Micropropagation of Feverfew (Tanacetum parthenium) and Quantification of Parthenolide Content in Its Micropropagated and Conventionally Grown Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. T. parthenium Micropropagation
2.1.1. Plant Materials and Reagents
2.1.2. Seed Surface Sterilization and Culture Initiation
2.1.3. Callus Induction
2.1.4. Shoot Induction
2.1.5. Rooting
2.1.6. Acclimatization
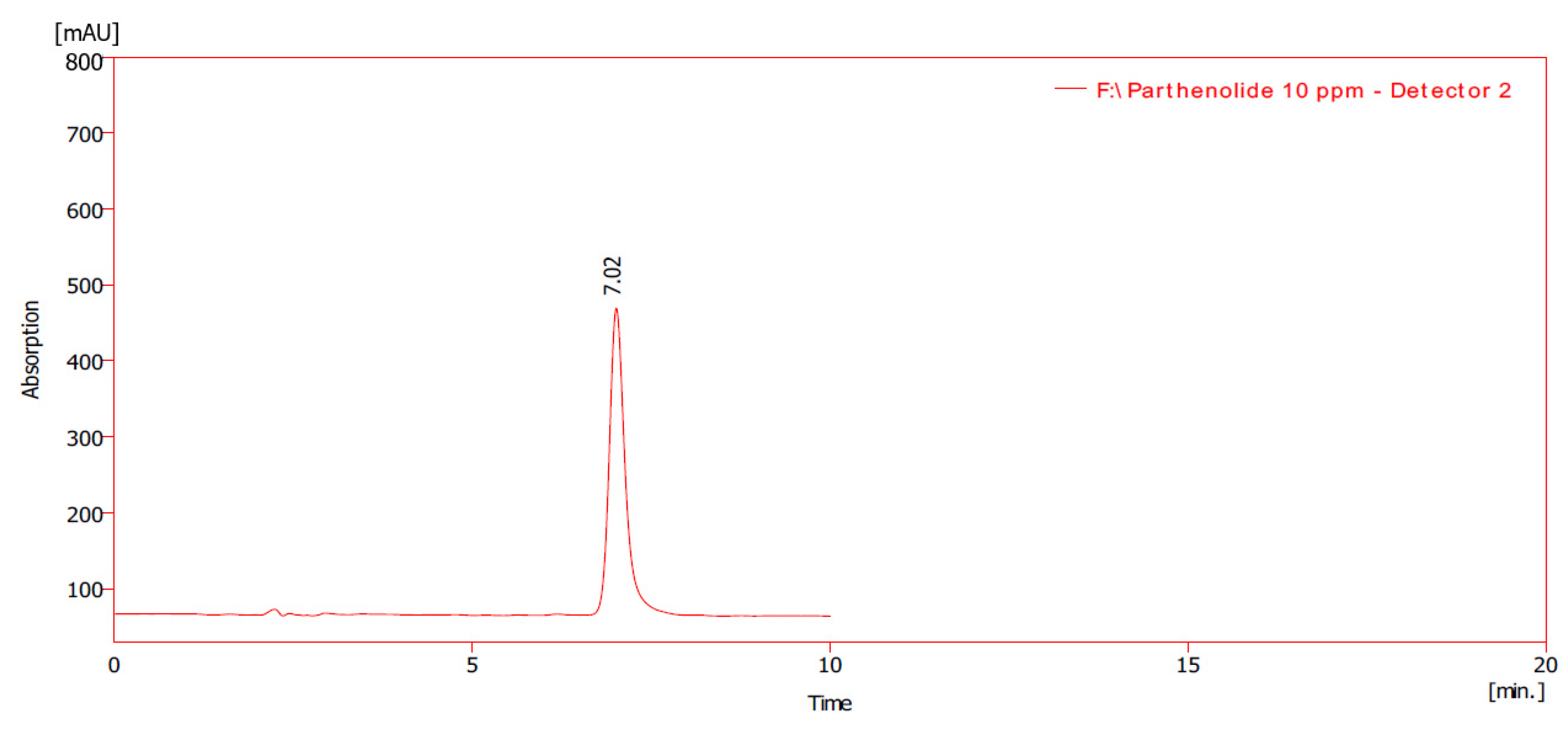
2.2. Parthenolide Quantification of the Feverfew Plant
2.2.1. Preparation of Sample and Standard
2.2.2. Quantification of Parthenolide Content
2.3. Statistical Analysis
3. Results and Discussion
3.1. Micropropagation of the Feverfew Plant
3.1.1. Seed Surface Sterilization
3.1.2. Callus Induction
3.1.3. Shoot Induction
3.1.4. Root Induction
3.1.5. Acclimatization of the Micropropagated Feverfew Plantlets
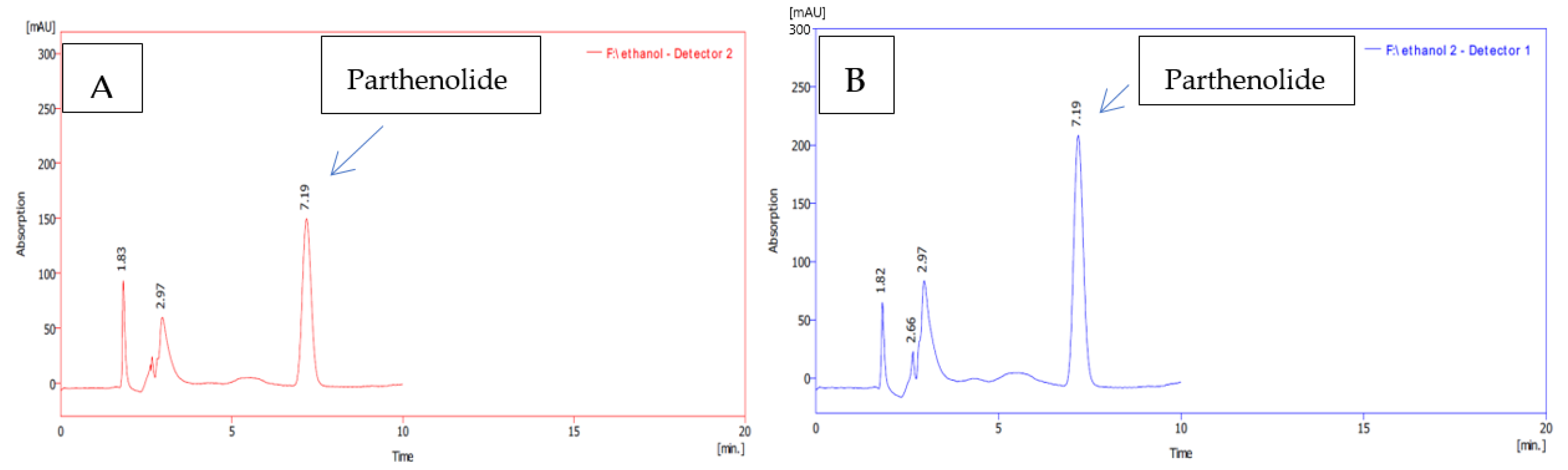
3.2. Quantification of Parthenolide Content of the Feverfew Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pareek, A.; Suthar, M.; Rathore, G.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Pourianezhad, F.; Tahmasebi, S.; Abdusi, V.; Nikfar, S.; Mirhoseini, M. Review on feverfew, a valuable medicinal plant. J. HerbMed. Pharmacol. 2016, 5, 45–49. [Google Scholar]
- Sadat-Hosseini, M.; Farajpour, M.; Boroomand, N.; Solaimani-Sardou, F. Ethnopharmacological studies of indigenous medicinal plants in the south of Kerman, Iran. J. Ethnopharmacol. 2017, 199, 194–204. [Google Scholar] [CrossRef]
- Mahmoodzadeh, Y.; Mazani, M.; Rezagholizadeh, L. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol. Rep. 2017, 4, 455–462. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Tenci, B.; Zanardelli, M.; Maidecchi, A.; Lugli, A.; Mattoli, L.; Ghelardini, C. Widespread pain reliever profile of a flower extract of Tanacetum parthenium. Phytomedicine 2015, 22, 752–758. [Google Scholar] [CrossRef]
