Yetisir, H.; Ulas, A. Root-growth characteristics contributing to nitrogen efficiency of reciprocally grafted potatoes (Solanum tuberosum L.) under Hydroponic conditions. Gesunde Pflanz. 2021, 73, 417–425. [Google Scholar] [CrossRef]
- Al Rubaye, O.A.M.; Yetisir, H.; Ulas, F.; Ulas, A. Enhancing Salt Stress Tolerance of Different Pepper (Capsicum annuum L.) Inbred Line Genotypes by Rootstock with Vigorous Root System. Gesunde Pflanz. 2021, 73, 375–389. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Fei, C.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef]
- Bie, Z.; Nawaz, M.A.; Huang, Y.; Lee, J.M.; Colla, G. Introduction of vegetable grafting. In Vegetable Grafting: Principles and Practices; Colla, G., Alfocea, F.P., Schwarz, D., Eds.; CABI Publishing: Oxfordshire, UK, 2017; pp. 1–21. [Google Scholar]
- Bansal, R. Heterosis in watermelon [Citrullus lanatus (Thunb.) Mansf.]. Environ. Ecol. 2002, 20, 976–979. [Google Scholar]
- Albacete, A.; Martinez-Anujar, C.; Marinez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unravelling rootstock-scion interactions to improve food Security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef]
- Lee, J.M. Cultivation of grafted vegetables I: Current status, grafting methods and benefits. HortScience 1994, 29, 235–239. [Google Scholar] [CrossRef]
- Sattelmacher, B.; Horst, W.J.; Becker, H.C. Factors that contribute to genetic variation for nutrient efficiency of crop plants. J. Plant Nutr. Soil Sci. 1994, 157, 215–224. [Google Scholar] [CrossRef]
- Gerendás, J.; Abbadi, J.; Sattelmacher, B. Potassium efficiency of safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.). J. Plant Nut Soil Sci. 2008, 171, 431–439. [Google Scholar] [CrossRef]
- Labconco, C. A Guide to Kjeldahl Nitrogen Determination Methods and Apparatus; Labconco Corporation: Houston, TX, USA, 1998. [Google Scholar]
- Harley, S.M. Use of a simple colorimetric assay to determine conditions for induction of nitrate reductase in plants. Am. Biol. Teach. 1993, 55, 161–164. [Google Scholar] [CrossRef]
- Adam, M.B. Genotypic Differences in Nitrogen Efficiency and Rootstock Potential of Some Local Tomato Varieties of Ghana and Turkey. Master’s Thesis, Erciyes University, Graduate School of Natural and Applied Sciences, Kayseri, Turkey, 2018. [Google Scholar]
- Nawaz, M.A.; Wang, L.; Jiao, Y.; Chen, C.; Zhao, L.; Mei, M.; Yu, Y.; Bie, Z.; Huang, Y. Pumpkin rootstock improves nitrogen use efficiency of watermelon scion by enhancing nutrient uptake, cytokinin content, and expression of nitrate reductase genes. Plant Growth Regul. 2017, 82, 233–246. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Han, X.; Chen, C.; Zheng, Z.; Shireen, F.; Bie, Z.; Huang, Y. Nitrogen use efficiency of watermelon grafted onto 10 wild watermelon rootstocks under low nitrogen conditions. Agronomy 2018, 8, 259. [Google Scholar] [CrossRef]
- Colla, G.; Suarez, C.M.C.; Cardarelli, M. Improving nitrogen use efficiency in melon by grafting. HortScience 2010, 45, 559–565. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Mirabelli, C.; Cardarelli, M. Nitrogen-use efficiency traits of mini-watermelon in response to grafting and nitrogen-fertilization doses. J. Plant Nutr. Soil Sci. 2011, 174, 933–941. [Google Scholar] [CrossRef]
- Clarkson, D.T. Factors affecting mineral nutrient acquisition by plants. Annu. Rev. Plant Physiol. 1985, 36, 77–115. [Google Scholar] [CrossRef]
