Abstract
The use of commercial food waste in the Korean agricultural industry is increasing due to its capacity to act as an ecofriendly fertilizer. However, the high salt content of food waste can be detrimental to plant health and increase salinity levels in agricultural fields. In the current study, we introduced halotolerant rhizobacteria to neutralize the negative impact of food waste-related salt stress on crop productivity. We isolated halotolerant rhizobacteria from plants at Pohang beach, and screened bacterial isolates for their plant growth-promoting traits and salt stress-mitigating capacity; consequently, the bacterial isolate Bacillus pumilus MAK9 was selected for further investigation. This isolate showed higher salt stress tolerance and produced indole-3-acetic acid along with other organic acids. Furthermore, the inoculation of B. pumilus MAK9 into Chinese cabbage plants alleviated the effects of salt stress and enhanced plant growth parameters, i.e., it increased shoot length (32%), root length (41%), fresh weight (18%), dry weight (35%), and chlorophyll content (13%) compared with such measurements in plants treated with food waste only (control). Moreover, relative to control plants, inoculated plants showed significantly decreased abscisic acid content (2-fold) and increased salicylic acid content (11.70%). Bacillus pumilus MAK9-inoculated Chinese cabbage plants also showed a significant decrease in glutathione (11%), polyphenol oxidase (17%), and superoxide anions (18%), but an increase in catalase (14%), peroxidase (19%), and total protein content (26%) in comparison to the levels in control plants. Inductively coupled plasma mass spectrometry analysis showed that B. pumilus MAK9-inoculated plants had higher calcium (3%), potassium (22%), and phosphorus (15%) levels, whereas sodium content (7%) declined compared with that in control plants. Similarly, increases in glucose (17%), fructose (11%), and sucrose (14%) contents were recorded in B. pumilus MAK9-inoculated plants relative to in control plants. The bacterial isolate MAK9 was confirmed as B. pumilus using 16S rRNA and phylogenetic analysis. In conclusion, the use of commercially powered food waste could be a climate-friendly agricultural practice when rhizobacteria that enhance tolerance to salinity stress are also added to plants.
1. Introduction
Food waste refers to all uneaten but disposed of food substances or leftovers from households, markets, supermarkets, and various eating establishments [1]. More than one third of food generated for human consumption [2,3] and nearly 1.3 billion tons of food are estimated to be lost in the food supply chain [4,5]. Up to 42% of food waste is produced by households, whereas 39% comes from food manufacturing industries, 14% from the food service sector, and 5% from the distribution process [6]. Food waste levels are expected to increase within 25 years due to economic and population growth, especially in Asian countries, rising from 278 to 416 million tons from 2005 to 2025 [4,7]. In Japan and Taiwan, ~19 million and 16.5 million tons of food is wasted annually, respectively [7,8]. In Korea, food waste reportedly comprises >22% (4.3 million tons) of municipal solid waste [9]. Together with other waste products, food waste can give rise to problems such as emanating odors, attracting vermin, emitting toxic gas, and contaminating groundwater [10]. Thus, such waste must be removed to ensure that the environment is clean and healthy.
Composting is a simple, economical, and natural biodegradation process that can convert food waste into a stable, hygienic, humus-rich, and nutrient-rich food for plants [7], which can also stabilize and enhance the properties of the soil to which it is applied [11]. Recycling food waste is now a public concern as it is an environmentally sound and cost effective use of waste biomass [12,13]. In Japan, food waste recycling accounts for only 6.7% of recycling compared with 78% for industrial waste recycling [14]. Nevertheless, food waste contains important nutrients that could be efficiently reused in agriculture [12,15], i.e., to improve soil properties, the growth and development of plants, and agricultural productivity [16,17]. The constituents of food waste generally include carbohydrates, protein, and inorganic compounds, which serve as food for microbes [18]. Thus, the integration of biofertilizers with organic fertilizers can be beneficial [14].
However, the application of food waste as a fertilizer for agricultural purposes has some disadvantages [19]. For example, it has been reported that soil salinity increases due to the decomposition of food waste [20]. Lee et al. [20] reported respective average water and salinity contents of 72.00% and 2.03% in collected food waste from Korea. Soil salinity can be a devastating environmental stressor: it can reduce cultivated land area, crop productivity, and crop quality [21]. Salinity stress also effects the morphological, physiological, and biochemical process of plants, including their seed germination, growth, and nutrient uptake [6]. Salinity stress is estimated to affect 20% and 33% of all cultivated and irrigated land worldwide, respectively [22]. Under salt stress conditions, reactive oxygen species (ROS), such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide, are produced and accumulate in plant cellular compartments, thereby causing oxidative damage [23]. To counteract ROS-mediated oxidative stress, plants have developed enzymatic and nonenzymatic systems that protect their cells and help maintain ROS at normal levels [23]. Plants under salinity stress accumulate Na and suffer inhibition of the uptake of nutrients such as Ca, K, and P, which are vital to the maintenance of cell membranes [22]. In soybean and tomato plants, salinity stress decreases growth attributes and productivity [24,25]. As a potential solution, plant growth-promoting rhizospheric bacteria have been reported to mitigate salinity stress and enhance crop yield in various plants [24,26,27].
Therefore, in current study, we hypothesized that the use of powdered food waste with the inoculation of salt-tolerant plant growth-promoting bacteria could be an ecofriendly agricultural practice that enhances the salinity stress tolerance of plants. Specifically, rhizospheric bacteria were isolated and screened for plant growth-promoting traits, e.g., siderophore production, phosphate solubilization, indole-3-acetic acid (IAA) production, and exopolysaccharide (EPS) production, as well as tolerance to NaCl stress. Based on multiple plant growth-promoting traits and salt tolerance, the bacterial isolate MAK9 was selected and identified using 16S rRNA analysis. The objective of the current study was then to understand the effects on the growth and chlorophyll content of Chinese cabbage plants when applying food waste with and without inoculation of the isolate MAK9. In addition, we aimed to elucidate the translocation of ion uptake (Na+, K+, Ca, and P), antioxidant production, and phytohormone content (abscisic acid [ABA] and salicylic acid [SA]) of Chinese cabbage plants treated with food waste with and without the isolate MAK9.
