Effects and Benefits of Orchid Mycorrhizal Symbionts on Dendrobium officinale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Fungal Strains
2.2. Electron Microscopic Determination of Mycorrhizal Fungi Symbiosis
2.3. Determination of Relative Conductivity
2.4. Determination of the Osmoregulatory Substance and Antioxidant Enzyme Activity in D. officinale
2.5. Measurement of Morphological Indices of D. officinale
2.6. Determination of Photosynthetic Pigment Content
2.7. Determination of the Polysaccharide Content of Functional Components in Stems of D. officinale
2.8. Statistical Analysis
3. Results
3.1. Colonization of Orchid Mycorrhizal Fungi
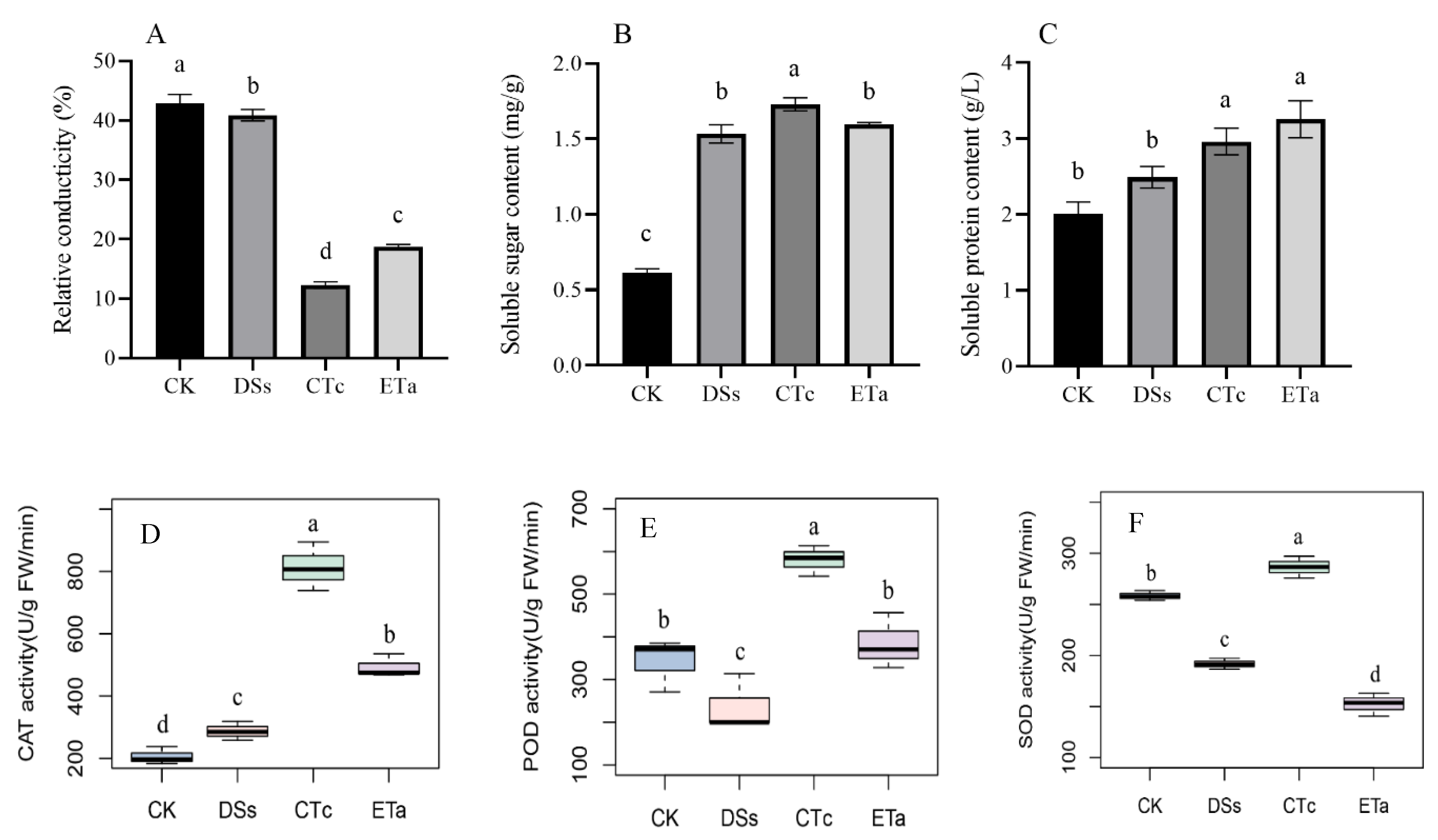
3.2. Comparison of Relative Conductivity, Osmoregulatory Substance Content and Antioxidant Enzyme Activities between Co-Cultured and Control Groups
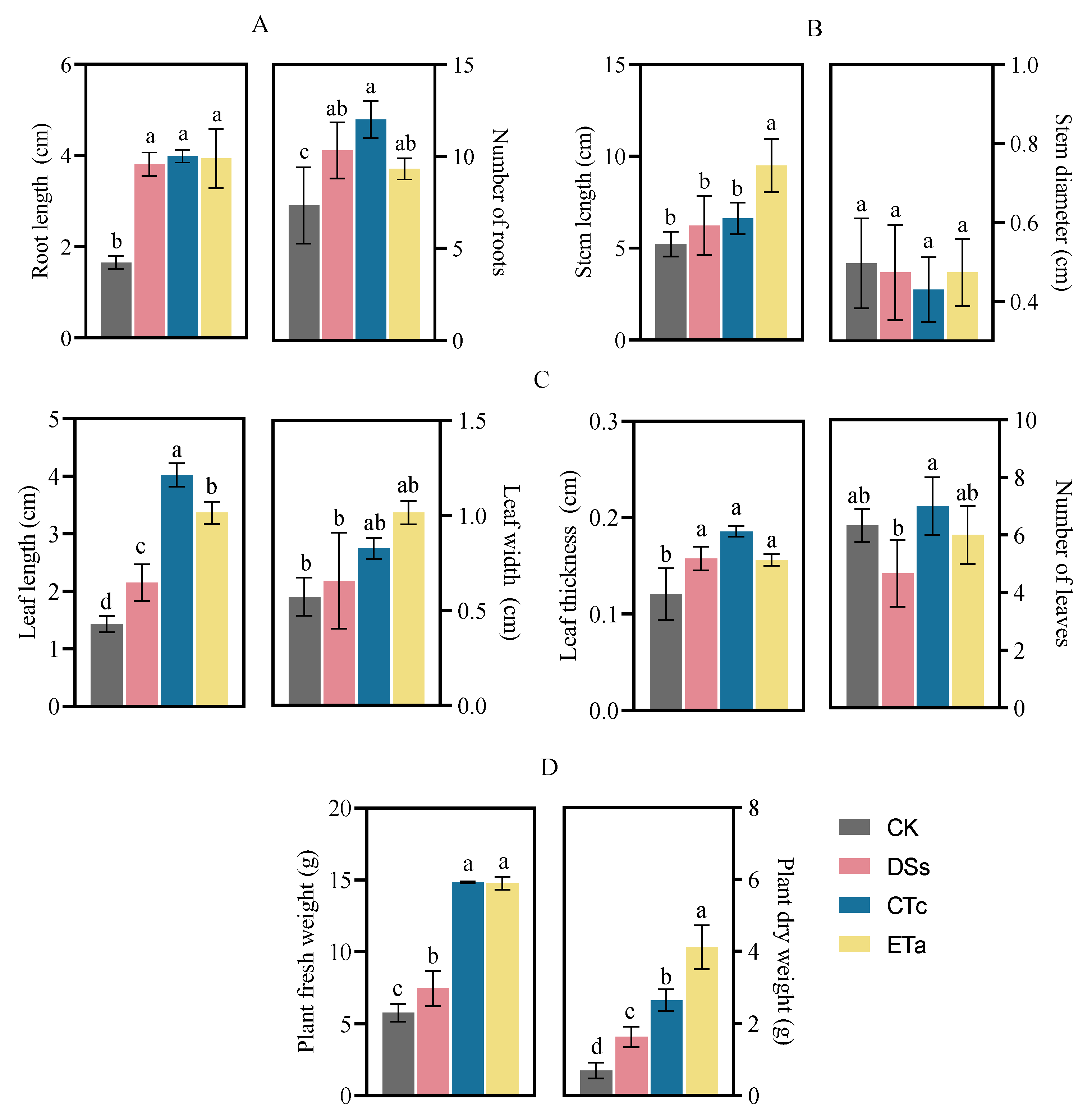
3.3. Effects of Orchid Mycorrhizal Fungi on D. officinale Morphology and Growth
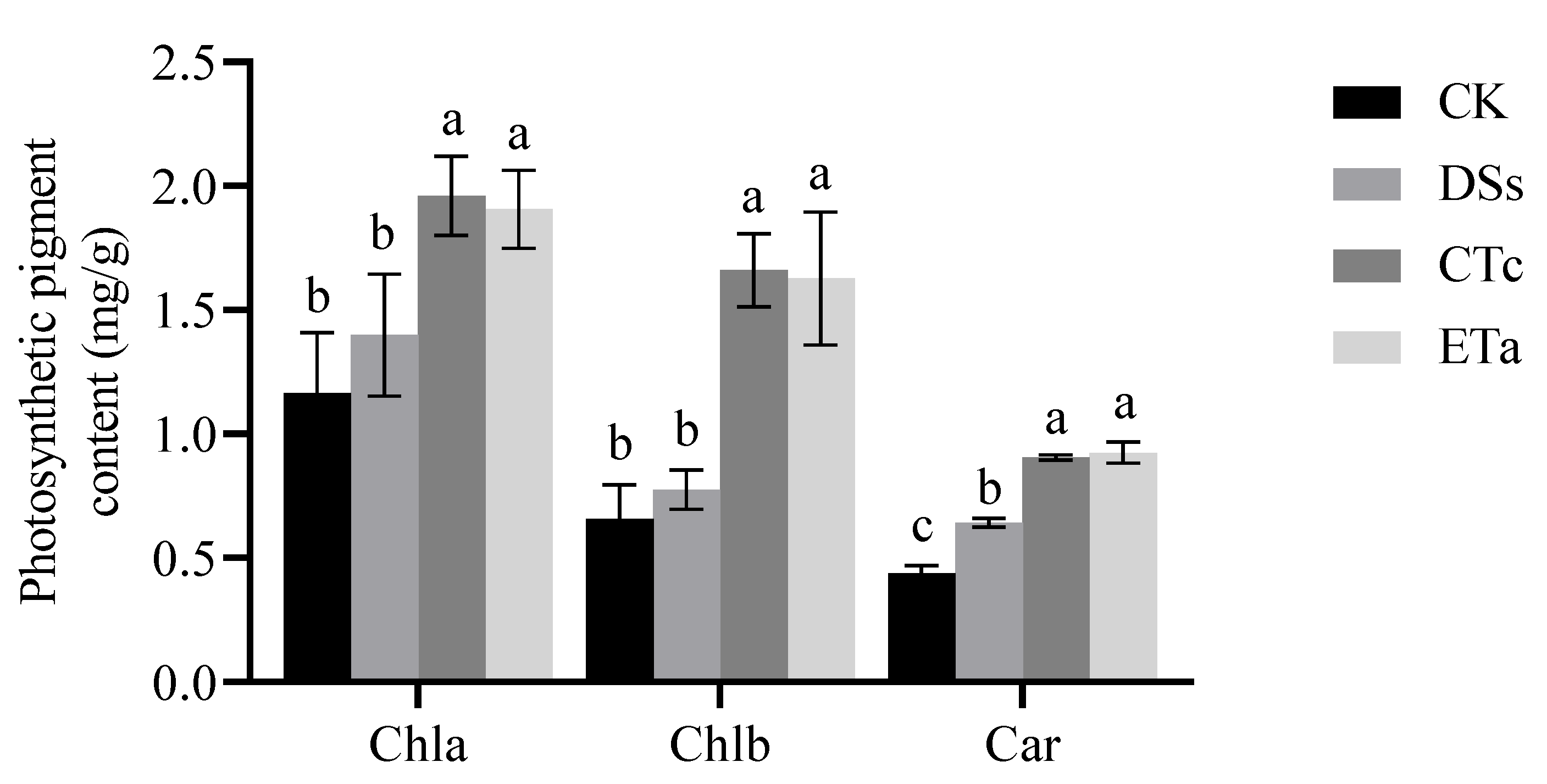
3.4. Orchid Mycorrhizal Fungi Promote an Increase in the Content of Photosynthetic Pigments in Leaves of D. officinale
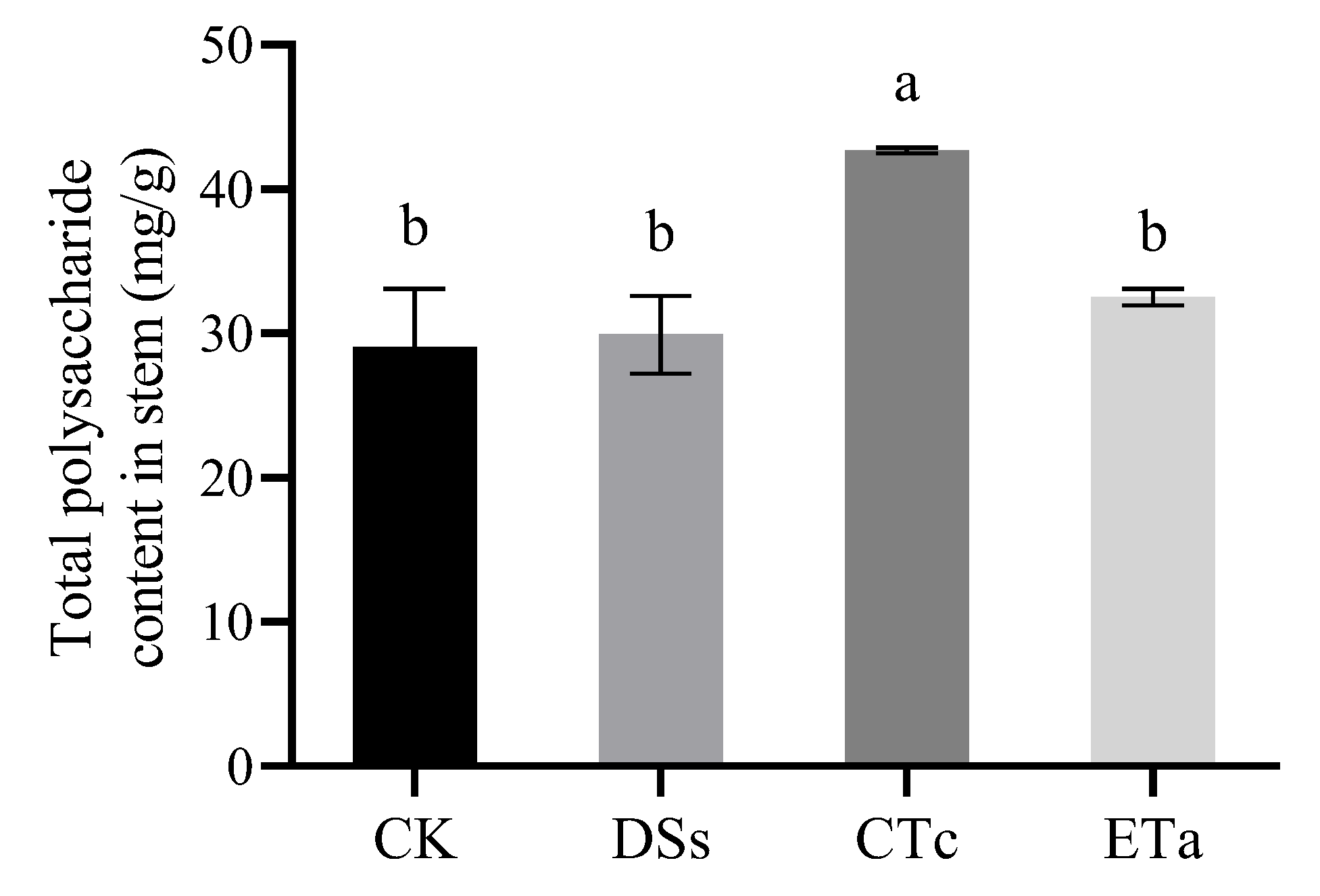
3.5. Effects of Orchid Mycorrhizal Fungi on Stem Polysaccharide Content of D. officinale
4. Discussion
4.1. Adaptability of D. officinale in a Symbiotic Environment by Resistance Index
4.2. Effects of Symbiotic Fungi on Growth and Morphology of D. officinale
4.3. Effect of Different Symbiotic Fungi on Total Polysaccharide Accumulation in D. officinale Stems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, L.; Liu, J.; Liang, J.; Si, J.; Wu, S. Dendrobium officinale Leaves as a New Antioxidant Source. J. Funct. Foods 2017, 37, 400–415. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Z.; Teixeira da Silva, J.A.; Luo, J.; Duan, J. Influence of Low Temperature on Physiology and Bioactivity of Postharvest Dendrobium Officinale Stems. Postharvest Biol. Technol. 2019, 148, 97–106. [Google Scholar] [CrossRef]
- Zeng, Q.; Ko, C.H.; Siu, W.S.; Li, L.F.; Han, X.Q.; Yang, L.; Bik-San Lau, C.; Hu, J.M.; Leung, P.C. Polysaccharides of Dendrobium officinale Kimura & Migo Protect Gastric Mucosal Cell Against Oxidative Damage-Induced Apoptosis In Vitro and In Vivo. J. Ethnopharmacol. 2017, 208, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.X.; Ren, Z.Y.; Zhang, X.F.; Xing, S.; Tao, S.; Liu, C.; Wei, G.; Yuan, Y.; Lei, Z. Structural Characterization of Polysaccharides From Dendrobium officinale and Their Effects on Apoptosis of hela Cell Line. Molecules 2018, 23, 2484. