Optimization of an In Vitro Embryo Rescue Protocol for Breeding Seedless Table Grapes (Vitis vinifera L.) in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials: Parent Vines and Hybridization
2.2. Emasculation and Pollination
2.3. Sampling Time
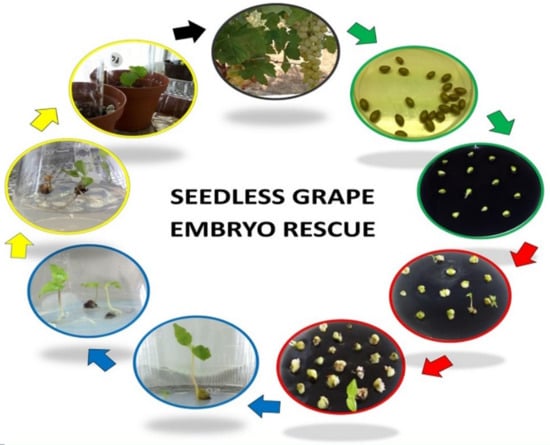
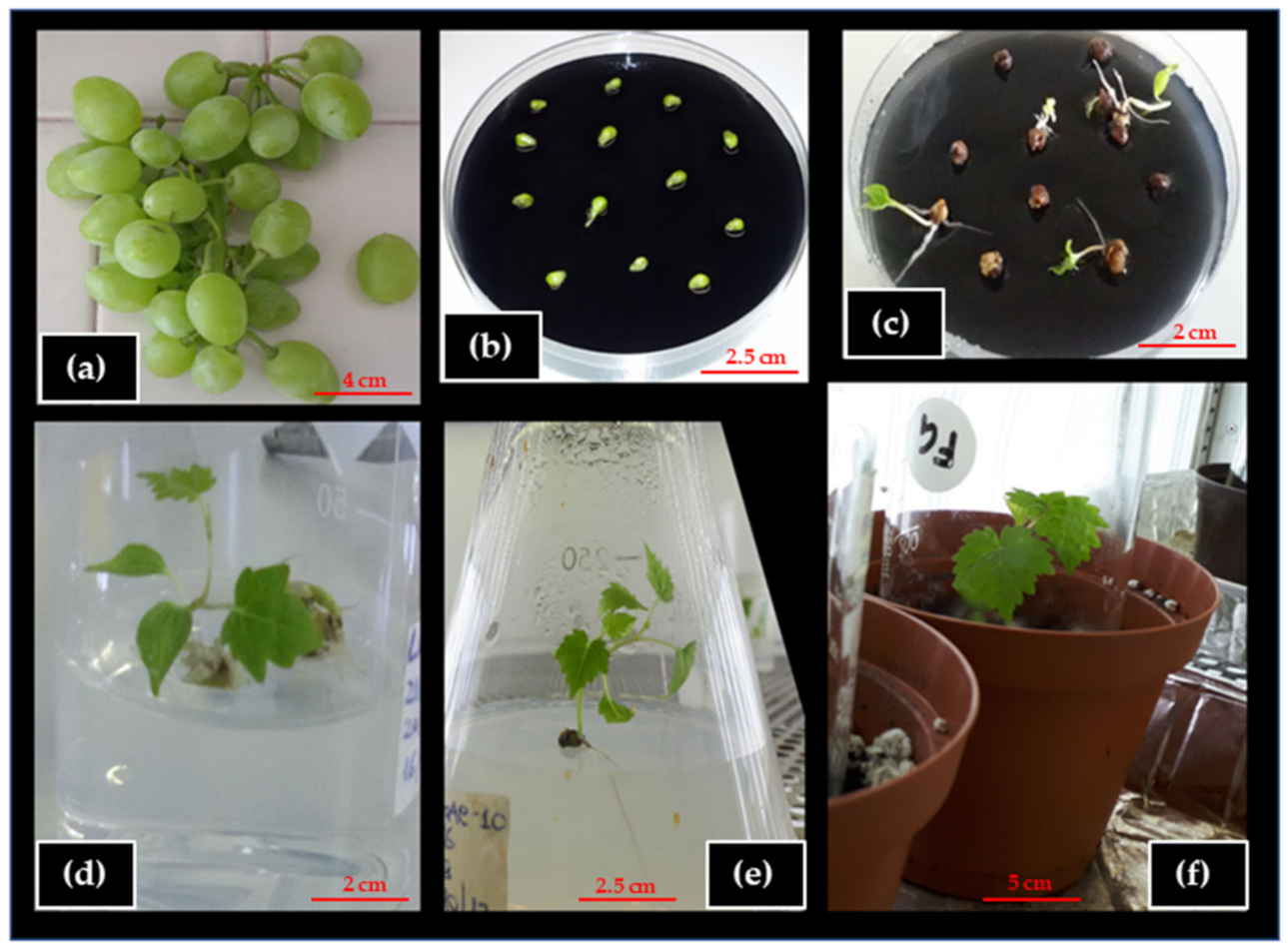
2.4. Ovule Culture and Embryo Growth
2.5. Embryo Germination, Rooting, Plantlet Formation, and Acclimation
2.6. Culture Media Composition
2.7. Experimental Design and Statistical Analyses
3. Results and Discussion
3.1. Effect of Genotype and Sampling Time on Ovule Fertilization Rate
3.2. Effect of Genotype and Sampling Time on Embryo Formation and Germination
3.3. Effect of Genotype and Sampling Time on Shoot Rooting and Plantlet Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Geng, X.; Chen, S.; Liu, K.; Yu, S.; Wang, X.; Zhang, C.; Zhang, J.; Wan, Y.; Luo, Q.; et al. The co-expression of genes involved in seed coat and endosperm development promotes seed abortion in grapevine. Planta 2021, 254, 87. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.V.; Singh, S.K.; Singh, A.K. Standardization of embryo rescue technique and bio-hardening of grape hybrids (Vitis vinifera L.) using Arbuscular mycorrhizal fungi (AMF) under subtropical conditions. Vitis 2011, 50, 115–118. [Google Scholar]
- Liu, S.M.; Sykes, S.R.; Clingeleffer, P.R. Improved in ovulo embryo culture for stenospermocarpic grapes (Vitis vinifera L.). Aust. J. Agric. Res. 2003, 54, 869–876. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, B.; Li, P.; Shu, X.; Zhang, X.; Zhang, J. New coldresistant, seedless grapes developed using embryo rescue and marker-assisted selection. Plant Cell Tissue Organ Cult. 2020, 140, 551–562. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.-M.; Renault, J.-H.; Clément, C.; Martiny, L.; Delmas, D.; et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a Novel Anti-Herpes Simplex Virus Nutraceutical Agent: An Overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef]
- Kumar, S.; Chang, Y.-C.; Lai, K.-H.; Hwang, T.-L. Resveratrol, a molecule with anti-inflammatory and anti-cancer activities: Natural product to chemical synthesis. Curr. Med. Chem. 2021, 28, 3773–3786. [Google Scholar] [CrossRef]
- Li, G.R.; Ji, W.; Wang, G.; Zhang, J.X.; Wang, Y.J. An improved embryo-rescue protocol for hybrid progeny from seedless Vitis vinifera grapes x wild Chinese Vitis species. In Vitro Cell. Dev. Biol.-Plant. 2014, 50, 110–120. [Google Scholar] [CrossRef]
