Effects of Different Rootstocks and Storage Temperatures on Postharvest Quality of Eggplant (Solanum melongena L. cv. Madonna)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments, and Storage
2.2. Analysis of Fruit Quality during Storage
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.P.; Luthria, D.; Wilson, T.; Vorsa, N.; Singh, V.; Banuelos, G.S.; Pasakdee, S. Polyphenols content and antioxidant capacity of eggplant pulp. Food Chem. 2009, 114, 955–961. [Google Scholar] [CrossRef]
- Concellón, A.; Zaro, M.J.; Chaves, A.R.; Vicente, A.R. Changes in quality and phenolic antioxidants in dark purple American eggplant (Solanum melongena L. cv. Lucía) as affected by storage at 0 °C and 10 °C. Postharvest Biol. Technol. 2012, 66, 35–41. [Google Scholar] [CrossRef]
- Singh, S.; Khemariya, P.; Rai, A.; Rai, A.C.; Koley, T.K.; Singh, B. Carnauba wax- based edible coating increase the shelf-life and retain quality of egg plant (Solanum melongena) fruits. Food Sci. Technol. 2016, 74, 420–426. [Google Scholar]
- Karthiayini, K.; Philomina, P.T. Effect of temperature and relative humidity on the haematology of chicken. Indian Vet. J. 2010, 87, 232–235. [Google Scholar]
- Sahoo, N.R.; Bal, L.M.; Pal, U.S.; Sahoo, D. Effect of packaging conditions on quality and shelf-life of fresh pointed gourd (Trichosanthes dioica Roxb.) during storage. Food Packag. Shelf Life 2015, 5, 56–62. [Google Scholar] [CrossRef]
- Gao, H.; Kang, L.; Liu, Q.; Cheng, N.; Wang, B.; Cao, W. Effect of 24-epibrassinolide treatment on the metabolism of eggplant fruits in relation to development of pulp browning under chilling stress. J. Food Sci. Technol. 2015, 52, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front. Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Mozafarian, M.; Kappel, N. Effect of grafting on the quality and appearance of eggplant fruit. Prog. Agric. Eng. Sci. 2021, 16, 153–161. [Google Scholar]
- Gisbert, C.; Prohens, J.; Raigón, M.D.; Stommel, J.R.; Nuez, F. Eggplant relatives as sources of variation for developing new rootstocks: Effects of grafting on eggplant yield and fruit apparent quality and composition. Sci. Hortic. 2011, 128, 14–22. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F.; Mineo, V.; Planeta, D.; D’Anna, F. Effect of grafting on yield and quality of Eggplant (Solanum melongena L.). Sci. Hortic. 2013, 4, 108–114. [Google Scholar] [CrossRef]
- Kacjan Maršic, N.; Mikulic-Petkovšek, M.; Stampar, F. Grafting influences phenolic profile and carpometrictraits of fruits of greenhouse-grown eggplant (Solanum melongena L.). J. Agric. Food Chem. 2014, 62, 10504–10514. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Maggio, A.; D’Anna, E.; Bruno, M.; D’Anna, F. Grafting affects yield and phenolic profile of Solanum melongena L. landraces. J. Integr. Agric. 2016, 15, 1017–1024. [Google Scholar] [CrossRef]
- Passam, H.C.; Stylianou, M.; Kotsiras, A. Performance of eggplant grafted on tomato and eggplant rootstocks. Eur. J. Hort. Sci. 2005, 70, 130–134. [Google Scholar]
- Khah, E.M. Effect of grafting on growth, performance and yield of aubergine (Solanum melongena L.) in greenhouse and open-field. Intern. J. Plant Prod. 2011, 5, 359–366. [Google Scholar]
- Pebriana, E.; Dawam Maghfoer, M.O.C.H.; Widaryanto, E.E. Effect of grafting using wild eggplant as rootstock on growth and yield of four eggplant (Solanum melongena L.) cultivars. Biosci. Res. 2018, 15, 337–347. [Google Scholar]
- Rahman, M.A.; Rashid, M.A.; Hossain, M.M.; Salam, M.A.; Masum, A.S.M.H. Grafting compatibility of cultivated eggplant varieties with wild Solanum species. Pak. J. Biol. Sci. 2002, 5, 755–757. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, B.; Ozer, H. Effects of Grafting and Green Manure Treatments on Postharvest Quality of Tomatoes. J. Soil Sci. Plant Nutr. 2019, 19, 780–792. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Khah, E.M.; Christakou, E.C.; Bletsos, F.A. Effect of grafting and modified atmosphere packaging on eggplant quality parameters during storage. Int. J. Food Sci. Technol. 2005, 40, 311–322. [Google Scholar] [CrossRef]
- Larrigaudiere, C.; Lentheric, I.; Vendrell, M. Relationship between enzymatic browning and internal disorders in controlled-atmosphere stored pears. J. Sci. Food Agric. 1988, 78, 232–236. [Google Scholar] [CrossRef]
- Cantwell, M.; Suslow, T. Eggplant: Recommendations for Maintaining Postharvest Quality. 2000. Available online: http://afghanag.ucdavis.edu/a_horticulture/row-crops/eggplant/FS_Veg_Eggplant_Postharvest_UCD_PHTC.pdf (accessed on 18 January 2014).