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving Production of Plant Secondary Metabolites Through Biotic and Abiotic Elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Riahi, L.; Cherif, H.; Miladi, S.; Neifar, M.; Bejaoui, B.; Chouchane, H.; Masmoudi, A.S.; Cherif, A. Use of Plant Growth Promoting Bacteria as an Efficient Biotechnological Tool to Enhance the Biomass and Secondary Metabo-lites Production of the Industrial Crop Pelargonium Graveolens L’Hér. under Semi-Controlled Conditions. Ind. Crops Prod. 2020, 154, 112721. [Google Scholar] [CrossRef]
- Suetsugu, K.; Kawakita, A.; Kato, M. Avian Seed Dispersal in a Mycoheterotrophic Orchid Cyrtosia septentrionalis. Nat. Plants 2015, 1, 15052. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, Q.; Gong, Q.F.; Qiu, S.; He, J.X.; Huang, J.; He, Y.Q. First Report of Stem and Root Rot of the Medicinal Herb Dendrobium officinale (Orchidaceae) Caused by Ceratobasidium sp. AG-R in Guangxi, China. Plant Dis. 2017, 101, 1679. [Google Scholar] [CrossRef]
- Lin, Y.; Li, J.; Li, B.; He, T.; Chun, Z. Effects of Light Quality on Growth and Development of Protocorm-Like Bodies of Dendrobium Officinale in Vitro. Plant Cell Tiss. Organ Cult. 2011, 105, 329–335. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Zhang, X.; Sun, P.; Jiang, X.; Han, Z. The Mycorrhizal Fungi of Cymbidium Promote the Growth of Dendrobium officinale by Increasing Environmental Stress Tolerance. PeerJ 2021, 12, e12555. [Google Scholar] [CrossRef]
- Wang, M.Y.; Tao, X.; Ke-Yan, L.I.; Wang, Y.L.; Yang, T.D.; Xiong, Z. Analysis of Community Structure of Endophytic Fungi From Dendrobium officinale. J. West China For. Sci. 2014, 43, 106–111. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y. Research Progress in Dendrobium candidum Mycorrhiza. J. Chongqing Univ. Arts Sci. 2015, 34, 99–102. [Google Scholar] [CrossRef]
- Ma, X.; Kang, J.; Nontachaiyapoom, S.; Wen, T.; Hyde, K.D. Non-mycorrhizal endophytic fungi from orchids. Curr. Sci. 2015, 109, 72–87. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid Mycorrhiza: Implications of a Mycophagous Life Style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Khamchatra, N.-M.; Dixon, K.; Chayamarit, K.; Apisitwanich, S.; Tantiwiwat, S. Using in Situ Seed Baiting Technique to Isolate and Identify Endophytic and Mycorrhizal Fungi from Seeds of a Threatened Epi-phytic Orchid, Dendrobium Friedericksianum Rchb.f. (Orchidaceae). Agric. Nat. Resour. 2016, 50, 8–13. [Google Scholar] [CrossRef]