- Akkurt, M.; Tahmaz, H.; Veziroglu, S. Recent developments in seedless grapevine breeding. S. Afr. J. Enol. Vitic. 2019, 40, 1. [Google Scholar] [CrossRef]
- Ramming, D.W.; Emershad, R.L.; Tarailn, R.A. stenospermocarpic, seedless Vitis vinifera × Vitis rotundifolia hybrid developed by embryo rescue. Hort. Sci. 2000, 3, 732–734. [Google Scholar] [CrossRef]
- Notsuka, K.; Tsuru, T.; Shiraishi, M. Seedless-seedless grape hybridization via in-ovulo embryo culture. J. Jpn. Soc. Hort. Sci. 2001, 70, 7–15. [Google Scholar] [CrossRef]
- Angulo, O.; Fidelibus, M.W.; Heymann, H. Grape cultivar and drying method affect sensory characteristics and consumer preference of raisins. J. Sci. Food Agric. 2007, 87, 865–870. [Google Scholar] [CrossRef]
- Aujla, K.M.; Shah, N.A.; Ishaq, M.; Farooq, A. Post-harvest losses and marketing of grapes in Pakistan. Sarhad J. Agric. 2011, 27, 485–490. [Google Scholar]
- Li, S.; Li, Z.; Zhao, Y.; Zhao, J.; Luo, Q.; Wang, Y. New disease-resistant, seedless grapes are developed using embryo rescue and molecular markers. 3 Biotech 2020, 10, 4. [Google Scholar] [CrossRef]
- Stout, A.B. Seedlessness in grapes. Hortic. Rev. 1936, 11, 159–184. [Google Scholar]
- Lahogue, F.; This, P.; Bouquet, A. Identification of a codominant scar marker linked to the seedlessness character in grapevine. Theor. Appl. Genet. 1998, 97, 950–959. [Google Scholar] [CrossRef]
- Gray, D.J.; Mortensen, J.A.; Benton, C.M.; Durham, R.E.; Moore, G.A. Ovule culture to obtain progeny from hybrid seedless bunch grapes. J. Am. Soc. Hortic. Sci. 1990, 115, 1019–1024. [Google Scholar] [CrossRef]
- Agüero, C.B.; Riquelme, C.; Tizio, R. Embryo rescue from seedless grapevines (Vitis vinifera L.) treated with growth retardants. Vitis 1995, 34, 73–76. [Google Scholar]
- Valdez, J.G.; Ulanovsky, S.M. In vitro germination of stenospermic seeds from reciprocal crosses (Vitis vinifera L.) applying different techniques. Vitis 1997, 36, 105–107. [Google Scholar]
- Tian, L.L.; Wang, Y.J.; Niu, L.; Tang, D.M. Breeding of disease resistant seedless grapes using Chinese wild Vitis spp. I. In Vitro embryo rescue and plant development. Sci. Hortic. 2008, 117, 136–141. [Google Scholar] [CrossRef]
- Gribaudo, I.; Zanetti, R.; Botta, R.; Vallania, R.; Eynard, I. In Ovulo embryo culture of stenospermocarpic grapes. Vitis 1993, 32, 9–14. [Google Scholar]
- Bharathy, P.V.; Karibasappa, G.S. Influence of pre-bloom sprays of benzyladenine on in vitro recovery of hybrid embryos from crosses of thompson seedless and 8 seeded varieties of grape (Vitis spp.). Vitis 2003, 42, 199–202. [Google Scholar]