2. Materials and Methods
2.1. Plant Material and Isolation of Rhizospheric Bacteria
Different plants species (Artemisia princeps, Echinochloa crus-galli, Chenopodium ficifolium, and Oenothera biennis) were collected from the sand dunes of Pohang beach (36°7′56.2″ N, 129°23′55.1″ E) in Korea. The plants were packed in sterilized polyethylene zip bags along with rhizospheric soil. Subsequently, 1 g of rhizospheric soil taken from the root of individual plants was used for serial dilution. Petri plates were covered with Parafilm and kept in an incubator at 27 °C.
2.2. Screening Rhizobacterial Isolates for Plant Growth Promotion
Rhizospheric bacterial isolates were grown for IAA production in 50 mL of LB media at 30 °C with shaking at 120 rpm for 6 days. Salkowski reagent was used for IAA detection and UV absorbance was assessed with a UV spectrophotometer (Multiskan GO, Thermo Fisher Scientific, Vantaa, Finland) at 530 nm [28]. For phosphate solubilization and siderophore production, rhizospheric bacteria were inoculated into Petri dishes with Pikovskaya’s medium and Chrome Azurol S (CAS) medium, and incubated for 5 days at 27 °C according to the method of Chadha et al. [28]. For detection of bacterial EPS, we followed the method of Yu-Na et al. [29].
2.3. Screening of MAK9 for Halotolerance and Bacterial Identification
Rhizospheric bacterial isolates were tested for halotolerance at different NaCl concentrations. In LB medium, the bacterial isolates were grown and supplemented with different levels of NaCl (0%, 3%, 6%, 9%, and 12%) to assess their ability to mitigate salt stress. For 48 hrs, the flasks were incubated at 27 °C and 120 rpm in a shaking incubator, after which the OD (600 nm) was determined using a spectrophotometer. For bacterial identification, genomic DNA was extracted from bacterial fluid samples (200 mL) using a QIAmp DNA Minikit (Qiagen) according to the manufacturer’s instructions, and with a final eluted volume of 100 μL. Extracted DNA was then amplified for PCR, which was performed using 16S rRNA gene-specific primers: 27F primer (5′-AGAGTTTGATCACTGGCTCAG-3′) and 1492R primer (5′-CGGCTTACCTTGTTACGACTT-3′). PCR amplifications were performed with 1 × Ex Taq buffer (Takara Bio Inc, Japan), 0.8-mM dNTP, 0.02 unit’s μL−1 of Ex Taq polymerase, 0.4-mg mL−1 bovine serum albumin, and 1.0 μM of each primer. The amplifications were also performed at an annealing temperature of 55 °C (40 s), an initial denaturation temperature of 94 °C (5 min), with 30 amplification cycles including denaturation at 94 °C (60 s), annealing (30 s), and extension at 72 °C (60 s), and finally the last extension at 72 °C (10 min). A Wizard® PCR Preps DNA Purification System was used to purify the PCR product (Promega, Madison, WI, USA). Purified double-stranded PCR fragments were directly sequenced using a Big Dye Terminator Cycle sequencing kit (Applied Biosystems, Forster City, CA, USA) following the manufacturer’s instructions. Chromas Lite 2.01 (http://www.technelysium.com.au/chromas.html, 11 June 2020) was used to alter sequences for each zone. For the 16S region of bacteria, BLAST (http://www.ncbi.nlm.nih.gov/BLAST, 1 August 2021) was used to compare the sequence homology of nucleotides. To construct a neighboring tree, ClustalW was used to align the sequences along with MEGA (6.0) (Department of Biological Sciences, Tokyo Metropolitan University, Tokyo, Japan). To statistically support the nodes in the phylogenetic trees, bootstrap replication (1000 replications) was also performed.
2.4. Screening of MAK9 Culture Filtrate for Organic Acid and IAA Production
The bacterial isolate MAK9 was grown in LB media (10-g tryptone, 5-g yeast extract, and pH 7.2) for 3 days, after which it was centrifuged (5000× g for 10 min) and the culture filtrate was analyzed for organic acid and IAA production [30]. For organic acid content analysis, the bacterial culture broth was filtered through a 0.45 μm Millipore filter (DISMIC-25CS, ADVANTE, Tokyo, Japan) and 10 μL of each sample was injected into a high-performance liquid chromatography (HPLC) column (waters 600 E; column: RSpak KC-811 [8.0 × 300 mm2]; eluent: 0.1% H3PO4/H2O; flow rate: 1.0 mL/min; temperature: 40 °C). The retention times and peak areas of the chromatograms were compared with standards from Sigma-Aldrich, St. Louis, MO, USA (Table S1). For IAA analysis, the culture filtrates were acidified to a pH of 2.8 and supplemented with 40 µL of (D5)-IAA as an IAA internal standard. The acidified and standard-supplemented free culture cells were extracted, methylated, and injected into a GC/MS–SIM (6890N network GC system and 5973 network mass selective detector; Agilent Technologies, Santa Clara, CA, USA) to identify and quantify IAA (Table S2).