- Penthala, N.R.; Janganati, V.; Alpe, T.L.; Apana, S.M.; Berridge, M.S.; Crooks, P.A.; Borrelli, M.J. N-[11CH3]Dimethylaminoparthenolide (DMAPT) uptake into orthotopic 9LSF glioblastoma tumors in the rat. Bioorganic. Med. Chem. Lett. 2016, 26, 5883–5886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyz, M.; Lesiak-Mieczkowska, K.; Koprowska, K.; Szulawska-Mroczek, A.; Wozniak, M. Cell context-dependent activities of parthenolide in primary and metastatic melanoma cells. Br. J. Pharmacol. 2010, 160, 1144–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranello, M.P.; Bauer, L.; Jordan, C.T.; Benoit, D.S.W. Micelle delivery of parthenolide to acute myeloid leukemia cells. Cell. Mol. Bioeng. 2015, 8, 455–470. [Google Scholar] [CrossRef] [Green Version]
- Flores-Lopez, G.; Moreno-Lorenzana, D.; Ayala-Sanchez, M.; Aviles-Vazquez, S.; Torres-Martinez, H.; Crooks, P.A.; Guzman, M.L.; Mayani, H.; Chávez-González, A. Parthenolide and DMAPT induce cell death in primitive CML cells through reactive oxygen species. J. Cell. Mol. Med. 2018, 22, 4899–4912. [Google Scholar] [CrossRef] [PubMed]
- Czyz, M.; Koprowska, K.; Sztiller-Sikorska, M. Parthenolide reduces the frequency of ABCB5-positive cells and clonogenic capacity of melanoma cells from anchorage independent melanospheres. Cancer Biol. Ther. 2013, 14, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majdi, M.; Abdollahi, M.R.; Maroufi, A. Parthenolide accumulation and expression of genes related to parthenolide biosynthesis affected by exogenous application of methyl jasmonate and salicylic acid in Tanacetum parthenium. Plant Cell Rep. 2015, 34, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, M.; Rahaie, M.; Ebrahimi, A. The effect of TiO2 and SiO2 nanoparticles and salinity stress on expression of genes involved in parthenolide biosynthesis in feverfew (Tanacetum parthenium L.). Caryologia 2019, 72, 3–14. [Google Scholar]
- Nieto-Trujillo, A.; Buendía-González, L.; García-Morales, C.; Román-Guerrero, A.; Cruz-Sosa, F.; Estrada-Zúñiga, M.E. Phenolic compounds and parthenolide production from in vitro cultures of Tanacetum parthenium. Rev. Mex. Ing. Quim. 2017, 16, 371–383. [Google Scholar]
- Mahood, H.E. Effect of plant growth regulators and explant source on the induction of callus of Dianthus caryophyllus L. Basrah J. Agric. Sci. 2021, 34, 100–106. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Pourianezhad, F.; Rahnama, H.; Mousavi, A.; Khosrowshahli, M.; Mafakheri, S. Parthenolide production in cell suspension culture of feverfew. Bioresour. Bioprocess. 