- Concellon, A.; Anon, M.C.; Chaves, A.R. Effect of low temperature storage on physical and physiological characteristics of eggplant fruit (Solanum melongena L.). Food Sci. Technol. 2007, 40, 389–396. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Temperini, O.; Rea, E.; Salerno, A.; Pierandrei, F. Influence of grafting on yield and fruit quality of pepper (Capsicum annuum L.) grown under greenhouse conditions. Acta Hortic. 2008, 782, 359–363. [Google Scholar] [CrossRef]
- Khah, E.M.; Katsoulas, N.; Tchamitchian, M.; Kittas, C. Effect of grafting on eggplant leaf gas exchanges under mediterranean greenhouse conditions. Int. J. Plant Prod. 2011, 5, 121–134. [Google Scholar]
- Di Gioia, F.; Serio, F.; Buttaro, D.; Ayala, O.; Santamaria, P. Vegetative growth, yield, and fruit quality of ‘Cuore di Bue’, an heirloom tomato, as influenced by rootstock. J. Hortic. Sci. Biotechnol. 2010, 85, 477–482. [Google Scholar] [CrossRef]
- Nicoletto, C.; Tosini, F.; Sambo, P. Effect of grafting and ripening conditions on some qualitative traits of ‘Cuore di bue’ tomato fruits. J. Sci. Food Agric. 2013, 93, 1397–1403. [Google Scholar] [CrossRef]
- Jha, S.N.; Matsuoka, T.; Miyauchi, K. Surface gloss and weight of eggplant during storage. Biosyst. Eng. 2002, 81, 407–412. [Google Scholar] [CrossRef]