- This, P.; Lahogue, F.; Adam-Blondon, A.F.; Doligez, A. Towards marker-assisted selection for seedlessness in grapevine. Acta Hort. 2000, 528, 221–229. [Google Scholar] [CrossRef]
- Ramming, D.W. The use of embryo culture in fruit breeding. Hort. Sci. 1990, 25, 393–398. [Google Scholar] [CrossRef]
- Liu, S.M.; Sykes, S.R.; Clingeleffer, P.R. Effect of culture medium, genotype, and year of cross on embryo development and recovery from in vitro cultured ovules in breeding stenospermocarpic seedless grape varieties. Aust. J. Agric. Res. 2008, 59, 175–182. [Google Scholar] [CrossRef]
- Narayanaswami, S.; Norstog, K. Plant embryo culture. Bot. Rev. 1964, 30, 587–628. [Google Scholar] [CrossRef]
- Emershad, R.L.; Ramming, D.W. In-ovulo embryo culture of Vitis vinifera L. cv. ‘Thompson Seedless’. Hort. Sci. 1982, 17, 576. [Google Scholar]
- Ponce, M.T.; Agüero, C.B.; Gregori, M.T.; Tizio, R. Factors aaffecting the development of stenospermic grape (Vitis vinifera) embryos cultured in vitro. In VII International Symposium on Grapevine Genetics and Breeding; ISHS Acta Horticulturae 528; ISHS: Montpellier, France, 2000; Volume 2, pp. 667–672. [Google Scholar]
- Tang, D.; Wang, Y.; Cai, J.; Zhao, R. Effects of exogenous application of plant growth regulators on the development of ovule and subsequent embryo rescue of stenospermic grape (Vitis vinifera L.). Sci. Hortic. 2009, 120, 51–57. [Google Scholar] [CrossRef]
- Sharma, D.R.; Kaur, R.; Kumar, K. Embryo rescue in plants-a review. Euphytica 1996, 89, 325–337. [Google Scholar] [CrossRef]
- Li, Z.; Li, T.; Wang, Y.; Xu, Y. Breeding new seedless grapes using in ovulo embryo rescue and marker-assisted selection. In Vitro Cell Dev. Biol. Plant 2015, 51, 241–248. [Google Scholar] [CrossRef]
- Wang, A.L.; Wang, Y.J.; Tang, D.M.; Zhang, C.H. Research on improvement of seedling rate in embryo rescue of seed less grape. Sci. Agric. Sin. 2010, 43, 4238–4245. [Google Scholar]
- Ji, W.; Li, Z.Q.; Zhou, Q.; Yao, W.K.; Wang, Y.J. Breeding new seedless grape by means of in vitro embryo rescue. Genet. Mol. Res. 2013, 12, 859–869. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, G.; Yan, A.; Xu, H. The study of triploid progenies crossed between different ploidy grapes. Afr. J. Biotechnol. 2011, 10, 5967–5971. [Google Scholar]
- Yang, D.; Li, W.; Li, S.; Yang, X.; Wu, J.; Cao, Z. In vitro embryo rescue culture of F1 progenies from crosses between diploid and tetraploid grape varieties. Plant Growth Regul. 2007, 51, 63–71. [Google Scholar] [CrossRef]