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Pourianezhad, F.; Rahnama, H.; Mousavi, A.; Khosrowshahli, M.; Mafakheri, S. Effects of combined elicitors on parthenolide production and expression of parthenolide synthase (TpPTS) in Tanacetum parthenium hairy root culture. Plant Biotechnol. Rep. 2019, 13, 211–218. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hakiman, M.; Maziah, M. Non enzymatic and enzymatic antioxidant activities in aqueous extract of different Ficus deltoidea accessions. J. Med. Plants Res. 2009, 3, 120–131. [Google Scholar]
- Haida, Z.; Syahida, A.; Ariff, S.M.; Maziah, M.; Hakiman, M. Factors affecting cell biomass and flavonoid production of Ficus deltoidea var. kunstleri in cell suspension culture system. Sci. Rep. 2019, 9, 9533. [Google Scholar] [CrossRef]
- Aburashed, E.A.; Khan, I.A. Determination of parthenolide in selected feverfew products by liquid chromatography. J. AOAC Int. 2000, 83, 789–801. [Google Scholar] [CrossRef] [Green Version]
- García-Pérez, P.; Lozano-Milo, E.; Landín, M.; Gallego, P.P. Machine learning technology reveals the concealed interactions of phytohormones on medicinal plant in vitro organogenesis. Biomolecules 2020, 10, 746. [Google Scholar] [CrossRef]
- Telci, C.; Yildiz, M.; Pelit, S.; Onol, B.; Erkilic, E.G.; Kendir, H. The effect of surface-disinfection process on dormancy-breaking, seed germination, and seedling growth of Lathyrus chrysanthus bois. under in vitro conditions. Propag. Ornam. Plants 2011, 11, 10–16. [Google Scholar]
- Cui, J.; Wei, X.; Deng, M.; Chen, J. Regeneration of Gynura aurantiaca ‘Purple Passion’ via indirect shoot organogenesis. Sci. Hortic. 2019, 246, 176–181. [Google Scholar] [CrossRef]
- Sié, R.S.; Charles, G.; Sakhanokho, H.F.; Toueix, Y.; Djè, Y.; Sangaré, A.; Branchard, M. Protocols for callus and somatic embryo initiation for Hibiscus sabdariffa L. (Malvaceae): Influence of explant type, sugar, and plant growth regulators. Aust. J. Crop Sci. 2010, 4, 98–106. [Google Scholar]
- Dewir, Y.H.; Nurmansyah; Naidoo, Y.; da Silva, J.A.T. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.L.; Wei, Y.H. Thidiazuron: A multi-dimensional plant growth regulator. African J. Biotechnol. 2011, 10, 8984–9000. [Google Scholar]