2.5. Effects of MAK9 on the Growth Attributes of Chinese Cabbage Plants
Chinese cabbage plant seeds were surface sterilized with 70% ethanol for 10 min and then washed three times with autoclaved distilled water. Petri dishes with two layers of filter paper were autoclaved; Chinese cabbage seeds were kept on wet sterile filter paper and incubated for 3 days at 27 °C for germination. Uniformly germinated seedlings were transferred to pots (10 × 9 cm2) containing autoclaved soil. Treatments were as follows: (1) control Chinese cabbage plants provided with autoclaved water, (2) bacteria-inoculated Chinese cabbage plants, (3) plants treated with food waste powder (Seyen Co., Ltd., Kyungsan, Korea) (8%), and (4) plants treated with food waste powder and inoculated with the isolate MAK9. After being transferred to pots, each plant was supplied with 10 mL of MAK9 cell suspension. The seedlings were normally lifted for the mutualistic association of bacteria and planted for about 2 weeks in the pots. Food waste was introduced as a treatment after two weeks. Food waste extract solution (10 mL; 8%) was applied to the pots every 3 days and this treatment was continued for ~2 weeks. The growth characteristics of the harvested plants, i.e., fresh and dry weight, root measurements, and shoot length were assessed after 20 days. Chlorophyll content was measured using a SPAD meter (SPAD-502 Minolta, Tokyo, Japan).
2.6. Estimation of Enzymatic Activities
To determine catalase (CAT) content, the method of Imran et al. [31] was used; 20 mg of leaves were homogenized in 50-mM Tris-HCl buffer (pH 7.0) containing 1-mM EDTA, 3-mM MgCl2, and 1.0% PVP and then centrifuged at 15,000 rpm and 22 °C for 15 min. Subsequently, 10-mM phosphate buffer (pH 7.0) and 0.5 mL of 0.2-M H2O2 were added to the supernatant. An absorption decrease occurred at 240 mm and the activity of the enzyme was determined. One unit of CAT was given as μg of H2O2 released by mg of protein min−1. To determine peroxidase (POD) content, the established protocol of Kubi et al. [27] was used; the reaction mixture was incubated at 25 °C for 5 min, and then 5% 0.5 mL H2SO4 was used to stop the reaction. Absorptive measurements were then taken at 420 nm. The same reaction mixture used for POD was used for polyphenol oxidase (PPO) activity but without H2O2; the results were also measured at 420 nm. Protein content was estimated using the Bradford assay [32] at an OD of 595 nm. Ascorbic peroxidase (APX) activity was measured following the method of Kim et al. [33]. The plant sample was added to a reaction mixture (50-mM potassium phosphate [pH 7], 0.5-mM ascorbate, 0.1-mM hydrogen peroxide, and 0.1-mM EDTA), and the decrease in absorbance was measured from 10 to 30 s at 290 nm using a spectrophotometer. The method of Khan et al. [34] was used to assess superoxide dismutase (SOD) activity. Briefly, leaf samples (100 mg) were homogenized with 0.01-M phosphate buffer at pH 7 and then centrifuged (17,000× g for 15 min at 4 °C). The supernatant, which was used as a crude enzyme extract, was passed through a reaction mixture containing Tris-HCl buffer (2 mL; pH 8.2), double-distilled water (2 mL), and 2-mM pyrogallol (0.5 mL). The absorption of the assay mixture and a blank (lacking pyrogallol or tissue homogenate) was measured at 470 nm using a spectrophotometer (Multiskan GO; Thermo Fisher Scientific, Waltham, MA, the United States) at 180 s intervals; data are expressed as units/mg of protein. To determine the reduction in glutathione (GSH) concentration, each sample (500 mg) was treated with 2 mL of 10% trichloroacetic acid and centrifuged at 10,000 rpm for 15 min at 4 °C. The resulting supernatant (1 mL) was combined with 0.5 mL of Ellman’s reagent and 3 mL of 15-mM sodium phosphate buffer (pH 7.4) and incubated for 5 min at 30 °C. Thereafter, the absorbance was measured at 412 nm using a spectrophotometer. For analysis of superoxide anion production, 1 g of fresh plant powder sample was immersed in 10-mM sodium phosphate buffer (pH 7.0) containing 0.05% (w/v) NBT and 10-mM NaN3 for 1 h at room temperature. Afterwards, 5 mL of the solution was transferred into a new test tube and heated for 15 min at 85 °C in a water bath. Immediately after heating, the solution was cooled down in ice, vacuum filtered, and analyzed at 580 nm using a spectrometer. Superoxide anion scavenging activity was calculated using the following equation: Scavenging (%) = (A580Control − A580Sample / A580Control) × 100 [35,36]. The experiment was repeated in triplicate.
2.7. Quantification of Endogenous ABA and SA
For the quantification of endogenous ABA content in the freeze-dried samples, a GC-MS (5973 Network Mass Selective Detector and 6890N Network Gas Chromatograph, Agilent Technologies, Santa Clara, CA, USA) method was used (Table S3). Specifically, Lab-Base (ThermoQuest, Manchester, UK) data system software was used to monitor responses to ions at m/z 162 and 190 for Me-ABA and m/z 166 and 194 for Me-[2H6]-ABA. Analysis of SA was conducted according to the method of Jan et al. [37]; the freeze-dried areal parts of the plants were used for HPLC analysis with a Shimadzu system equipped with a fluorescence detector (RF-10AXL, Shimadzu, Japan), including excitation and emission at 305 and 365 nm, respectively, and fitted with a C18 reverse phase HPLC column (HP Hypersil ODS; particle size: 5 μm, pore size: 120 A; Waters, MA, USA) (Table S4). Flow rate was maintained at 1 mL/min.