- Dadzie, R.G.; Amoah, R.S.; Ampofo-Asiama, J.; Quaye, B.; Abano, E.E. Physicochemical properties of eggplant (Solanum aethiopicum L.) fruits as affected by cassava starch coating during low temperature storage: Optimisation of coating conditions. Int. J. Postharvest Technol. Innov. 2019, 6, 276–300. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Nanjappa, C.; Ashok, N.; Ravi, N.; Roopa, N.; Raju, P.S. Shellac and aloevera gel based surface coating for shelf life extension of tomatoes. J. Food Sci. Technol. 2015, 52, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, C.; Giuffrida, F. Variation of plant growth and macronutrient uptake in grafted tomatoes and eggplants on three different rootstocks. Eur. J. Hortic. Sci. 2006, 71, 97–101. [Google Scholar]
- Cassaniti, C.; Giuffrida, F.; Scuderi, D.; Leonardi, C. Effect of rootstock and nutrient solution concentration on eggplant grown in a soilless system. J. Food Agric. Environ. 2011, 9, 252–256. [Google Scholar]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Turhan, A.; Ozmen, N.; Serbeci, M.S.; Seniz, V. Effects of grafting on different rootstocks on tomato fruit yield and quality. Hortic. Sci. 2011, 38, 142–149. [Google Scholar] [CrossRef]