- Jackson, W.A.; Pan, W.L.; Moll, R.H.; Kamprath, E.J. Uptake, translocation, and reduction of nitrate. Biochem. Basis Plant Breed. 1986, 2, 73–108. [Google Scholar]
- Claassen, N.; Syring, K.M.; Jungk, A. Verification of a mathematical model by simulating potassium uptake from soil. Plant Soil 1986, 95, 209–220. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Hua, B.; Liu, Z.; Fan, M.; Bie, Z. Grafting onto different rootstocks as a means to improve watermelon tolerance to low potassium stress. Sci. Hortic. 2013, 149, 80–85. [Google Scholar] [CrossRef]
- Ofori, E. Influence of Grafting on the Growth, Yield, Quality and Shelf Life of Tomatoes (Solanum lycopersicum L.) Grafted onto Three Solanum Species. Ph.D. Thesis, Department of Crop Science, School of Agriculture and Consumer Science, University of Ghana, Nyankpala, Ghana, 2015. [Google Scholar]
- Yücel, Y.C. Farklı Patlıcan (Solanum melongena L.) Genotiplerinin Azot Etkinlik Bakımından Agronomik, Fizyolojik ve Morfolojik Karekterizasyonu ve Anaçlık Potansiyellerinin Belirlenmesi; Agronomical, Physiological and Morphological Characterization of Some Selected Eggplant (Solanum melongena L.) Genotypes for Nitrogen Efficiency and Rootstock Potential. Master’s Thesis, Erciyes University, Graduate School of Natural and Applied Sciences, Kayseri, Turkey, 2020. [Google Scholar]
- Bannari, A.; Khurshid, K.S.; Staenz, K.; Schwarz, J.W. A comparison of hyperspectral chlorophyll indices for wheat crop chlorophyll content estimation using laboratory reflectance measurements. IEEE Trans. Geosci. Remote Sens. 2007, 45, 3063–3074. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Chen, C.; Shireen, F.; Zheng, Z.; Jiao, Y.; Sohail, H.; Afzal, M.; Imtiaz, M.; Ali, M.A.; Huang, Y.; et al. Improving vanadium stress tolerance of watermelon by grafting onto bottle gourd and pumpkin rootstock. Plant Growth Regul. 2018, 85, 41–56. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lu, X.M.; Yan, B.; Li, B.; Sun, J.; Guo, S.R.; Tezuka, T. Bottle gourd rootstock-grafting affects nitrogen metabolism in NaCl stressed watermelon leaves and enhances short-term salt tolerance. J. Plant Physiol. 2013, 170, 653–661. [Google Scholar] [CrossRef]

| Shoot Fresh Weight (g/plant) | Root Fresh Weight (g/plant) | Main Stem Length (cm/plant) | ||||
|---|---|---|---|---|---|---|
| Graft Combinations (S/R) | Low N | High N | Low N | High N | Low N | High N |
| CT | ||||||
| CT/CT | 13.8 i | 37.8 g | 6.2 j | 7.6 i | 30.3 g | 48.5 ef |
| CT/PI 296341 | 19.9 h | 62.7 e | 10.6 g | 19.5 a | 38.8 efg | 75.3 bc |
| CT/PI 271769 | 18.8 h | 51.9 f | 14.0 d | 16.0 c | 37.0 fg | 75.5 bc |
| CT/CG | 18.3 h | 82.2 b | 10.6 g | 18.6 b | 45.5 ef | 85.3 ab |
| CT/(CG × PI 296341) | 19.0 h | 69.1 d | 7.4 i | 16.3 c | 47.7 ef | 88.2 ab |
| CT/(CG × PI 271769) | 20.7 h | 78.3 c | 11.1 fg | 18.5 b | 54.0 de | 98.3 a |
| CT/Cha. G | 19.3 h | 50.9 f | 9.3 h | 12.3 e | 52.5 e | 67.2 cd |
| CT/(Cha. G × PI 296341) | 21.1 h | 92.4 a | 11.6 ef | 19.0 ab | 41.7 efg | 95.3 a |
| F-Test | ||||||
| Graft comb. | *** *** *** | *** *** *** | *** *** ** | |||
| N rate | ||||||
| Graft comb. × N dose | ||||||
| Shoot Dry Weight (g/plant) | Root Dry Weight (g/plant) | Root: Shoot Ratio (g/g) | ||||
|---|---|---|---|---|---|---|
| Graft Combinations (S/R) | Low N | High N | Low N | High N | Low N | High N |
| CT/CT | 1.05 h | 2.78 f | 0.12 h | 0.23 g | 0.11 d | 0.08 e |
| CT/PI 296341 | 1.63 g | 4.27 d | 0.32 efg | 0.70 a | 0.19 b | 0.16 c |
| CT/PI 271769 | 1.68 g | 3.37 e | 0.41 def | 0.43 de | 0.24 a | 0.13 d |