- Erland, L.A.E.; Giebelhaus, R.T.; Victor, J.M.R.; Murch, S.J.; Saxena, P.K. The morphoregulatory role of thidiazuron: Metabolomics-guided hypothesis generation for mechanisms of activity. Biomolecules 2020, 10, 1253. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Sun, H.; Wusiman, N.; Sun, W.; Li, B.; Gao, Y.; Kong, J.; Zhang, D.; Zhang, X.; et al. Crosstalk between cytokinin and ethylene signaling pathways regulates leaf abscission in cotton in response to chemical defoliants. J. Exp. Bot. 2019, 70, 1539–1551. [Google Scholar] [CrossRef] [Green Version]
- Zahid, N.A.; Jaafar, H.Z.E.; Hakiman, M. Micropropagation of ginger (Zingiber officinale Roscoe) ‘Bentong’ and evaluation of its secondary metabolites and antioxidant activities compared with the conventionally propagated plant. Plants 2021, 10, 630. [Google Scholar] [CrossRef]
- Bakhshipour, M.; Mafakheri, M.; Kordrostami, M.; Zakir, A.; Rahimi, N.; Feizi, F.; Mohseni, M. In vitro multiplication, genetic fidelity and phytochemical potentials of Vaccinium arctostaphylos L.: An endangered medicinal plant. Ind. Crops Prod. 2019, 141, 111812. [Google Scholar] [CrossRef]
- Najhah, M.Y.; Jaafar, H.Z.E.; Nakasha, J.J.; Hakiman, M. Shoot multiplication and callus induction of labisia pumila var. Alata as influenced by different plant growth regulators treatments and its polyphenolic activities compared with the wild plant. Molecules 2021, 26, 3229. [Google Scholar] [CrossRef]
- Zahid, N.A.; Jaafar, H.Z.E.; Hakiman, M. Alterations in microrhizome induction, shoot multiplication and rooting of ginger (Zingiber officinale Roscoe) var. Bentong with regards to sucrose and plant growth regulators application. Agronomy 2021, 11, 320. [Google Scholar] [CrossRef]
- Jogam, P.; Sandhya, D.; Shekhawat, M.S.; Alok, A.; Manokari, M.; Abbagani, S.; Allini, V.R. Genetic stability analysis using DNA barcoding and molecular markers and foliar micro-morphological analysis of in vitro regenerated and in vivo grown plants of Artemisia vulgaris L. Ind. Crops Prod. 2020, 151, 112476. [Google Scholar] [CrossRef]
- Pradhan, N.; Dwivedi, P. In vitro shoot multiplication of Stevia rebaudiana, an important plant with high economic and medicinal values. Vegetos 2016, 29, 1000180. [Google Scholar] [CrossRef]