- Krumbein, A.; Schwarz, D. Grafting: A possibility to enhance health-promoting and flavour compounds in tomato fruits of shaded plants? Sci. Hortic. 2013, 149, 97–107. [Google Scholar] [CrossRef]
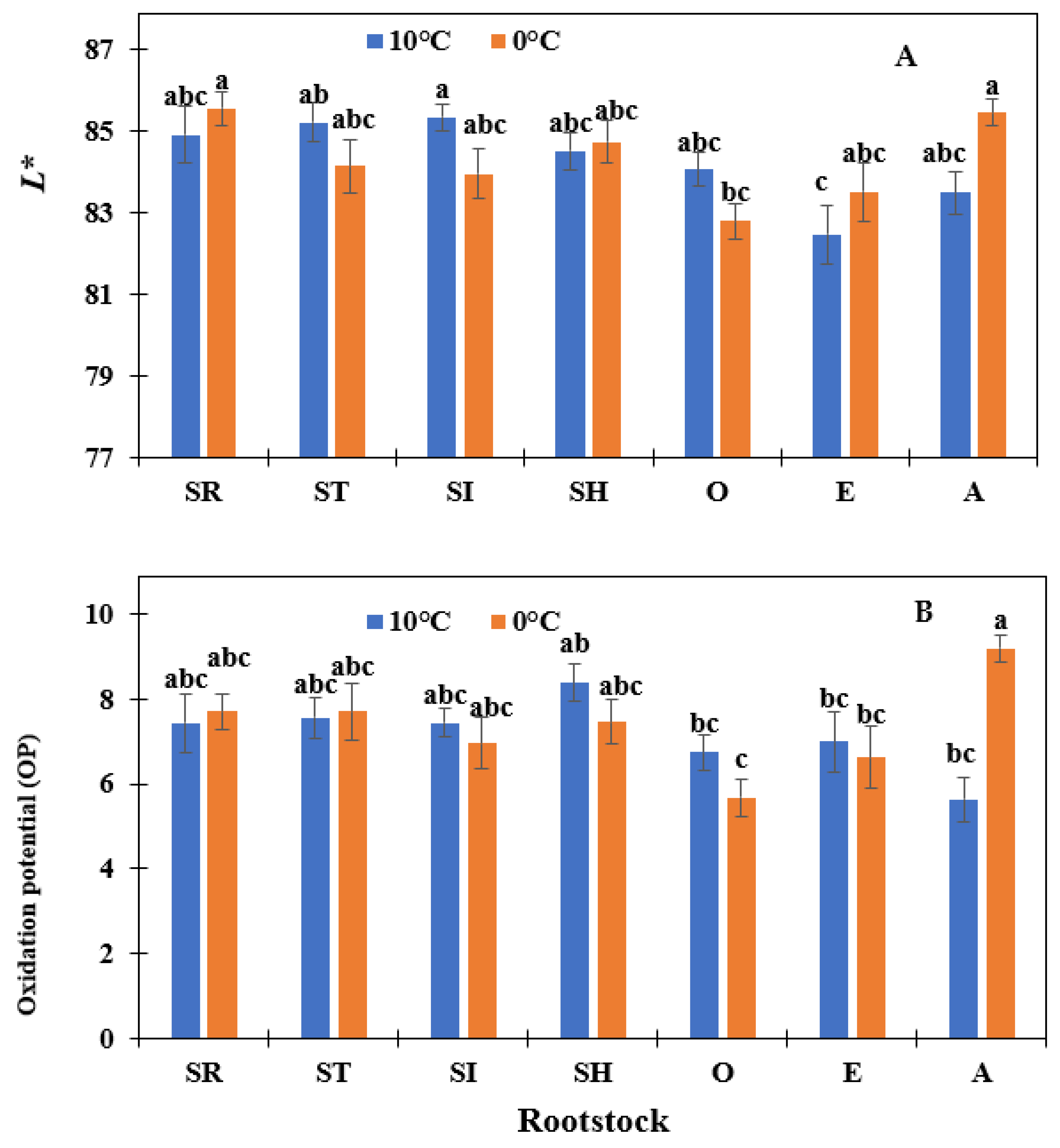



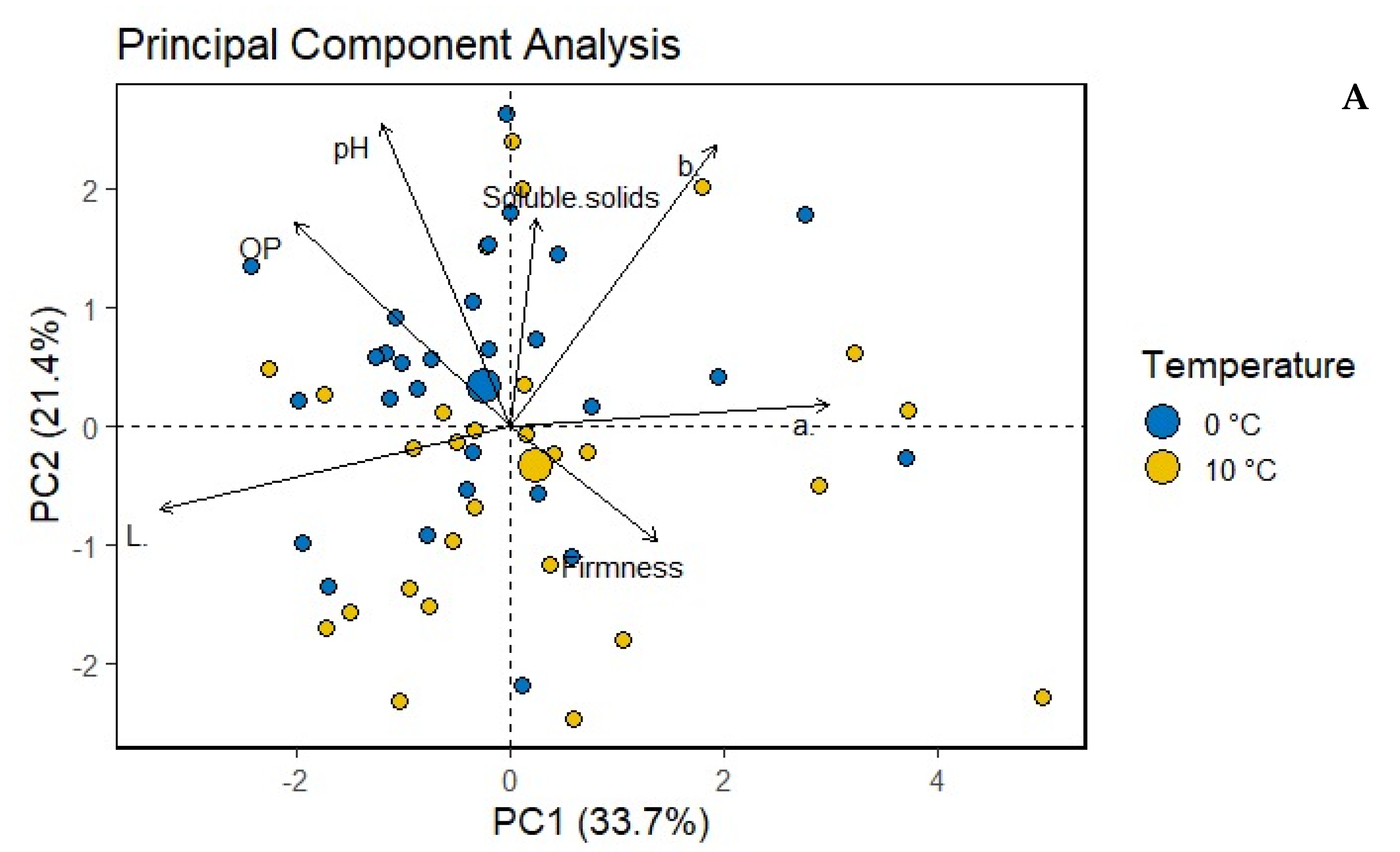
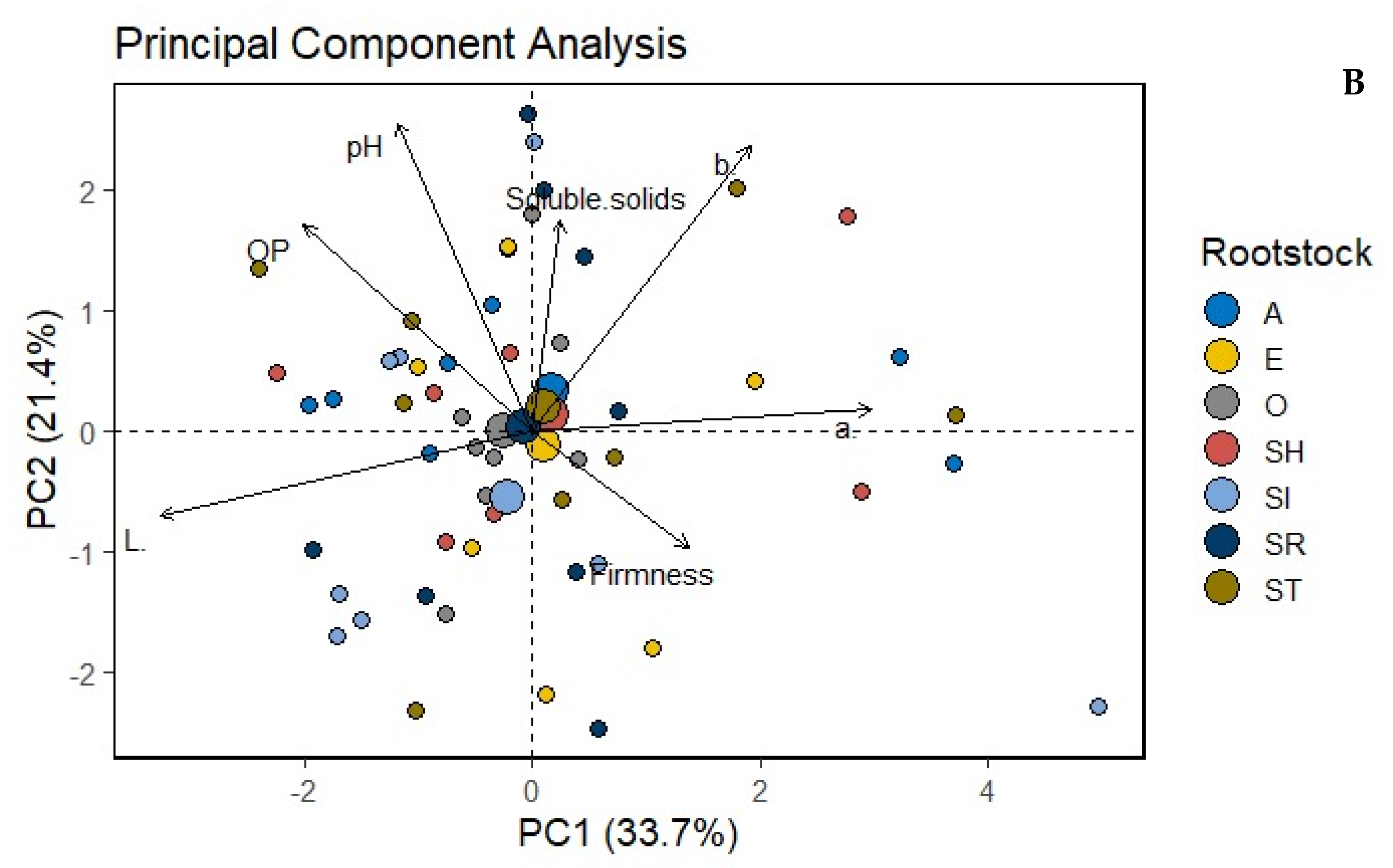
| Treatments | L* | a* | b* | Oxidation Potential (OP) | pH | Firmness (kg/cm3) | Soluble Solids (Brix) | |
|---|---|---|---|---|---|---|---|---|
| Storage day | 0 | 84.70 a | −3.98 a | 29.59 a | 7.72 a | 5.50 a | 4.17 a | 5.10 a |
| 3 | 83.80 b | −3.56 a | 28.55 ab | 7.61 ab | 5.29 c | 3.75 b | 4.90 b | |
| 6 | 83.45 b | −3.96 a | 27.75 b | 5.86 c | 5.37 b | 3.53 bc | - | |
| 9 | 85.15 a | −3.70 a | 25.39 c | 6.83 bc | 5.42 b | 3.58 c | - | |
| Storage temperature | 10 °C | 84.30 a | −3.70 a | 27.27 b | 7.14 a | 5.34 b | 3.78 a | 5.08 a |
| 0 °C | 84.07 a | −3.94 a | 28.09 a | 7.08 a | 5.44 a | 3.68 a | 4.92 a | |
| Rootstock | SR | 85.22 a | −4.13 a | 26.33 b | 7.57 a | 5.39 ab | 3.99 a | 4.74 ab |
| ST | 84.71 a | −3.65 a | 27.68 ab | 7.63 a | 5.37 b | 3.88 a | 5.06 ab | |
| SI | 84.65 a | −3.62 a | 28.19 a | 7.20 ab | 5.38 b | 3.77 a | 4.85 b | |
| SH | 84.61 a | −3.75 a | 27.61 ab | 7.97 a | 5.36 b | 4.04 a | 5.38 a | |
| O | 83.37 ab | −3.97 a | 27.87 ab | 6.16 b | 5.41 ab | 3.42 b | 4.59 b | |
| E | 83.01 b | −3.86 a | 28.00 ab | 6.8 ab | 5.43 ab | 3.41 b | 4.89 ab | |