- Guo, Y.S.; Li, K.; Zhao, Y.H.; Guo, X.W. Study of embryo rescue on cross between tetraploid table grape (Vitis vinifera L.) and Vitis amurensis Rupr. In Proceedings of the 2nd Conference Horticulture Science Technology, Beijing, China, 18–19 December 2010; pp. 147–149. [Google Scholar]
- Ramming, D.W.; Walker, M.A.; Tenscher, A.; Krivanek, A.F. Breeding table and raisin grapes with increased fruit quality while retaining Pierce’s disease resistance. Acta Hortic. 2009, 827, 445–450. [Google Scholar] [CrossRef]
- Druart, P. Aneuploids and variants of apple (Malus domestica Borkh.) through in vitro culture techniques. Acta Hortic. 2000, 520, 301–307. [Google Scholar] [CrossRef]
- Xie, K.D.; Yuan, D.Y.; Wang, W.; Xia, Q.M.; Wu, X.M.; Chen, C.W.; Chen, C.L.; Grosser, J.W.; Guo, W.W. Citrus triploid recovery based on 2x × 4x crosses via an optimized embryo rescue approach. Sci. Hortic. 2019, 252, 104–109. [Google Scholar] [CrossRef]
- Uma, S.; Saraswathi, M.S.; Akbar, A.; Mustaffa, M.M. Embryo rescue and plant regeneration in banana (Musa spp.). Plant Cell Tissue Organ Cult. 2011, 105, 105–111. [Google Scholar] [CrossRef]
- Krishna, H.; Singh, S.K. Biotechnological advances in mango (Mangifera indica L.) and their future implication in crop improvement: A review. Biotechnol. Adv. 2007, 25, 223–243. [Google Scholar] [CrossRef]
- Hu, D.; Tang, X.; Zhang, Q.; Luo, Z. Cross compatibilities of oriental persimmon ‘Mopanshi’ and ‘Luotian-tianshi’ with ‘Zenjimaru’. Acta Hortic. 2013, 996, 165–170. [Google Scholar] [CrossRef]
- Yamada, A.; Tao, R. Controlled pollination with sorted reduced and unreduced pollen grains reveals unreduced embryo sac formation in Diospyros kaki Thunb. ‘Fujiwaragosho’ J. Jpn. Soc. Hortic. Sci. 2007, 76, 133–138. [Google Scholar] [CrossRef]
- Pommer, C.V.; Ramming, D.W.; Emershad, R.L. Influence of grape genotype, ripening season, seed trace size, and culture date on in ovule embryo development and plant formation. Bragantia 1995, 54, 237–249. [Google Scholar] [CrossRef]
- Emershad, R.L.; Ramming, D.W.; Serpe, M.D. In Ovulo embryo development and plant formation from stenospermic genotypes of Vitis vinifera. Am. J. Bot. 1989, 76, 397–402. [Google Scholar] [CrossRef]
- Burger, P.; Trautmann, I.A. Manipulations of ovules to improve in vitro development of Vitis vinifera L. embryos. Acta Hortic. 2000, 528, 613–619. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Li, K.; Liu, Z.; Lin, H.; Guo, X.; Li, C. In vitro embryo rescue culture of F1 progenies from crosses between tetraploid grape and Vitis amurensis Rupr. Afr. J. Agric. Res. 2011, 6, 4906–4909. [Google Scholar]