2.8. Inductively Coupled Plasma (ICP) Analysis of Ion Uptake
To determine ionic uptake, the method of Khan et al. [38] was followed using freeze-dry samples. All samples (0.2 g) were first digested with 5 mL of HNO3 and 3 mL of H2O2 in a microwave, and then 3% HNO3 was added to further digest the samples. For the quantification of calcium (Ca), sodium (Na+), and potassium (K+) content, samples were injected through an ICP mass spectrometry system.
3. Analysis of Sugars
The sugar content (glucose, fructose, and sucrose) in freeze-dried powder (0.5 g) from shoots was solubilized using 80% ethanol and kept for 24 h at room temperature. Samples were then placed in a water bath at 80 °C for 30 min, after which they were cooled, centrifuged, and the extract was then dried using a vacuum dryer. The dried residue was redissolved in 2 mL of deionized water and then passed through 0.45-μm Nylon-66 syringe filters. Subsequently, the filtrated samples were injected into a HPLC Waters system (Millipore Corp., Waters Chromatography, Milford, MA, USA) comprising a Sugar-Pak column (300 mm; a model 60 controller) and sugar signals were detected using a Waters 410 refractive index detector (Table S5). Sucrose, fructose, and glucose were quantified by comparing their peaks area with those of specific standards.
4. Statistical Analysis
Data were statistically analyzed using Duncan’s multiple range test in Statistical Analysis Software (SAS: V 9.2, Institute Inc, Cary, NC, USA). Unless otherwise stated, the experiments were repeated twice with 15 replicates per treatment and data collected from each replicate were pooled. GraphPad Prism (version 6.01; San Diego, CA, USA) was used to create graphical presentations.
5. Results
5.1. Isolation, Screening, and Identification
In total, 27 isolates were isolated and these were screened for plant growth-promoting traits (siderophore, phosphate, IAA, and EPS production). Based on the screening results, 16, 10, 12, and 12 isolates were positive for IAA production, siderophore activity, EPS production, and phosphate solubilization, respectively (Figure S1). Isolate MAK9 showed high tolerance to salt stress (Figure S2) and was, therefore, further investigated for molecular identification. Analysis showed that isolate MAK9 shared sequence identity with Bacillus pumilus; it was submitted to NCBI with the accession number MZ675645 (Figure 1).
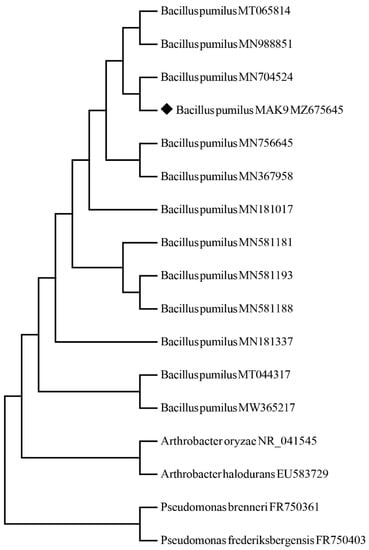
Figure 1.
Phylogenetic tree of isolate MAK9 constructed using 16S rRNA sequences as well as neighbor-joining and maximum-likelihood methods. ♦ represents the bacterial strain.
5.2. In Vitro IAA and Organic Acid Production of Isolate MAK9
Isolate MAK9 was grown in LB media for 5 days, centrifuged, and the culture filtrate was tested for IAA and organic acid production (Figure 2). MAK 9 produced IAA (Figure 2A) as well as acetic acid, succinic acid, and lactic acid. Moreover, the highest amount of acetic acid was observed in the culture filtrate of isolate MAK9 (Figure 2B).
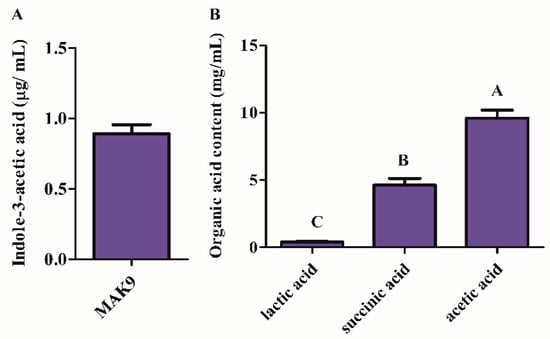
Figure 2.
Quantification of indole-3-acetic acid (IAA) and organic acids produced by isolate MAK9. (A) GC/MS–SIM analysis of the IAA content in the culture broth of isolate MAK9. (B) Organic acids were detected and quantified using high-performance liquid chromatography relative to their respective standards. Different letters indicate significant differences between the mean values of three replicates ± standard deviation.
5.3. Bacterial Isolates Regulate Chinese Cabbage Growth under Food Waste
The application of food waste (8%) was found to reduce the growth attributes of Chinese cabbage plants (Figure 3): shoot length (9.16 ± 0.70), root length (5.5 ± 0.5), fresh weight (1.8 ± 0.05), dry weight (0.14 ± 0.05), and chlorophyll content (21.6 ± 1.6) were decreased in Chinese cabbage plants treated with 8% food waste compared with the shoot length (17.20 ± 1.0), root length (12.0 ± 1.0), fresh weight (2.6 ± 0.1), dry weight (0.22 ± 0.05), and chlorophyll content (27.1 ± 1.1) of control plants (Table 1). However, microbe inoculation inhibited the effect of salt stress caused by food waste; it increased shoot length (12.1 ± 0.7), root length (7.8 ± 0.7), fresh weight (2.1 ± 0.05), dry weight (0.19 ± 0.05), and chlorophyll content (24.5 ± 0.7) in plants treated with food waste and bacterial inoculation compared with those treated with food waste alone (Table 1).