| A | 84.45 ab | −3.65 a | 27.88 ab | 7.37 ab | 5.36 b | 3.88 a | 5.32 a | |
| F value | ||||||||
| Storage day | ** | ns | ** | ** | ** | ** | ** | |
| Temperature | ns | * | ** | ns | ** | ns | ns | |
| Rootstocks | ** | ns | ns | ** | * | ** | ** | |
| Storage day × Temperature | ns | ns | ** | ns | ** | ns | ns | |
| Storage day × Rootstock | ** | ** | ** | ** | ** | ** | ** | |
| Temperature × Rootstock | ** | ns | ns | ** | ns | ns | ns | |
| Storage day × Temperature × Rootstock | ns | * | ns | * | ** | ** | ns | |
| Coefficient of variation (%) | 2.74 | 17.96 | 8.43 | 19.12 | 1.62 | 14.95 | 9.46 | |
| Storage Day | Rootstock | L* | a* | b* | Oxidation Potential (OP) | pH | Firmness (kg/cm3) |
|---|---|---|---|---|---|---|---|
| 0 | SR | 84.89 a-d | −4.1 a–d | 29.08 a–c | 9.97 a | 5.55 ab | 4.93 a |
| ST | 84.03 a-d | −2.86 a–c | 30.14 ab | 9.67 a | 5.53 a–c | 3.51 c–f | |
| SI | 85.26 a-c | −4.56 cd | 29.05 a–c | 7.13 a–c | 5.44 a–f | 3.56 c–f | |
| SH | 84.62 a–d | −4.01 a–d | 29.15 a–c | 8.82 ab | 5.46 a–e | 4.56 ab | |
| O | 83.91 a–d | −3.69 a–d | 31.39 a | 5.81 a–c | 5.53 a | 2.43 g | |
| E | 86.36 ab | −4.71 d | 27.69 a–e | 5.81 a–c | 5.56 a | 2.77 fg | |
| A | 84.65 a–d | −4.24 a–d | 28.82 a–c | 7.90 a–c | 5.38 c–h | 4.04 a–d | |