- Zettler, L.W.; Poulter, S.B.; McDonald, K.I.; Stewart, S.L. Conservation-Driven Propagation of an Epiphytic Orchid (Epidendrum Nocturnum) With a Mycorrhizal Fungus. Hortic. Sci. 2007, 42, 135–139. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial Orchid Conservation in the Age of Extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-Y.; Shao, S.-C.; Liu, S.-J.; Gao, J.-Y. Do the Fungi Associated With Roots of Adult Plants Support Seed Germination? A Case Study on Dendrobium exile (Orchidaceae). Glob. Ecol. Conserv. 2019, 17, e00582. [Google Scholar] [CrossRef]
- Brundrett, M.C. Scientific Approaches to Australian Temperate Terrestrial Orchid Conservation. Aust. J. Bot. 2007, 55, 293–307. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Waud, M.; Merckx, V.S.; Lievens, B.; Brys, R. Mycorrhizal Diversity, Seed Germination and Long-Term Changes in Population Size Across Nine Populations of the Terrestrial Orchid Neottia ovata. Mol. Ecol. 2015, 24, 3269–3280. [Google Scholar] [CrossRef]
- Zi, X.M.; Sheng, C.L.; Goodale, U.M.; Shao, S.C.; Gao, J.Y. In Situ Seed Baiting to Isolate Germination-Enhancing Fungi for an Epiphytic Orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 2014, 24, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Lv, Y.; Gao, C.; Guo, S.X. Mycena sp. a Mycorrhizal Fungi of the Orchid Dendrobium officinale. Mycol. Prog. 2012, 11, 395–401. [Google Scholar] [CrossRef]
- Gao, W.W.; Guo, S.X. Effect of Three Endophytic Fungi on Growth of Dendrobium candidum and Anoectochilus roxburghii. Chin. Trad. Herb. Drugs 2002, 33, 543–545. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms Underlying Beneficial Plant-Fungus Interactions in Mycorrhizal Symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H.; Dee, M.; Vu, A.; Köllner, T.G.; Chen, F. Colonization by Arbuscular Mycorrhizal and Endophytic Fungi Enhanced Terpene Production in Tomato Plants and Their Defense Against A Herbivorous Insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal Ecology and Evolution: The Past, the Present, and the Future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Zhao, X.L.; Yang, J.Z.; Liu, S.; Chen, C.L.; Zhu, H.Y.; Cao, J.X. The Colonization Patterns of Different Fungi on Roots of Cymbidium hybridum Plantlets and Their Respective Inoculation Effects on Growth and Nutrient Uptake of Orchid Plantlets. World J. Microbiol. Biotechnol. 