| CT/CG | 1.65 g | 6.20 b | 0.25 g | 0.55 bc | 0.15 c | 0.09 e |
| CT/(CG × PI 296341) | 1.55 g | 4.42 d | 0.25 g | 0.47 cd | 0.16 c | 0.11 d |
| CT/(CG × PI 271769) | 1.72 g | 5.78 c | 0.40 def | 0.65 ab | 0.23 a | 0.11 d |
| CT/Cha. G | 1.72 g | 3.22 e | 0.30 fg | 0.38 def | 0.17 c | 0.12 d |
| CT/(Cha. G × PI 296341) | 1.70 g | 6.67 a | 0.38 def | 0.60 ab | 0.23 a | 0.09 e |
| F-Test | ||||||
| Graft comb. | *** *** *** | *** *** ** | *** *** *** | |||
| N rate | ||||||
| Graft comb. × N dose | ||||||
| Nitrogen Uptake Efficiency (mg N/mg N) | Nitrogen Use Efficiency (g DW/g N) | Biological Production Efficiency (g DW/g N) | ||||
|---|---|---|---|---|---|---|
| Graft Combinations (S/R) | Low N | High N | Low N | High N | Low N | High N |
| CT/CT | 0.542 e | 0.224 i | 31.25 b | 8.28 f | 57.62 f | 36.91 k |
| CT/PI 296341 | 0.656 cd | 0.307 h | 48.61 a | 12.70 de | 74.07 b | 41.31 hi |
| CT/PI 271769 | 0.755 ab | 0.253 hi | 50.10 a | 10.02 ef | 66.31 d | 39.66 ij |
| CT/CG | 0.637 d | 0.419 fg | 49.11 a | 18.40 c | 77.10 a | 43.91 gh |
| CT/(CG × PI 296341) | 0.690 bcd | 0.311 h | 46.13 a | 17.21 de | 66.85 d | 45.08 hi |
| CT/(CG × PI 271769) | 0.694 bcd | 0.382 g | 51.09 a | 13.14 cd | 73.72 b | 42.31 g |
| CT/Cha. G | 0.808 a | 0.244 hi | 51.09 a | 9.33 ef | 63.42 e | 38.22 ijk |
| CT/(Cha. G × PI 296341) | 0.714 bc | 0.487 ef | 50.60 a | 19.84 c | 70.82 c | 40.73 hi |
| F-Test | ||||||
| Graft comb. | *** *** *** | *** *** ** | *** *** *** | |||
| N rate | ||||||
| Graft comb. × N dose | ||||||
| Total Root Length (m/plant) | Total Root Volume (cm3/plant) | Av. Root Diameter (mm) | ||||
|---|---|---|---|---|---|---|
| Graft Combinations (S/R) | Low N | High N | Low N | High N | Low N | High N |
| CT/CT | 313.2 i | 1010.3 g | 191.0 ef | 187.3 ef | 0.284 fgh | 0.345 b |
| CT/PI 296341 | 702.1 h | 3500.1 a | 112.0 g | 245.3 d | 0.297 e | 0.258 hi |
| CT/PI 271769 | 861.1 gh | 1871.1 de | 118.0 f | 244.3 d | 0.313 d | 0.324 c |
| CT/CG | 710.0 h | 1665.6 ef | 770.0 a | 312.0 c | 0.284 fg | 0.414 a |
| CT/(CG × PI 296341) | 845.9 gh | 2069.3 d | 213.7 e | 266.3 d | 0.324 cd | 0.274 hi |
| CT/(CG × PI 271769) | 1869.2 de | 2829.4 b | 392.7 b | 248.0 d | 0.350 b | 0.320 cd |
| CT/Cha. G | 938.0 g | 1499.6 f | 210.0 ef | 225.0 e | 0.287 ef | 0.276 ghi |
| CT/(Cha. G × PI 296341) | 1737.4 e | 2302.8 c | 329.0 c | 378.7 b | 0.353 b | 0.267 ij |
| F-Test | ||||||
| Graft comb. | *** *** *** | *** *** *** | *** n.s. *** | |||
| N rate | ||||||
| Graft comb. × N dose | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulas, A. Crossbreeding Rootstocks Improve Nitrogen Efficiency of Grafted Watermelon by Inducing Leaf Physiological and Root Morphological Responses. Horticulturae 2022, 8, 879. https://doi.org/10.3390/horticulturae8100879
Ulas A. Crossbreeding Rootstocks Improve Nitrogen Efficiency of Grafted Watermelon by Inducing Leaf Physiological and Root Morphological Responses. Horticulturae. 2022; 8(10):879. https://doi.org/10.3390/horticulturae8100879
Chicago/Turabian StyleUlas, Abdullah. 2022. "Crossbreeding Rootstocks Improve Nitrogen Efficiency of Grafted Watermelon by Inducing Leaf Physiological and Root Morphological Responses" Horticulturae 8, no. 10: 879. https://doi.org/10.3390/horticulturae8100879
APA StyleUlas, A. (2022). Crossbreeding Rootstocks Improve Nitrogen Efficiency of Grafted Watermelon by Inducing Leaf Physiological and Root Morphological Responses. Horticulturae, 8(10), 879. https://doi.org/10.3390/horticulturae8100879