| 3 | SR | 82.76 b–e | −3.42 a–d | 28.76 a–c | 6.43 a–c | 5.27 gh | 4.04 a–d |
| ST | 83.84 a–d | −3.80 a–d | 28.22 a–e | 7.25 a–c | 5.26 h | 4.18 a–d | |
| SI | 85.40 a–c | −3.72 a–d | 29.06 a–c | 5.68 a–c | 5.29 f–h | 4.28 a–d | |
| SH | 84.19 a–d | −3.62 a–d | 27.45 b–e | 8.26 a–c | 5.30 f–h | 4.14 a–d | |
| O | 85.33 a–c | −4.42 b–d | 27.66 b–e | 4.68 bc | 5.31 f–h | 4.21 a–c | |
| E | 79.30 e | −2.70 ab | 30.05 ab | 3.80 c | 5.33 e–h | 4.16 a–d | |
| A | 84.15 a–d | −2.60 a | 29.33 ab | 6.08 a–c | 5.29 f–h | 4.15 a–d | |
| 6 | SR | 85.56 a–c | −4.24 a–d | 24.97 de | 7.25 a–c | 5.41 b–f | 3.52 d–f |
| ST | 84.31 a–d | −4.21 a–d | 28.20 bc | 8.05 a–c | 5.35 e–h | 3.87 b–d | |
| SI | 83.88 a–d | −3.57 a–d | 28.96 ab | 7.05 a–c | 5.37 c–h | 3.93 b–d | |
| SH | 84.13 a–d | −3.54 a–d | 27.80 a–d | 8.43 a–c | 5.35 d–h | 3.58 c–f | |
| O | 81.86 de | −4.19 a–d | 27.83 bc | 7.16 a–c | 5.36 e–h | 3.52 d–f | |
| E | 82.56 c–e | −3.72 a–d | 28.16 bc | 8.73 a | 5.38 d–h | 3.11 e–g | |
| A | 83.70 a–d | −3.85 a–d | 28.59 a–c | 8.07 a–c | 5.36 d–h | 3.60 c–f | |
| 9 | SR | 86.91 a | −4.54 cd | 24.36 de | 7.06 a–c | 5.32 e–h | 3.97 b–d |
| ST | 86.05 ab | −3.37 a–d | 25.38 c–e | 6.20 a–c | 5.37 d–h | 3.94 b–d | |
| SI | 84.65 a–d | −3.07 a–d | 26.44 b–e | 8.25 a | 5.41 b–g | 3.45 c–f | |