2014, 30, 1993–2003. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Shen, Y.; Nartey Korkor, L.; Pu, Q.; Lu, J.; Shakeela, B.; Kong, D.; Li, O.; Zeng, G.; et al. Physiological Responses of Dendrobium officinale Under Exposure to Cold Stress With Two Cultivars. Phyton 2020, 89, 599–617. [Google Scholar] [CrossRef]
- Zou, H.; Lin, J.B.; Dai, Y.M.; Wang, W.Y. Effects of Endophyte on the Drought Resistance of Dendrobium officinale Under Drought Stress. North. Hortic. 2020, 6, 119–125. [Google Scholar]
- He, H.Q.; Peng, X.Y.; Tao, S.; Wang, Y.N.; Xu, Y.; Lin, J.X. Effects of Arbuscular Mycorrhizal Fungi on Growth and Antioxidant Enzyme Activities of Leymus chinensis Seedlings Under Salt (Alkali) and Drought Stress. Mod. Agric. Sci. Technol. 2019, 12, 149–150, 152. [Google Scholar] [CrossRef]
- Cheng, S.F.; Yeh, C.H.; Chen, H.J.; Chang, D. Growth and Development of Phaius tankervilleae (Banks) Blume When Inoculated with Orchid Mycorrhizal Fungi. Afr. J. Agric. Res. 2012, 7, 5644–5652. [Google Scholar] [CrossRef]
- Lee, M.C.; Chang, D.C.N.; Wu, C.W.; Wang, Y.T.; Chang, Y.S. Phalaenopsis Efficiently Acclimate to Highlight Environment Through Orchid Mycorrhization. Sci. Hortic. 2014, 179, 184–190. [Google Scholar] [CrossRef]
- Swangmaneecharern, P.; Serivichyaswat, P.; Nontachaiyapoom, S. Promoting Effect of Orchid Mycorrhizal Fungi Epulorhiza Isolates on Seed Germination of Dendrobium Orchids. Sci. Hortic. 2012, 148, 55–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.Y.; Chen, X.M.; Guo, S.X.; Lee, Y.I. Effect of Different Mycobionts on Symbiotic Germination and Seedling Growth of Dendrobium officinale, an Important Medicinal Orchid. Bot. Stud. 2020, 61, 2. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.Y.; Yang, H.T.; Xi, G.J. Screening of Excellent Mycorrhizal Fungi From Dendrobium officinale. Jiangsu Agric. Sci. 2017, 45, 108–110. [Google Scholar] [CrossRef]
- Sun, J.Q.; Liu, R.J.; Li, M. Advances in the Study of Increasing Plant Stress Resistance and Mechanisms by Arbuscular Mycorrhizal Fungi. Plant Physiol. J. 2012, 48, 845–852. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Guo, S.X. Isolation and Identification of Endophytic and Mycorrhizal Fungi From Seeds and Roots of Dendrobium (Orchidaceae). Mycorrhiza 2012, 22, 297–307. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Vega, F.E. The Use of Fungal Entomopathogens as Endophytes in Biological Control: A Review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in Plant Response to Native and Exotic Arbuscular Mycorrhizal Fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Fan, L.; Guo, S.X. Research Progress of Mycorrhizal Fungi in Orchidaceae. Microbiol. Bull. 1998, 4, 227–230. [Google Scholar]