- Valdez, J.G. Immature embryo rescue of grapevine (Vitis vinifera L.) after an extended period of seed trace culture. Vitis 2005, 44, 17–23. [Google Scholar]
- de Menezes, E.F.; de Silva, E.M.; Yano-Melo, A.M.; de Souza Leão, P.C.; de Melo, N.F. Immature embryo rescue and in vitro development evaluation of intraspecific hybrids from brazilian seedless Grapevine “Superior × Thompson” clones. Am. J. Plant Sci. 2014, 5, 1956–1960. [Google Scholar] [CrossRef][Green Version]
- Ji, W.; Li, G.R.; Luo, Y.X.; Ma, X.H.; Wang, M.; Ren, R. In vitro embryo rescue culture of F1 progenies from crosses between different ploidy grapes. Gen. Mol. Res. 2015, 14, 18616–18622. [Google Scholar] [CrossRef]
- Fanizza, G.; Lamaj, F.; Costantini, L.; Chaabane, R.; Grand, M.S. QTL analysis for fruit yield components in table grapes (Vitis vinifera). Theor. Appl. Genet. 2005, 111, 658–664. [Google Scholar] [CrossRef]
- Cabezas, J.A.; Cervera, M.T.; Ruiz-Garcia, L.; Carreno, J.; Martínez-Zapater, J.M. A genetic analysis of seed and berry weight in grapevine. Genome 2006, 49, 1572–1585. [Google Scholar] [CrossRef]
- Costantini, L.; Battilana, J.; Lamaj, F.; Fanizza, G.; Grando, M.S. Berry and phenology-related traits in grapevine (Vitis vinifera L.): From quantitative trait loci to underlying genes. BMC Plant Biol. 2008, 8–38. [Google Scholar] [CrossRef]
- Mejía, N.; Soto, B.; Guerrero, M.; Casanueva, X.; Houel, C.; Miccono, M.; Ramos, R.; Le Cunff, L.; Boursiquot, J.M.; Hinrichsen, P.; et al. Molecular, genetic and transcriptional evidence for a role of VvAGL11 in stenospermocarpic seedless in grapevine. BMC Plant Biol. 2011, 11, 57. [Google Scholar] [CrossRef]
- Karaagac, E.; Vargas, A.M.; de Andrés, M.T.; Carreño, I.; Ibáñez, J.; Carreño, J.; Martínez-Zapater, J.M.; Cabezas, J.A. Marker assisted selection for seedlessness in table grape breeding. Tree Gen. Genom. 2012, 8, 1003–1015. [Google Scholar] [CrossRef]
- Muñoz-Espinoza, C.; Di Genova, A.; Sánchez, A.; Correa, J.; Espinoza, A.; Meneses, C.; Maass, A.; Orellana, A.; Hinrichsen, P. Identification of SNPs and InDels associated with berry size in table grapes integrating genetic and transcriptomic approaches. BMC Plant Biol. 2020, 20, 365. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of Mountain Laurel, Kalmia latifolia, by use of shoot-tip culture. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Emershad, R.L.; Ramming, D.W. Somatic embryogenesis and plant development from immature zygotic embryos of seedless grapes (Vitis vinifera L.). Plant Cell Rep. 1994, 14, 6–12. [Google Scholar] [CrossRef]