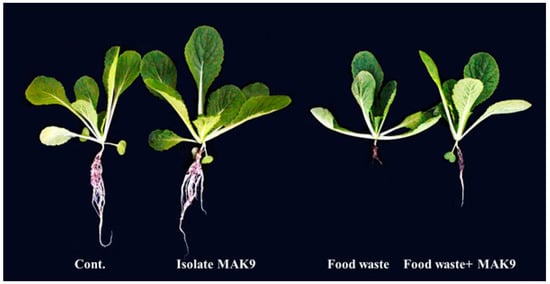
Figure 3.
Effects of bacterial isolate MAK9 on the growth of Chinese cabbage and on the effects of food waste (8%) application.

Table 1.
Effect of Bacillus pumilus MAK9 on the growth attributes of Chinese cabbage.
5.4. Effect of Food Waste on ABA and SA Content
Chinese cabbage plants treated with food waste or with food waste and bacterial inoculation were also tested to quantify endogenous SA and ABA content. The salt content in food waste significantly increased ABA content (3-fold); however, Chinese cabbage plants also inoculated with isolate MAK9 showed a significant decrease in ABA content (2-fold) compared with those treated with food waste alone (Figure 4A). In contrast, endogenous SA content decreased in food waste-treated plants; however, bacterial inoculated Chinese cabbage plants showed an increase in SA content (11%) compared with that in plants treated only with food waste (Figure 4B).
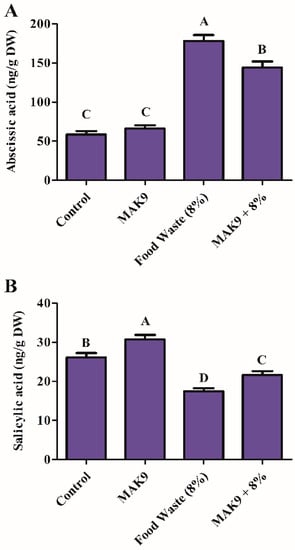
Figure 4.
Endogenous abscisic acid (A) and salicylic acid (B) content in Chinese cabbage after food waste (8%) treatment and/or inoculation with isolate MAK9. Different letters indicate differences between the mean values of three replicates ± standard deviation.
5.5. Quantification of Antioxidants in Chinese Cabbage under Food Waste Treatments
To investigate the influence of food waste on the antioxidant defense system of Chinese cabbage, the activities of major ROS scavenging enzymes, i.e., CAT, POD, PPO, GSH, and SOD, were investigated. No differences in antioxidant regulation were found between bacterial inoculated Chinese cabbage and the control plants. However, increases in CAT (26%), POD (1-fold), GSH (40%), PPO (66%), and SOA (67%) were observed in food waste-treated plants compared with control plants, whereas a decrease in total protein content (36%) was detected in treated plants (Figure 5). However, Chinese cabbage plants inoculated with isolate MAK9 showed a decrease in GSH (11%), PPO (17%), and SOA (18%) levels compared with those observed in food waste-treated plants. Moreover, increases in CAT (14%), POD (19%), and total protein content (26.96%) were observed in food waste-treated Chinese cabbage plants that were also inoculated with isolate MAK9 (Figure 5F).
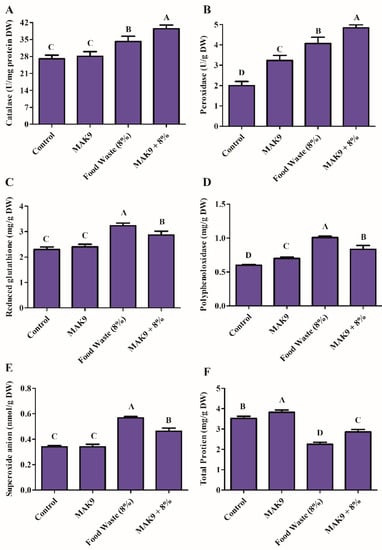
Figure 5.
Effects of isolate MAK9 on antioxidant levels in Chinese cabbage with and without food waste (8%) application. (A) Catalase, (B) peroxidase, (C) glutathione, (D) polyphenol oxidase, (E) superoxide anions, and (F) total protein. Different letters indicate differences between the mean values of three replicates ± standard deviations.
5.6. ICP Analysis of Na, K, P, and Ca Content
The accumulation of Ca, Na, K, and P were investigated using ICP analysis in control and food waste-treated Chinese cabbage with and without isolate MAK9 inoculation (Figure 6). Decreases in Ca (11.34 mg/kg), K (26.42 mg/kg), and P (49.82 mg/kg) content were observed in food waste-treated Chinese cabbage plants compared with in control plants (Ca, 12.40 mg/kg; K, 32.88 mg/kg; and P, 51.79 mg/kg; Figure 6). However, inoculation with isolate MAK9 mitigated the inhibitory effect of food waste containing salt and enhanced the content of Ca (11.70 mg/kg), K (32.44 mg/kg), and P (57.52 mg/kg) in Chinese cabbage plants compared with the respective contents in food waste-treated plants. Conversely, a decrease in Na content (28.30 mg/kg) was observed in inoculated plants compared with those treated with food waste only (30.41 mg/kg) (Figure 6).
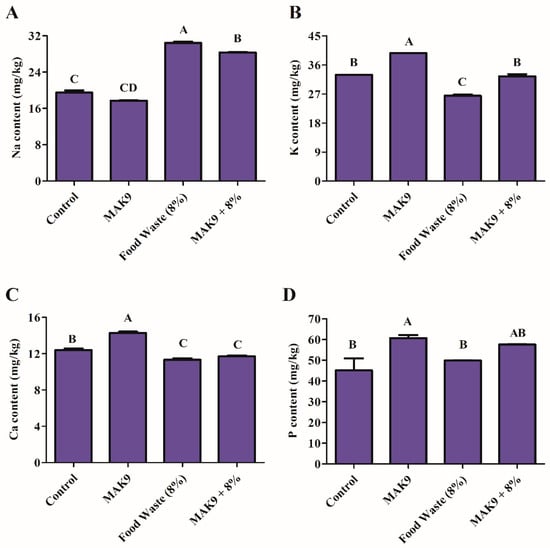
Figure 6.