- Xing, X.K.; Guo, S.X.; Chen, X.M.; Meng, Z.X. Mycorrhizal Microstructure of Dendrobium officinale Cultivated Under Artificial Conditions. J. Fungi 2005, 4, 88–93. [Google Scholar] [CrossRef]
- Meng, P.; Shao, S.C.; Peng, G.H. Effects of Different Endophytic Fungi on Dendrobium officinale Seedling Growth. Guizhou Agric. Sci. 2017, 45, 106–110. [Google Scholar]
- Zhu, Y.M.; Jin, J.Y.; Cui, Y.Y. Effects of Endophytic Fungi on the Growth of Dendrobium officinale Seedlings. Zhejiang Agric. Sci. 2019, 60, 1601–1605. [Google Scholar] [CrossRef]
- Chen, Y.L.; Brundrett, M.C.; Dell, B. Effects of Ectomycorrhizas and Vesicular-Arbuscular Mycorrhizas, Alone or in Competition, on Root Colonization and Growth of Eucalyptus globulus and E. urophylla. New Phytol. 2010, 146, 545–555. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Xu, L.J.; Xia, W.; Liang, L.X.; Bai, X.; Li, L.B.; Xu, L.; Liu, L. Mycorrhizal Compatibility and Germination-Promoting Activity of Tulasnella species in Two Species of Orchid (Cymbidium mannii and Epidendrum radicans). Horticulturae 2021, 7, 472. [Google Scholar] [CrossRef]
- Wang, Q.M.; Yuan, L.F.; Xu, X.C.; Wu, S.G. The Effect of Drought Stress on the Content of Osmotic Adjusting Substance in Leaves and Cell Membrane Permeability of Different Soybean Varieties in Seedling Period. J. Henan Agric. Sci. 2005, 8, 9–12. [Google Scholar] [CrossRef]
- Yu, X.L.; Hu, Q.L.; Huang, Y. Study on Extracting Methods and Characteristics of Chlorophyll in Peony. J. Luoyang Norm. Univ. 2005, 5, 113–115. [Google Scholar] [CrossRef]
- Jin, H.; Kang, Z.H.; Chen, H.; Han, S.F. Effects of Mycorrhizal Fungi on the Growth and Mineral Nutrition Absorption of Dendrobium officinale. J. Fujian For. Univ. 2007, 1, 80–83. [Google Scholar] [CrossRef]
- Jin, H.; Xu, Z.X.; Chen, J.H.; Han, S.F.; Ge, S. Nteraction Between Tissue-Cultured Seedlings of Dendrobium officinale and Mycorrhizal Fungi (Epulorhiza sp.) During Symbiotic Culture. J. Plant Ecol. 2009, 33, 433–441. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.J. Synergistic Association of Endophytic Fungi Enhances Glycine max L. Resilience to Combined Abiotic Stresses: Heavy Metals, High Temperature and Drought Stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- González-Teuber, M.; Urzúa, A.; Plaza, P.; Bascuñán-Godoy, L. Effects of Root Endophytic Fungi on Response of Chenopodium Quinoa to Drought Stress. Plant Ecol. 2018, 219, 231–240. [Google Scholar] [CrossRef]