- Bin, H.; Bird, S.; Kemble, R.; Miki, B.; Keller, W. Plant regeneration from microspore-derived embryos of Brassica napus: Effect of embryo age, culture temperature, osmotic pressure, and abscisic acid. In Vitro Cell. Dev. Biol. 1991, 27, 28–31. [Google Scholar]
- Midani, A.R.; Sharma, H.C.; Singh, S.K. Effect of ovule age on ovulo-embryo culture in seeded and seedless grape genotypes. Ind. J. Hortic. 2002, 59, 359–362. [Google Scholar]
- Li, G.R.; Wang, Y.J.; Tang, D.M.; Luo, Q.W. Study on the sampling dates of embryo rescue techniques for seedless grape. J. Agric. Univ. Hebei 2004, 27, 17–21. [Google Scholar]
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242. [Google Scholar] [CrossRef]
- Premachandran, A.; Dhayasree, K.; Kurien, S. Seedless fruits: Fruits of future. J. Pharmacogn. Phytochem. 2019, 8, 1053–1059. [Google Scholar]
- Liu, S.M.; Sykes, S.R.; Clingeleffer, P.R. Pollen fertility and berry setting behaviour of the grape variety Carina. Austr. J. Exp. Agric. 2007, 47, 877–882. [Google Scholar] [CrossRef]
| Cultivar | Species | Berry Characteristics | Cross | Id. |
|---|---|---|---|---|
| Luisa (♀) | Vitis vinifera L. | Seedless (herbaceous seed traces), white skin, elliptic shape, muscat taste | ||
| Thompson Seedless® (♂) | V. vinifera L. | Seedless, white skin, oval shape, medium size, neutral taste | Luisa × Thompson S. | SG-1 |
| White Seedless (♂) | V. vinifera L. | Seedless, white skin, medium size, neutral taste | Luisa × White S. | SG-2 |
| Superior Seedless® (♂) | V. vinifera L. | Seedless, white skin, medium-large size, crunchy, neutral taste | Luisa × Superior S. | SG-3 |
| Princess Seedless® (♂) | V. vinifera L. | Seedless, white skin, cylindrical shape, medium-large size, muscat taste | Luisa × Princess S. | SG-4 |
| Crimson Seedless (♂) | V. vinifera L. | Seedless, red skin, medium-large size, elliptic shape, crunchy, neutral taste | Luisa × Crimson S. | SG-5 |
| Regal Seedless (♂) | V. vinifera L. | Seedless, white skin, ovoidal shape, medium-large size, crunchy, neutral taste | Luisa × Regal S. | SG-6 |
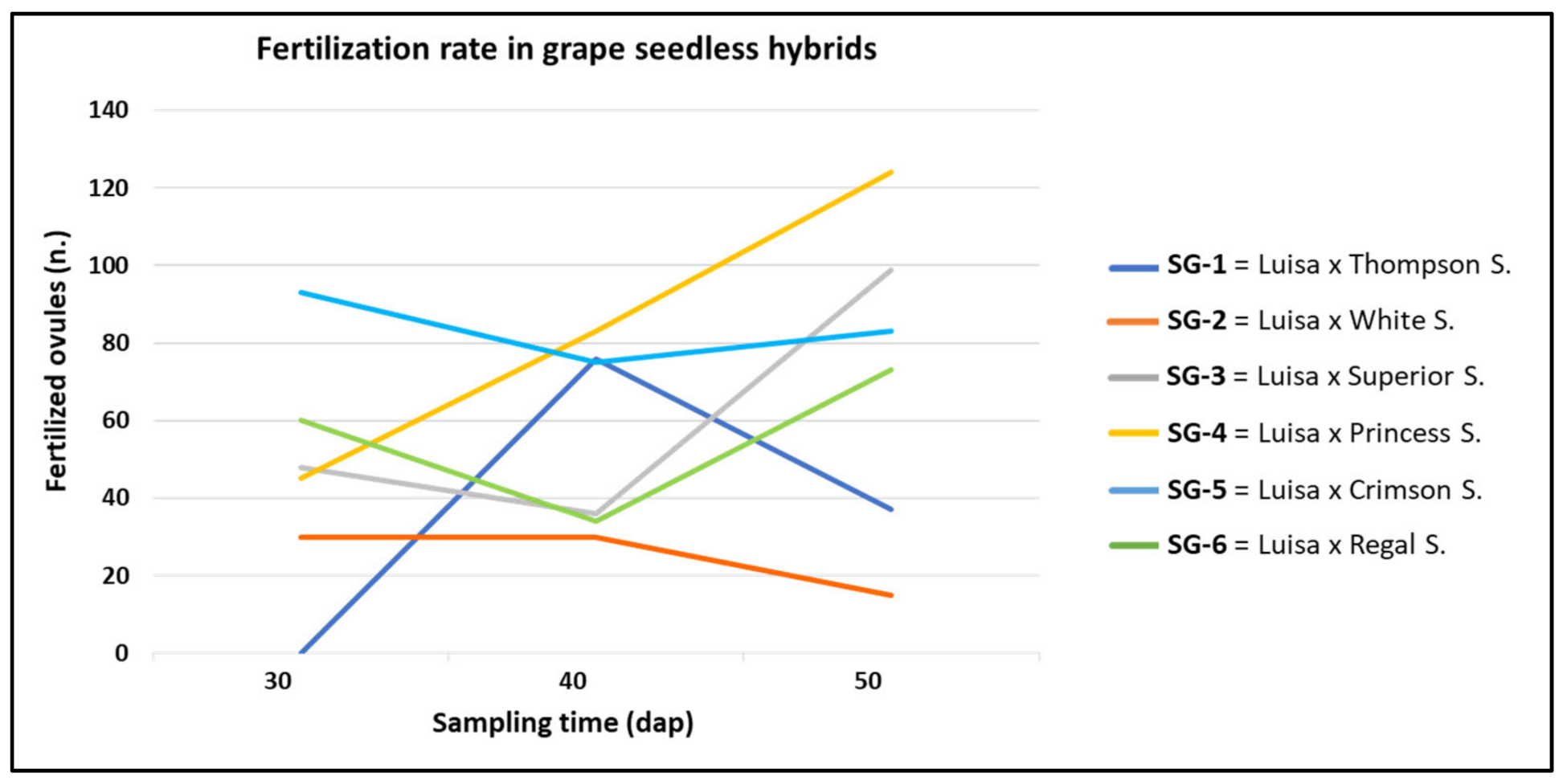
| Cross (♀ × ♂) | Id. Code | 30 DAP | 40 DAP | 50 DAP | Total (n.) | |||
|---|---|---|---|---|---|---|---|---|
| Total (n.) | Mean (n.) | Total (n.) | Mean (n.) | Total (n.) | Mean (n.) | |||
| Luisa × Thompson S. | SG-1 | 0 e | 0 | 76 b | 2.5 | 37 d | 1.2 | 113 c |