- Joshi, B.; Bhandari, A.; Panwar, G.S. An efficient micropropagation protocol for Vernonia amygdalina Delile—An economically valuable shrub. J. Herbs Spices Med. Plants 2020, 26, 267–274. [Google Scholar] [CrossRef]
- Prashanth, S.; Pooja, S.; Suchetha, K.N.; Vidya, V.K. Radical scavenging and antioxidant activities of ethanolic and aqueous extract from the leaves of feverfew (Tanacetum parthenium L.) and a synthetic compound parthenolide. J. Pharmacogn. Phytochem. 2015, 4, 223–227. [Google Scholar]
- Tadić, V.; Živković, J.; Bigović, D.; Žugić, A. Variation of parthenolide and phenolic compounds content in different parts of Tanacetum parthenium (L.) Schulz Bip., Asteraceae during 18 months storage. Lek. Sirovine 2019, 39, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Tomsone, L.; Kruma, Z. Comparison of different solvents for isolation of phenolic compounds from horseradish (Armoracia rusticana L.) leaves. Res. Rural Dev. 2013, 1, 104–110. [Google Scholar]
| NaOCl (%) | Aseptic Seeds (%) | Seed Germination (%) |
|---|---|---|
| 2 | 31.00 ± 1.00 d | 90.00 ± 1.49 a |
| 3 | 33.00 ± 1.53 d | 90.00 ± 2.11 a |
| 4 | 41.00 ± 1.80 c | 90.00 ± 2.58 a |
| 5 | 47.00 ± 2.13 b | 81.00 ± 1.80 b |
| 6 | 65.00 ± 2.69 a | 73.00 ± 2.60 b |
| 2,4-D (mg/L) | TDZ (mg/L) | Callus Induction% |
|---|---|---|
| 0 | 0.0 | 0.00 ± 0.00 i |
| 0 | 0.2 | 33.00 ± 3.19 g |
| 0 | 0.3 | 47.00 ± 2.53 ef |
| 0 | 0.4 | 62.00 ± 3.82 c |
| 1 | 0.0 | 0.00 ± 0.00 i |
| 1 | 0.2 | 43.00 ± 2.34 f |
| 1 | 0.3 | 72.00 ± 4.71 b |
| 1 | 0.4 | 75.00 ± 4.34 b |
| 2 | 0.0 | 0.00 ± 0.00 i |
| 2 | 0.2 | 23.00 ± 1.17 h |
| 2 | 0.3 | 84.00 ± 1.65 a |
| 2 | 0.4 | 86.00 ± 1.72 a |
| 3 | 0.0 | 0.00 ± 0.00 i |
| 3 | 0.2 | 53.00 ± 2.78 de |
| 3 | 0.3 | 61.00 ± 3.73 cd |
| 3 | 0.4 | 62.00 ± 2.36 c |
| Cytokinin (mg/L) | Shoot Induction (%) | Number of days to Shoot initiation | Number of Shoots/Explant | Shoot Length (cm) | Number of Leaves/Shoot |
|---|---|---|---|---|---|
| Control (0) | 60.00 ± 4.08 c | 12.00 ± 0.47 a | 2.10 ± 0.23 d | 2.00 ± 0.33 c | 1.25 ± 0. 16 c |
| Kinetin (5) | 72.50 ± 2.50 b | 7.00 ± 0.21 c | 3.00 ± 0.26 c | 2.10 ± 0.02 bc | 3.00 ± 0.15 a |