- Sabra, M.; Aboulnasr, A.; Franken, P.; Perreca, E.; Wright, L.P.; Camehl, I. Beneficial Root Endophytic Fungi Increase Growth and Quality Parameters of Sweet Basil in Heavy Metal Contaminated Soil. Front. Plant Sci. 2018, 9, 1726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Zhang, G.; Zhang, D.W.; Hsiao, Y.Y.; Guo, S.X. ESTs Analysis Reveals Putative Genes Involved in Symbiotic Seed Germination in Dendrobium officinale. PLoS ONE 2013, 8, e72705. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Chen, C.; Yang, J.; Zhu, H.; Liu, M.; Lv, F. Deep Sequencing-based Comparative Transcriptional Profiles of Cymbidium hybridum Roots in Response to Mycorrhizal and Non-mycorrhizal Beneficial Fungi. BMC Genom. 2014, 15, 747. [Google Scholar] [CrossRef]
- Perotto, S.; Rodda, M.; Benetti, A.; Sillo, F.; Ercole, E.; Rodda, M.; Girlanda, M.; Murat, C.; Balestrini, R. Gene Expression in Mycorrhizal Orchid Protocorms Suggests a Friendly Plant-fungus Relationship. Planta 2014, 239, 1337–1349. [Google Scholar] [CrossRef]
- Valadares, R.B.S.; Perotto, S.; Lucheta, A.R.; Santos, E.C.; Oliveira, R.M.; Lambais, M.R. Proteomic and Transcriptomic Analyses Indicate Metabolic Changes and Reduced Defense Responses in Mycorrhizal Roots of Oeceoclades maculata (Orchidaceae) Collected in Nature. J. Fungi 2020, 6, 148. [Google Scholar] [CrossRef]
- Katoch, M.; Singh, A.; Singh, G.; Wazir, P.; Kumar, R. Phylogeny, Antimicrobial, Antioxidant and Enzyme-Producing Potential of Fungal Endophytes Found in Viola odorata. Ann. Microbiol. 2017, 67, 529–540. [Google Scholar] [CrossRef]
- White, J.F., Jr.; Torres, M.S. Is Plant Endophyte-Mediated Defensive Mutualism the Result of Oxidative Stress Protection? Physiol. Plant 2010, 138, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Hagh-Doust, N.; Färkkilä, S.M.A.; Hosseyni Moghaddam, M.S.; Tedersoo, L. Symbiotic Fungi as Biotechnological Tools: Methodological Challenges and Relative Benefits in Agriculture and Forestry. Fungal Biol. Rev. 2022, in press. [CrossRef]