| Luisa × White S. | SG-2 | 30 d | 1 | 30 c | 1 | 15 e | 0.5 | 75 d |
| Luisa × Superior S. | SG-3 | 48 c | 1.6 | 36 c | 1.2 | 99 b | 3.3 | 183 b |
| Luisa × Princess S. | SG-4 | 45 c | 1.5 | 83 a | 2.8 | 124 a | 4.1 | 252 a |
| Luisa × Crimson S. | SG-5 | 93 a | 3.1 | 75 b | 2.5 | 83 c | 2.8 | 251 a |
| Luisa × Regal S. | SG-6 | 60 b | 2 | 34 c | 1.1 | 73 c | 2.4 | 167 b |
| Total n. | 276 C | 1.8 | 334 B | 1.9 | 431 A | 2.4 | 1041 | |
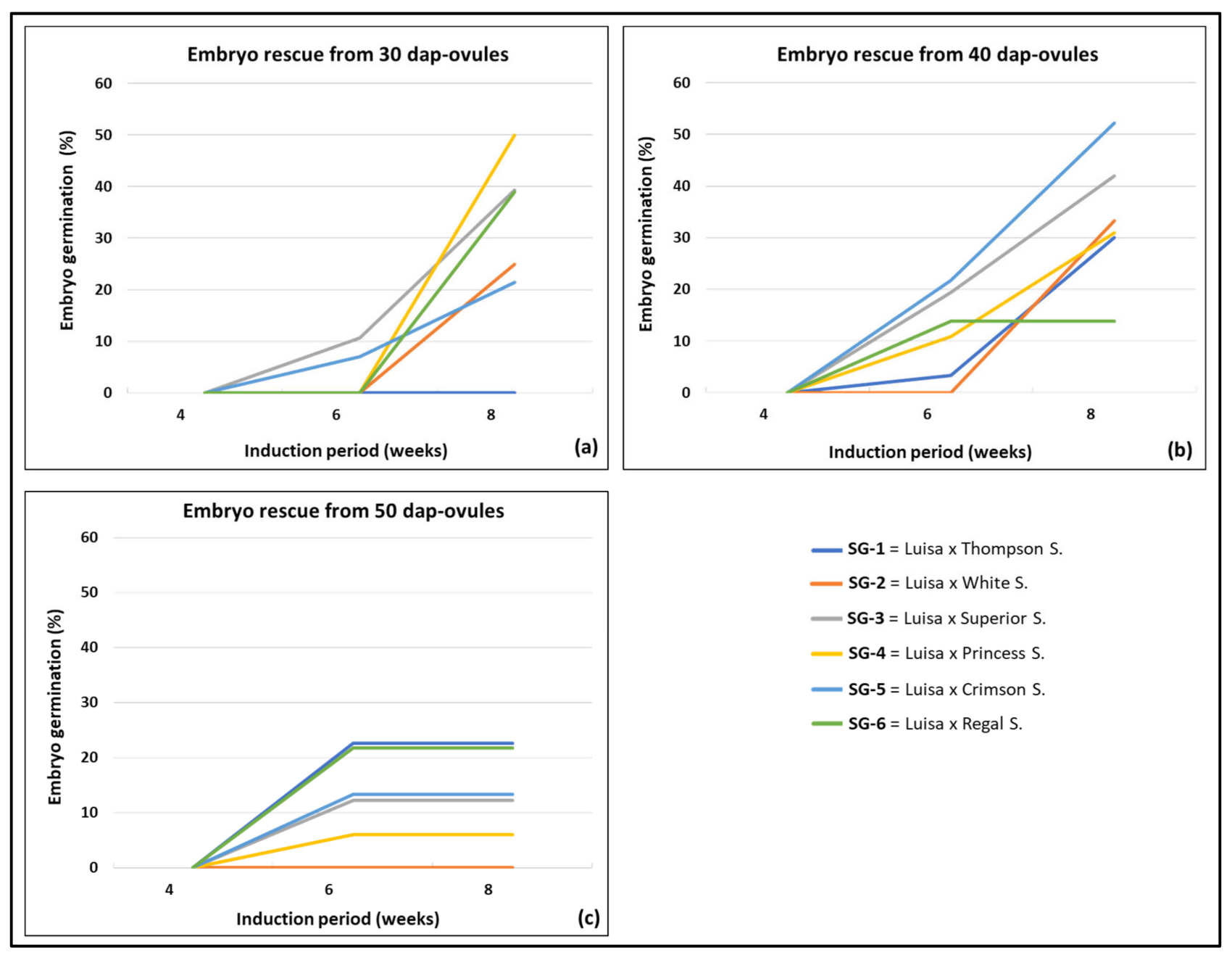
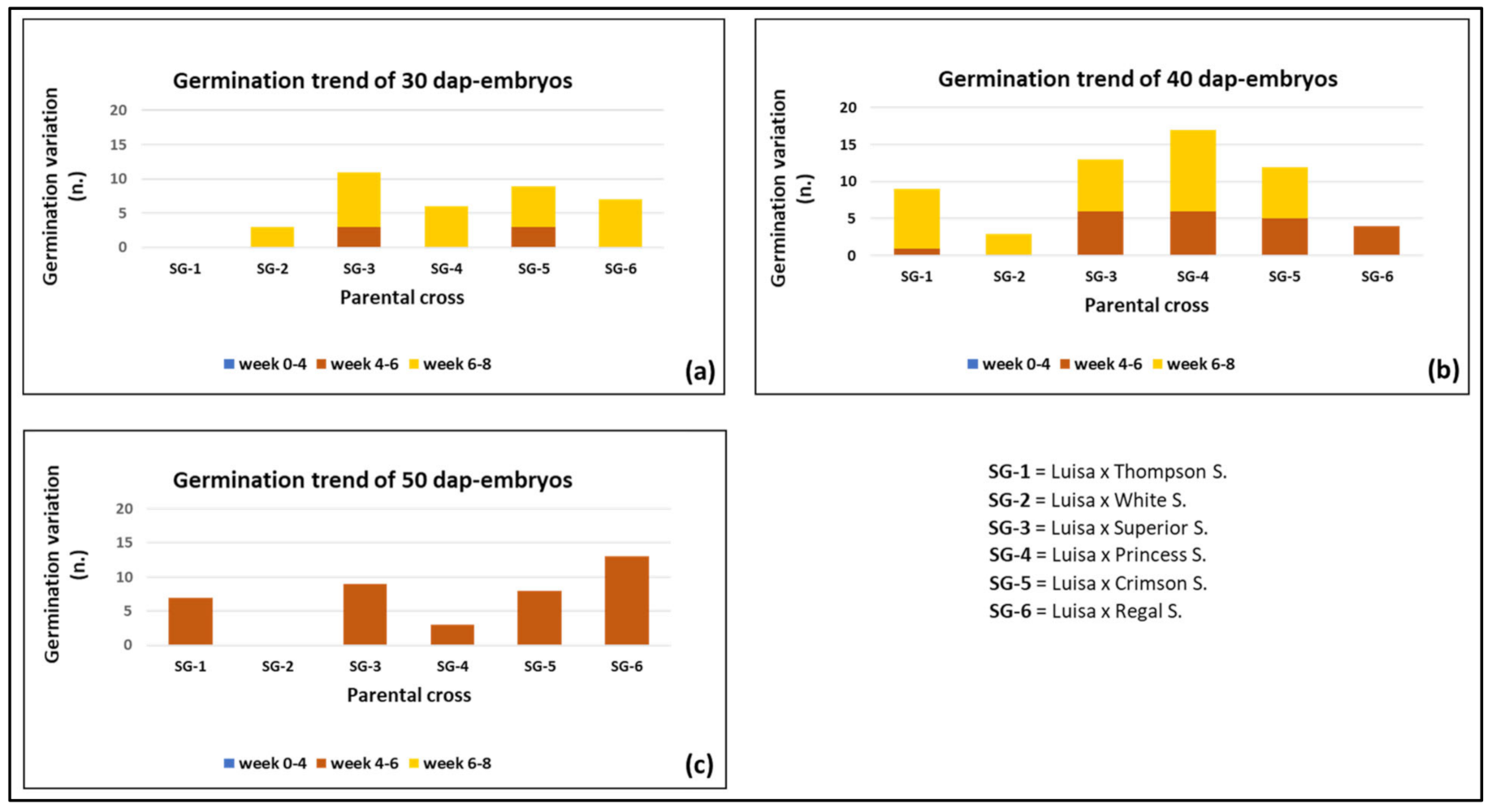
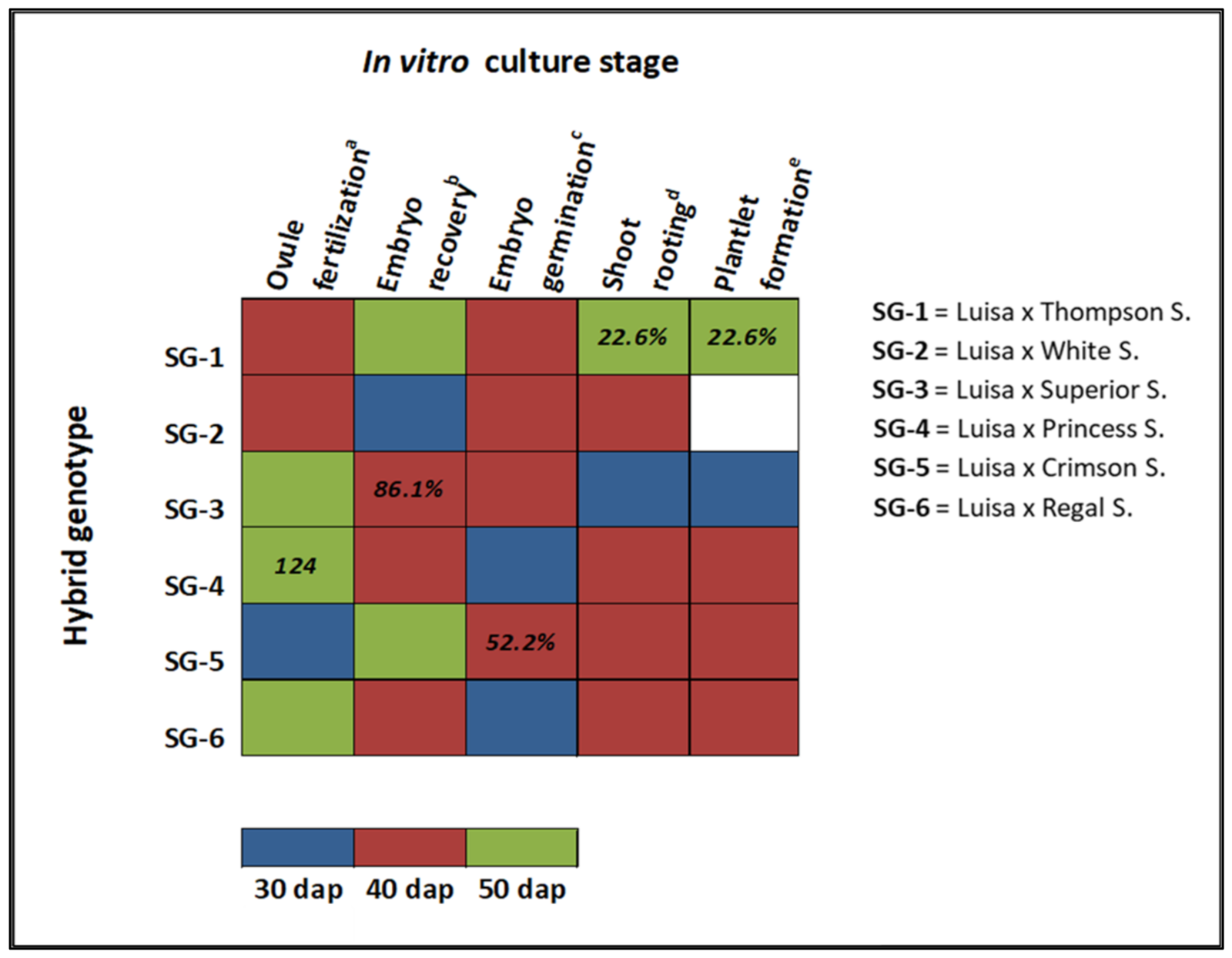
| Cross (♀ × ♂)- Sampling Time | Isolated Ovules a | Embryo Recovery b | Embryo Germination c | |||||
|---|---|---|---|---|---|---|---|---|
| (n.) | (n.) | (%) | 4 Weeks (n.) | 6 Weeks (n.) | 6 Weeks (%) | 8 Weeks (n.) | 8 Weeks (%) | |
| Luisa × Thompson S.-30 DAP | 0 c | 0 b | 0 | 0 | 0 b | 0 | 0 b | 0 |
| Luisa × Thompson S.-40 DAP | 76 a | 30 a | 39.5 | 0 | 1 b | 3.3 | 9 a | 30.0 |
| Luisa × Thompson S.-50 DAP | 37 b | 31 a | 83.8 | 0 | 7 a | 22.6 | 7 a | 22.6 |
| Luisa × White S.-30 DAP | 30 a | 12 a | 40.0 | 0 | 0 | 0 | 3 a | 25.0 |
| Luisa × White S.-40 DAP | 30 a | 9a b | 30.0 | 0 | 0 | 0 | 3 a | 33.3 |
| Luisa × White S.-50 DAP | 15 b | 5 b | 33.3 | 0 | 0 | 0 | 0 b | 0 |
| Luisa × Superior S.-30 DAP | 48 b | 28 b | 58.3 | 0 | 3 b | 10.7 | 11 ab | 39.3 |
| Luisa × Superior S.-40 DAP | 36 c | 31 b | 86.1 | 0 | 6 ab | 19.4 | 13 b | 41.9 |
| Luisa × Superior S.-50 DAP | 99 a | 74 a | 74.8 | 0 | 9 a | 12.2 | 9 a | 12.2 |
| Luisa × Princess S.-30 DAP | 45 c | 12 b | 26.7 | 0 | 0 c | 0 | 6 b | 50.0 |
| Luisa × Princess S.-40 DAP | 83 b | 55 a | 66.3 | 0 | 6 a | 10.9 | 17 a | 30.9 |
| Luisa × Princess S.-50 DAP | 124 a | 50 a | 40.3 | 0 | 3 b | 6.0 | 3 b | 6.0 |
| Luisa × Crimson S.-30 DAP | 93 a | 42 b | 45.2 | 0 | 3 b | 7.1 | 9 b | 21.4 |
| Luisa × Crimson S.-40 DAP | 75 b | 23 c | 30.7 | 0 | 5 b | 21.7 | 12 a | 52.2 |
| Luisa × Crimson S.-50 DAP | 83 ab | 60 a | 72.3 | 0 | 8 a | 13.3 | 8 b | 13.3 |
| Luisa × Regal S.-30 DAP | 60 b | 18 b | 30.0 | 0 | 0 c | 0 | 7 b | 38.9 |
| Luisa × Regal S.-40 DAP | 34 c | 29 b | 85.3 | 0 | 4 b | 13.8 | 4 b | 13.8 |
| Luisa × Rega S.l-50 DAP | 73 a | 60 a | 82.2 | 0 | 13 a | 21.7 | 13 a | 21.7 |
| Total n. of shoots from 30 DAP-ovules | 36 b | |||||||
| Total n. of shoots from 40 DAP-ovules | 40 b | |||||||
| Total n. of shoots from 50 DAP-ovules | 58 a | |||||||
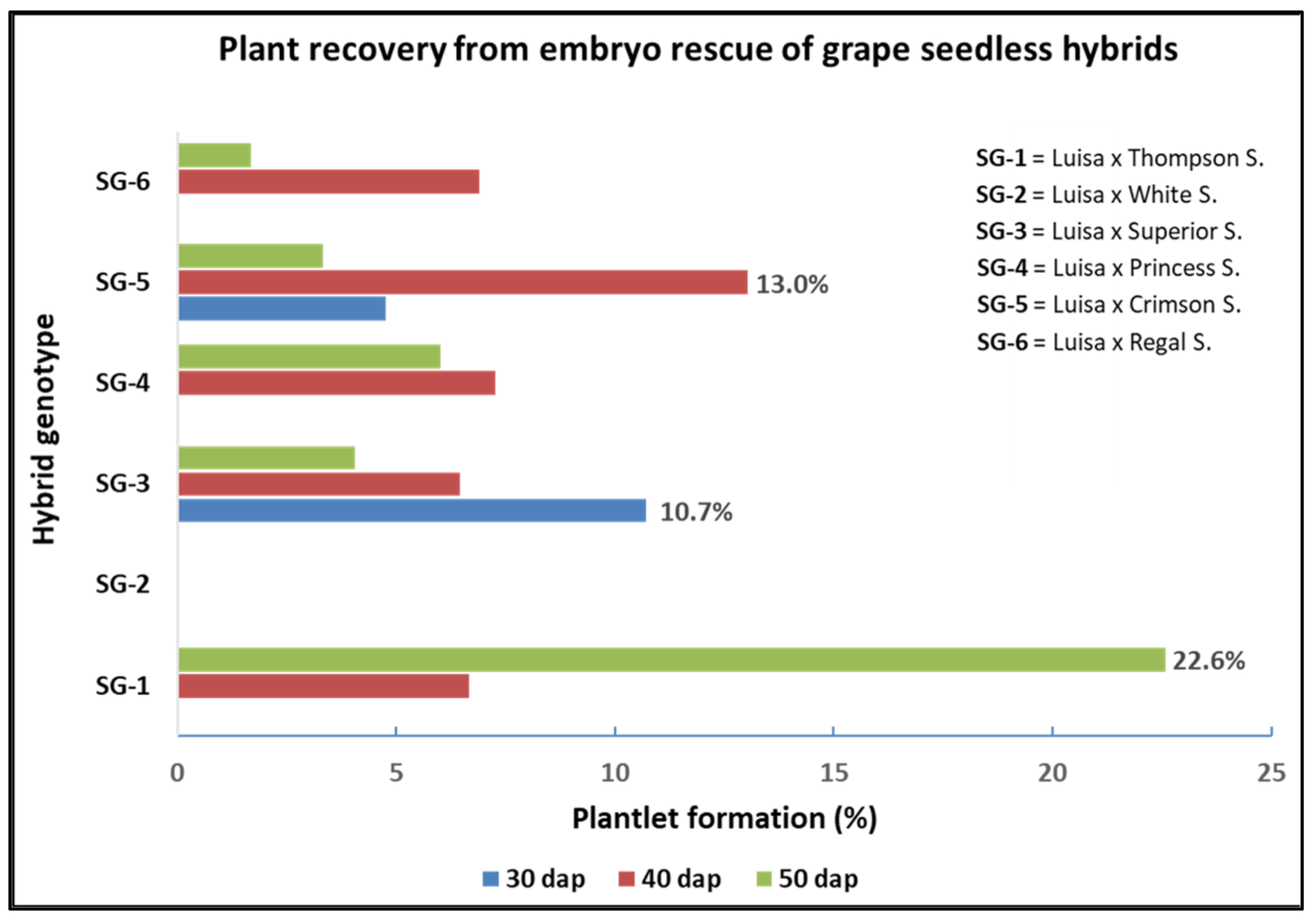
| Cross (♀ × ♂)- Sampling Time | Embryo Recovery a | Shoot Development b | Shoot Rooting c | Plantlet Formation d | |||