The effect of isolate MAK9 on the sodium (Na, (A)); potassium (K, (B)); calcium (C, (C)); and phosphorus (P, (D)) content of Chinese cabbage inoculated with isolate MAK9 and/or treated with food waste (8%). Different letters indicate differences between the mean values of three replicates ± standard deviations.
5.7. Sugar Content Analysis
The effects of food waste treatment and bacterial inoculation on Chinese cabbage plants’ sugar metabolism were evaluated by quantifying sucrose, glucose, and fructose content (Table 2). Decreases in glucose (26%), fructose (20%), and sucrose (16%) content were observed in food waste-treated Chinese cabbage plants compared with in control plants (Table 2). However, inoculation with isolate MAK9 apparently inhibited the effect of salt present in food waste and thereby enhanced the content of glucose (17%), fructose (11%), and sucrose (14%) in Chinese cabbage plants (Table 2).

Table 2.
Effects of Bacillus pumilus MAK9 on the total sugar content (glucose, fructose, and sucrose) of Chinese cabbage plants when administered with and without food waste treatment.
6. Discussion
Food waste-based compost provides organic matter to the soil that can substantially improve soil properties [39]. However, the major impediment to the use of food waste-based compost in agriculture is the risk of increasing salinity caused by the salt in the waste, which affects stabilization and lowers the efficiency of solid degradation during the composting process. For sustainable crop cultivation, the law indicates that NaCl content in food waste compost must be <2% [19]. Lee et al. [20] reported that the average salt content in 290 food waste samples collected from all over Korea was 2.03%. The inhibitory effect of salinity stress is largely due to ionic, osmotic, and oxidative stresses, which limit plant growth and reduce yield in crops [40,41]. Salinity stress influences some basic metabolic process in plants including photosynthesis, biosynthesis, lipid metabolism, and protein synthesis [40,42]. Moreover, increase in photosynthetic rate can ameliorate the harmful effects of salt stress and help maintain normal plant growth and development. In the current study, NaCl content in food waste negatively affected the growth attributes and chlorophyll content of Chinese cabbage (Figure 3; Table 1), similar to previous reports for soybean, maize, pepper, tomato, and cucumber under salinity stress [25,26,27,43,44,45]. Similarly, several halotolerant plant growth promoting bacterial inocula have been reported to alleviate salt stress and enhance plant growth in different crops, such as wheat, goat’s rue, rice, cotton, tomato, and pepper [46,47,48,49], that support our current results in isolate MAK9 inoculated Chinese cabbage treated with food waste (Figure 3; Table 1).
The plant cell membrane plays an important role in the maintenance of the cell microenvironment and metabolism. However, NaCl stress reduces the permeability of the cell membrane through Na accumulation, which competes with the binding sites of K by reducing the K outflow that inactivates enzymatic and metabolic plant processes [50,51]. Na shares an antagonistic relationship with K, which was confirmed by our finding that K uptake was reduced in food waste-treated Chinese cabbage (Figure 6A,B), similar to salinity induced reductions in K uptake previously reported in tomato [52] and lettuce [53] plants. High concentrations of Na in cellular tissues hamper various growth and metabolic process in plants [54]. Here, Na content was higher in food waste-treated plants, but bacterial inoculation reduced this Na content (Figure 6A). It is known that lowered Ca levels under salt stress can affect the ion balance of the cytoplasmic membrane. In the current study, Chinese cabbage maintained higher Ca content with bacterial inoculation of isolate MAK9 alongside food waste treatment compared with food waste treatment alone (Figure 6C). P is also an essential nutrient that is required for various metabolic processes including photosynthesis, energy transfer, and signal transduction; consequently, P deficiency limits plant growth [55]. Our ICP results showed that Chinese cabbage inoculated with bacteria had higher P content than plants that were not inoculated (Figure 6D). Similarly, previous studies on bacteria-inoculated tomato and mung bean plants showed the positive influence of bacteria on the growth of salinity stressed plants via improved P uptake [56,57].
Most of the soluble sugar in plants comes from the photosynthate of sucrose in leaves, which can be decomposed into fructose and glucose in sink cells [58,59]. Glucose, fructose, and sucrose are readily accessible and provide osmoprotection and radical scavenging under stress conditions. A previous study showed that salinity stress decreases total soluble sugar content in cucumber plants [60]. In Chinese cabbage, administering bacterial inoculation of MAK9 with food waste treatment significantly reduced the negative impact of salt stress by increasing total soluble sugar content (Table 2). Salt content in food waste likely suppresses Chinese cabbage growth by damaging thylakoid membranes, which, in turn, reduce chlorophyll content and affect photosynthetic efficiency, which ultimately leads to reduced production of soluble sugars. Enhanced synthesis of soluble sugars and polysaccharides has previously been found to impart stress tolerance to plants [61]. Our finding of increased soluble sugar content under salinity stress was similar to that reported for tomato [62], wheat [63], cucumber [60], cotton [64], rice [65], and date palm [66] plants.