- Ali, A.H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. Effect of ther Momyces Fungal Endophyte Isolated From Extreme Hot Desert-Adapted Plant on Heat Stress Tolerance of Cucumber. Appl. Soil Ecol. 2018, 124, 155–162. [Google Scholar] [CrossRef]
- Anith, K.N.; Aswini, S.; Varkey, S.; Radhakrishnan, N.V.; Nair, D.S. Root Colonization by the Endophytic Fungus Piriformospora indica Improves Growth, Yield and Piperine Content in Black Pepper (Piper Nigurm L.). Biocatal. Agric. Biotechnol. 2018, 14, 215–220. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.J. Endophytic Fungus Aspergillus Japonicus Mediates Host Plant Growth under Normal and Heat Stress Conditions. Biomed. Res. 2018, 11, 7696831. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, X.; Wang, J. Phillyrin Produced by Colletotrichum Gloeosporioides, an Endophytic Fungus Isolated From Forsythia suspensa. Fitoterapia 2012, 83, 1500–1505. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, L.H.; Zhong, J.; Duan, L.S. Effects of Different Mycorrhizal Fungi on Growth and Root System of Plug Seedlings of Dendrobium officinale. Acta Agric. Boreali-Occident. Sin. 2021, 37, 31–36. [Google Scholar] [CrossRef]
- Chen, X.M.; Yan, H.L.; Wang, C.L.; Tian, L.X.; Wang, A.R.; Guo, S.X. Effects of the Mycorrhizal Fungi Mycena sp. on the Growth and Chemical Properties of Dendrobium officinale. Sci. China Life Sci. 2016, 46, 872–879. [Google Scholar]
- Marković, S.M.; Živančev, D.; Horvat, D.; Torbica, A.; Jovankić, J.; Djukić, N.H. Correlation of Elongation Factor 1A Accumulation With Photosynthetic Pigment Content and Yield in Winter Wheat Varieties Under Heat Stress Conditions. Plant Physiol. Biochem. 2021, 166, 572–581. [Google Scholar] [CrossRef]
- Kang, Z.H.; Han, S.F.; Han, Z.M. Effects of Orchidaceous Rhizoctonias on the Growth of Dendrobium candidum. J. Nanjing For. Univ. Nat. Sci. Ed. 2007, 5, 49–52. [Google Scholar] [CrossRef]
- Cui, P.Q.; Jiang, W.B.; Weng, M.L.; Zhang, B.B.; Mao, N.N. Pigment Contents and Net Photosynthetic Rates of Leaves in Purple-Leaf Plum Seedling Under Shading. Northwest. Bot. J. 2010, 30, 2286–2292. [Google Scholar]
- He, T.G.; Yang, L.T.; Li, Y.R.; Wang, C.Q.; Hu, J.S. Effects of the Polysaccharides DCPPla-1 From Suspension Cultured Protocorm of Dendrobium officinale on Oxygen Radical and Lipid Peroxidation. Res. Dev. Nat. Prod. 2007, 3, 410–414. [Google Scholar] [CrossRef]
- He, T.G.; Yang, L.T.; Li, Y.R.; Wang, C.Q.; Huang, D.P. Physicochemical Properties and Antitumor Activity of Polysaccharide DCPP1a-1 From Suspension Cultured Protocorm of Dendrobium officinale. Res. Dev. Nat. Prod. 2007, 4, 578–583. [Google Scholar] [CrossRef]