| SH | 85.74 a–c | −3.95 a–d | 25.93 b–e | 6.14 a–c | 5.34 d–h | 4.13 a–d | |
| O | 83.88 a–d | −3.35 a–d | 24.64 e | 6.01 a–c | 5.50 a–f | 3.43 c–f | |
| E | 84.04 a–d | −4.33 a–d | 26.35 b–e | 6.90 a–c | 5.51 a–d | 3.78 b–e | |
| A | 85.55 a–c | −3.98 a–d | 24.77 de | 7.32 a–c | 5.43 a–h | 3.85 b–e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kappel, N.; Mozafarian, M. Effects of Different Rootstocks and Storage Temperatures on Postharvest Quality of Eggplant (Solanum melongena L. cv. Madonna). Horticulturae 2022, 8, 862. https://doi.org/10.3390/horticulturae8100862
Kappel N, Mozafarian M. Effects of Different Rootstocks and Storage Temperatures on Postharvest Quality of Eggplant (Solanum melongena L. cv. Madonna). Horticulturae. 2022; 8(10):862. https://doi.org/10.3390/horticulturae8100862
Chicago/Turabian StyleKappel, Noémi, and Maryam Mozafarian. 2022. "Effects of Different Rootstocks and Storage Temperatures on Postharvest Quality of Eggplant (Solanum melongena L. cv. Madonna)" Horticulturae 8, no. 10: 862. https://doi.org/10.3390/horticulturae8100862
APA StyleKappel, N., & Mozafarian, M. (2022). Effects of Different Rootstocks and Storage Temperatures on Postharvest Quality of Eggplant (Solanum melongena L. cv. Madonna). Horticulturae, 8(10), 862. https://doi.org/10.3390/horticulturae8100862