Phytohormones play important roles in the physiological responses and adaptation of plants to abiotic stresses such as salinity [67]. Among the phytohormones, ABA is a key mediator of osmotic stress responses; it regulates transpiration rate, controls stomatal opening, induces osmoprotactant accumulation, and activates ROS detoxification [68]. In the current study, the endogenous ABA levels of Chinese cabbage were significantly reduced by MAK9 inoculation alongside food waste treatment when compared with the levels detected with food waste treatment alone (Figure 4A). The decrease in ABA content in bacteria-inoculated plants is supported by previous findings for soybean [25], rice [65], and cucumber [45] plants. SA is also known to play a key role in modulating plant responses to a wide range of oxidative stresses [69]; it acts as a scavenger of ROS and induces systemic resistance that enhances stress tolerance [60]. Here, SA content was increased in food waste-treated plants that were also inoculated with isolate MAK9 (Figure 4B).
Higher reactive oxygen levels are produced during stress conditions, which lead to oxidative damage to proteins, lipids, and the plasma membrane [70]. With higher ROS production, plants produce several antioxidant enzymes to maintain the ROS balance in cells under stress conditions [70,71]. Our results showed that salinity stress from food waste increased the activity of antioxidant enzymes such as SOD, POD, and CAT in bacteria-inoculated Chinese cabbage plants (Figure 5). Similar results have been reported for desert bunchgrass [54] and alfalfa [72]. SOD is considered the first defensive enzyme as it scavenges superoxide radicals to hydrogen peroxide, which is further detoxified to water; furthermore, POD and CAT convert H2O2 to H2O. In a study of maize plants [27], higher antioxidant activities (including for SOD) were detected in bacteria-inoculated plants compared with those subjected to salt stress. Similarly, increased CAT activities were observed in soybean and rice plants inoculated with halotolerant bacteria under salinity stress. PPO, the enzyme that catalyzes the oxidation of phenolic compounds into highly reactive quinines, is also induced by salinity stress [72]. We found higher PPO content in bacteria-inoculated plants than that in control plants (Figure 5); thus, elevated PPO activity may reflect an increase in ROS scavenging capacity aimed at protecting plants from salinity stress.
7. Conclusions
Our results show that food waste can have detrimental effects on the physiological and biochemical parameters of Chinese cabbage due to the presence of high salt content. However, inoculation with isolate MAK9 in addition to food waste treatment was shown to enhance Chinese cabbage growth, reduce NaCl toxicity, inhibit Na+ uptake, and have positive antioxidant effects. These findings demonstrate that isolate MAK9 can alleviate salinity stress; therefore, it represents a valuable ecofriendly microorganism resource and a low-cost biotechnological approach to providing sustainable agriculture. It remains necessary to determine the specific application dose of food waste that avoids the negative impact of salinity in crop plants. Furthermore, long term evaluation of treatment with food waste plus halotolerant microbes is required with evaluation of soil quality parameters and plant growth under field conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae8010049/s1, Figure S1: Screening of bacterial isolates for different plant growth-promoting activities, Figure S2: Multiple plant growth-promoting traits produced by rhizospheric bacteria, Table S1: HPLC conditions used for analysis and quantification of organic acids, Table S2: GC/MS–SIM conditions used for analysis and quantification of IAA, Table S3: GC/MS–SIM conditions used for analysis and quantification of ABA, Table S4: HPLC conditions used for analysis and quantification of SA, Table S5: HPLC conditions used for the analysis and quantification of sugar content.
Author Contributions
Conceptualization and methodology, M.A.K. and I.-J.L.; software, K. and L.; formal analysis, M.I., S.S., E.-H.K. and S.-M.K.; resources, S.-H.K.; M.A.K.; writing—original draft preparation, M.A.K.; writing—review and editing, M.H.; supervision, I.-J.L.; funding acquisition, I.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Agenda Program (Project No. PJ015163032021) Rural Development Administration, Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bratovcic, A.; Zohorović, M.; Odobasic, A.; Šestan, I. Efficiency of food waste as an organic fertilizer. Int. J. Eng. Sci. Res. Technol. 2018, 7, 527–530. [Google Scholar] [CrossRef]
- FAO. Global food losses and food waste–Extent, causes and prevention. In SAVE FOOD: An Initiative on Food Loss and Waste Reduction; FAO: Rome, Italy, 2011. [Google Scholar]
- Schanes, K.; Dobernig, K.; Gözet, B. Food waste matters—A systematic review of household food waste practices and their policy implications. J. Clean. Prod. 2018, 182, 978–991. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. BioMed Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef]
- FAO. Towards the Future We Want: End Hunger and Make the Transition to Sustainable Agricultural and Food Systems; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; pp. 1–42. [Google Scholar]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Analysing global food waste problem: Pinpointing the facts and estimating the energy content. Cent. Eur. J. Eng. 2013, 3, 157–164. [Google Scholar] [CrossRef]
- Kawashima, T. The use of food waste as a protein source for animal feed—Current status and technological development in Japan. In Proceedings of the FAO Animal Production and Health Proceedings (FAO), Protein Sources for the Animal Feed Industry, Expert Consultation and Workshop, Bangkok, Thailand, 29 April–3 May 2002; pp. 303–311. [Google Scholar]