|---|---|---|---|---|---|---|---|
| (n.) | (n.) | (%) | (n.) | (%) | (n.) | (%) | |
| Luisa × Thompson S.-30 DAP | 0 b | 0b | 0 | 0 c | 0 | 0 c | 0 |
| Luisa × Thompson S.-40 DAP | 30 a | 9 a | 30 | 2 b | 6.7 | 2 b | 6.7 |
| Luisa × Thompson S.-50 DAP | 31 a | 7 a | 22.6 | 7 a | 22.6 | 7 a | 22.6 |
| Luisa × White S.-30 DAP | 12 a | 3 a | 25.0 | 0 | 0 | 0 | 0 |
| Luisa × White S.-40 DAP | 9 a | 3 a | 33.3 | 1 | 11.1 | 0 | 0 |
| Luisa × White S.-50 DAP | 5 b | 0 b | 0 | 0 | 0 | 0 | 0 |
| Luisa × Superior S.-30 DAP | 28 b | 11 ab | 39.3 | 3 b | 10.7 | 3 | 10.7 |
| Luisa × Superior S.-40 DAP | 31 b | 13 a | 41.9 | 3 b | 9.7 | 2 | 6.5 |
| Luisa × Superior S.-50 DAP | 74 a | 9 b | 12.2 | 6 a | 8.1 | 3 | 4.1 |
| Luisa × Princess S.-30 DAP | 12 b | 6 b | 50.0 | 0 c | 0 | 0 b | 0 |
| Luisa × Princess S.-40 DAP | 55 a | 17 a | 30.9 | 6 a | 10.9 | 4 a | 7.3 |
| Luisa × Princess S.-50 DAP | 50 a | 3 b | 6.0 | 3 b | 6.0 | 3 a | 6.0 |
| Luisa × Crimson S.-30 DAP | 42 b | 9 b | 21.4 | 2 b | 4.8 | 2 | 4.8 |
| Luisa × Crimson S.-40 DAP | 23 c | 12 a | 52.2 | 5 a | 21.7 | 3 | 13.0 |
| Luisa × Crimson S.-50 DAP | 60 a | 8 b | 13.3 | 2 b | 3.3 | 2 | 3.3 |
| Luisa × Regal S.-30 DAP | 18 c | 7 b | 38.9 | 0 b | 0 | 0 b | 0 |
| Luisa × Regal S.-40 DAP | 29 b | 4 c | 13.8 | 2 a | 6.9 | 2 a | 6.9 |
| Luisa × Regal S.-50 DAP | 60 a | 13 a | 21.7 | 2 a | 3.3 | 1 a | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giancaspro, A.; Mazzeo, A.; Carlomagno, A.; Gadaleta, A.; Somma, S.; Ferrara, G. Optimization of an In Vitro Embryo Rescue Protocol for Breeding Seedless Table Grapes (Vitis vinifera L.) in Italy. Horticulturae 2022, 8, 121. https://doi.org/10.3390/horticulturae8020121
Giancaspro A, Mazzeo A, Carlomagno A, Gadaleta A, Somma S, Ferrara G. Optimization of an In Vitro Embryo Rescue Protocol for Breeding Seedless Table Grapes (Vitis vinifera L.) in Italy. Horticulturae. 2022; 8(2):121. https://doi.org/10.3390/horticulturae8020121
Chicago/Turabian StyleGiancaspro, Angelica, Andrea Mazzeo, Antonio Carlomagno, Agata Gadaleta, Stefano Somma, and Giuseppe Ferrara. 2022. "Optimization of an In Vitro Embryo Rescue Protocol for Breeding Seedless Table Grapes (Vitis vinifera L.) in Italy" Horticulturae 8, no. 2: 121. https://doi.org/10.3390/horticulturae8020121
APA StyleGiancaspro, A., Mazzeo, A., Carlomagno, A., Gadaleta, A., Somma, S., & Ferrara, G. (2022). Optimization of an In Vitro Embryo Rescue Protocol for Breeding Seedless Table Grapes (Vitis vinifera L.) in Italy. Horticulturae, 8(2), 121. https://doi.org/10.3390/horticulturae8020121