- Chen, X.M.; Yan, H.L.; Wang, C.L.; Tian, L.X.; Wang, A.R.; Guo, S.X. Effects of Mycorrhizal Fungus Mycena sp. on the Growth and Polysaccharide Properties of Dendrobium officinale. Sci. China Life Sci. 2016, 59, 974–976. [Google Scholar] [CrossRef]
- Wu, L.S.; Dong, W.G.; Si, J.P.; Liu, J.J.; Zhu, Y.Q. Endophytic Fungi, Host Genotype, and Their Interaction Influence the Growth and Production of Key Chemical Components of Dendrobium Catenatum. Fungal Biol. 2020, 124, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Xu, Q.; Bian, C.; Tsai, W.C.; Yeh, C.M.; Liu, K.W.; Yoshida, K.; Zhang, L.S.; Chang, S.B.; Chen, F.; et al. The Dendrobium Catenatum Lindl. Genome Sequence Provides Insights Into Polysaccharide Synthase, Floral Development and Adaptive Evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Tsavkelova, E.A.; Zeng, S.; Ng, T.B.; Parthibhan, S.; Dobránszki, J.; Cardoso, J.C.; Rao, M.V. Symbiotic in Vitro Seed Propagation of Dendrobium: Fungal and Bacterial Partners and Their Influence on Plant Growth and Development. Planta 2015, 242, 1–22. [Google Scholar] [CrossRef]
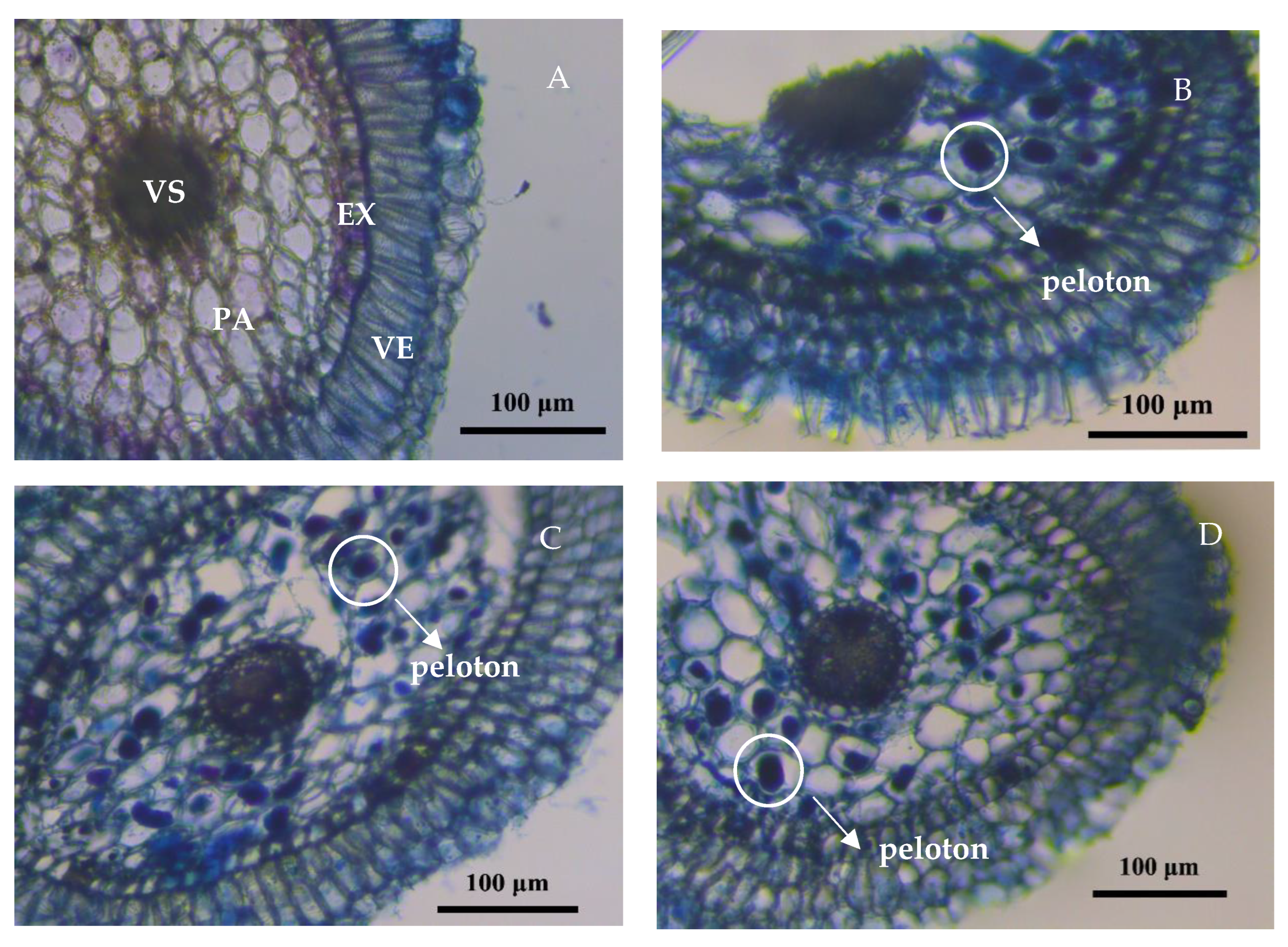

| Treatment Group | Species | ITS Sequence Accession Number (NCBI) | Plant Source |
|---|---|---|---|
| DSs | Serendipita sp. | OM778283 | Dendrobium officinale |
| CTc | Tulasnella calospora | OM756775 | Cymbidium goeringii |
| ETa | Tulasnella asymmetrica | OM778264 | Epidendrum radicans |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huo, W.; Hou, J.; Liu, L.; Yu, X.; Xu, L. Effects and Benefits of Orchid Mycorrhizal Symbionts on Dendrobium officinale. Horticulturae 2022, 8, 861. https://doi.org/10.3390/horticulturae8100861
Zhang Y, Huo W, Hou J, Liu L, Yu X, Xu L. Effects and Benefits of Orchid Mycorrhizal Symbionts on Dendrobium officinale. Horticulturae. 2022; 8(10):861. https://doi.org/10.3390/horticulturae8100861
Chicago/Turabian StyleZhang, Yifan, Wenwen Huo, Jiayi Hou, Lei Liu, Xiaoying Yu, and Lu Xu. 2022. "Effects and Benefits of Orchid Mycorrhizal Symbionts on Dendrobium officinale" Horticulturae 8, no. 10: 861. https://doi.org/10.3390/horticulturae8100861
APA StyleZhang, Y., Huo, W., Hou, J., Liu, L., Yu, X., & Xu, L. (2022). Effects and Benefits of Orchid Mycorrhizal Symbionts on Dendrobium officinale. Horticulturae, 8(10), 861. https://doi.org/10.3390/horticulturae8100861