| BA (5) | 80.00 ± 3.33 b | 9.00 ± 0.52 b | 3.00 ± 0.26 c | 2.40 ± 0.07 bc | 2.00 ± 0.21 b |
| Zeatin (5) | 100.00 ± 0.00 a | 7.00 ± 0.26 c | 5.00 ± 0.15 a | 2.50 ± 0.05 ab | 2.10 ± 0.18 b |
| TDZ (2) | 100.00 ± 0.00 a | 9.00 ± 0.21 b | 4.00 ± 0.26 b | 2.90 ± 0.02 a | 2.20 ± 0.13 ab |
| Treatment | Root Induction (%) | Time to Root Induction (Days) | Number of Roots/Plantlet | Root Length (cm) |
|---|---|---|---|---|
| Control | 90.00 ± 4.08 a | 7.00 ± 0.42 a | 4.10 ± 0.28 b | 2.00 ± 0.08 b |
| IAA | 90.00 ± 5.53 a | 7.00 ± 0.54 a | 5.00 ± 0.61 b | 2.20 ± 0.14 b |
| IBA | 90.00 ± 6.67 a | 7.00 ± 0.47 a | 8.90 ± 0.35 a | 3.80 ± 0.19 a |
| NAA | 90.00 ± 7.64 a | 7.00 ± 0.56 a | 8.10 ± 0.57 a | 3.40 ± 0.15 a |
| Propagation Method | Solvent | Parthenolide (mg/g DW) |
|---|---|---|
| Conventional | Aqueous | 23.23 ± 0.13 e |
| Ethanol | 28.40 ± 0.06 b | |
| Acetone | 25.60 ± 0.10 d | |
| Hexane | 17.30 ± 0.06 f | |
| Micropropagation | Aqueous | 25.50 ± 0.29 d |
| Ethanol | 30.60 ± 0.12 a | |
| Acetone | 27.90 ± 0.06 c | |
| Hexane | 17.50 ± 0.06 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahood, H.E.; Abbas, M.K.; Zahid, N.A. Micropropagation of Feverfew (Tanacetum parthenium) and Quantification of Parthenolide Content in Its Micropropagated and Conventionally Grown Plants. Horticulturae 2022, 8, 50. https://doi.org/10.3390/horticulturae8010050
Mahood HE, Abbas MK, Zahid NA. Micropropagation of Feverfew (Tanacetum parthenium) and Quantification of Parthenolide Content in Its Micropropagated and Conventionally Grown Plants. Horticulturae. 2022; 8(1):50. https://doi.org/10.3390/horticulturae8010050
Chicago/Turabian StyleMahood, Huda E., Majeed Kadhem Abbas, and Nisar Ahmad Zahid. 2022. "Micropropagation of Feverfew (Tanacetum parthenium) and Quantification of Parthenolide Content in Its Micropropagated and Conventionally Grown Plants" Horticulturae 8, no. 1: 50. https://doi.org/10.3390/horticulturae8010050
APA StyleMahood, H. E., Abbas, M. K., & Zahid, N. A. (2022). Micropropagation of Feverfew (Tanacetum parthenium) and Quantification of Parthenolide Content in Its Micropropagated and Conventionally Grown Plants. Horticulturae, 8(1), 50. https://doi.org/10.3390/horticulturae8010050