- Kim, J.D.; Park, J.S.; In, B.H.; Kim, D.; Namkoong, W. Evaluation of pilot-scale in-vessel composting for food waste treatment. J. Hazard. Mater. 2008, 154, 272–277. [Google Scholar] [CrossRef]
- Hamid, H.A.; Qi, L.P.; Harun, H.; Sunar, N.M.; Ahmad, F.H.; Muhamad, M.S. Development of Organic Fertilizer from Food Waste by Composting in UTHM Campus Pagoh. J. Des. Sustain. Environ. 2019, 1, 1–6. [Google Scholar]
- Tiwari, A.; Khawas, R. Food Waste and Agro By-Products: A Step towards Food Sustainability; IntechOpen: London, UK, 2021. [Google Scholar]
- Hossain, M.Z.; Von Fragstein, P.; Von Niemsdorff, P.; Heß, J. Effect of different organic wastes on soil properties and plant growth and yield: A review. Sci. Agric. Bohem. 2017, 48, 224–237. [Google Scholar]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Mahmood, A.; Iguchi, R.; Kataoka, R. Multifunctional food waste fertilizer having the capability of Fusarium-growth inhibition and phosphate solubility: A new horizon of food waste recycle using microorganisms. Waste Manag. 2019, 94, 77–84. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shaffique, S.; Kim, L.-R.; Kwon, E.-H.; Kim, S.-H.; Lee, Y.-H.; Kalsoom, K.; Aaqil Khan, M.; Lee, I.-J. Effects of Organic Fertilizer Mixed with Food Waste Dry Powder on the Growth of Chinese Cabbage Seedlings. Environments 2021, 8, 86. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture—Trends and Challenges; Annual Report; FAO: Rome, Italy, 2017; Volume 296. [Google Scholar]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, S.J.; Hwang, H.Y.; Kim, M.S.; Jung, H.i.; Luyima, D.; Hong, S.Y.; Oh, T.K.; Kim, S.H. Effects of food waste compost on the shift of microbial community in water saturated and unsaturated soil condition. Appl. Biol. Chem. 2019, 62, 36. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Kang, Y.-G.; Luyima, D.; Park, S.-J.; Oh, T.-K.; Lee, C.H. Characteristics of food waste: Water and salinity contents. Korean J. Agric. Sci. 2020, 47, 375–380. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Tartoura, K.A.H.; Youssef, S.A.; Tartoura, E.-S.A.A. Compost alleviates the negative effects of salinity via up-regulation of antioxidants in Solanum lycopersicum L. plants. Plant Growth Regul. 2014, 74, 299–310. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.A.; Lee, K.E.; Kang, S.M.; Dhungana, S.K.; Bhusal, N.; Lee, I.J. The Halotolerant Rhizobacterium-Pseudomonas koreensis MU2 Enhances Inorganic Silicon and Phosphorus Use Efficiency and Augments Salt Stress Tolerance in Soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Lubna; Asaf, S.; Hamayun, M.; Khan, A.L.; Waqas, M.; Khan, M.A.; Jan, R.; Lee, I.-J.; Hussain, A. Salt tolerance of Glycine max L. induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol. Biochem. 2018, 128, 13–23. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [Green Version]
- Kubi, H.A.A.; Khan, M.A.; Adhikari, A.; Imran, M.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Silicon and Plant Growth-Promoting Rhizobacteria Pseudomonas psychrotolerans CS51 Mitigates Salt Stress in Zea mays L. Agriculture 2021, 11, 272. [Google Scholar] [CrossRef]
- Chadha, N.; Prasad, R.; Varma, A. Plant Promoting Activities of Fungal Endophytes Associated with Tomato Roots from Central Himalaya, India and Their Interaction with Piriformospora Indica. Int. J. Pharma Bio Sci. 2015, 6, 333–343. [Google Scholar]
- Yu-Na, K.; Muhammad Aaqil, K.; Sang-Mo, K.; Muhammad, H.; In-Jung, L. Enhancement of Drought-Stress Tolerance of Brassica oleracea var. italica L. by Newly Isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500–1509. [Google Scholar] [CrossRef]
- Khan, M.A.; Hamayun, M.; Asaf, S.; Khan, M.; Yun, B.-W.; Kang, S.-M.; Lee, I.-J. Rhizospheric Bacillus spp. Rescues Plant Growth Under Salinity Stress via Regulating Gene Expression, Endogenous Hormones, and Antioxidant System of Oryza sativa L. Front. Plant Sci. 2021, 12, 1145. [Google Scholar] [CrossRef]
- Imran, M.; Aaqil Khan, M.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.-W.; Khan, A.L.; Lee, I.-J. Melatonin Ameliorates Thermotolerance in Soybean Seedling through Balancing Redox Homeostasis and Modulating Antioxidant Defense, Phytohormones and Polyamines Biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, Y.; Mun, B.-G.; Khan, A.L.; Waqas, M.; Kim, H.-H.; Shahzad, R.; Imran, M.; Yun, B.-W.; Lee, I.-J. Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS ONE 2018, 13, e0192650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Ullah, I.; Waqas, M.; Hamayun, M.; Khan, A.L.; Asaf, S.; Kang, S.-M.; Kim, K.-M.; Jan, R.; Lee, I.-J. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 2019, 77, 9–21. [Google Scholar] [CrossRef]
- De Sousa, A.; AbdElgawad, H.; Asard, H.; Pinto, A.; Soares, C.; Branco-Neves, S.; Braga, T.; Azenha, M.; Selim, S.; Al Jaouni, S.; et al. Metalaxyl Effects on Antioxidant Defenses in Leaves and Roots of Solanum nigrum L. Front. Plant Sci. 2017, 8, 1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.L.; Gilani, S.A.; Waqas, M.; Al-Hosni, K.; Al-Khiziri, S.; Kim, Y.-H.; Ali, L.; Kang, S.-M.; Asaf, S.; Shahzad, R.; et al. Endophytes from medicinal plants and their potential for producing indole acetic acid, improving seed germination and mitigating oxidative stress. J. Zhejiang Univ. Sci. B 2017, 18, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Lee, I.-J.; Kim, K.M. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 2020, 15, e0232228. [Google Scholar] [CrossRef]
- Juteau, P. Review of the use of aerobic thermophilic bioprocesses for the treatment of swine waste. Livest. Sci. 2006, 102, 187–196. [Google Scholar] [CrossRef]
- Hamayun, M.; Afzal Khan, S.; Khan, A.; Shinwari, Z.; Hussain, J.; Sohn, E.-Y.; Kang, S.-M.; Kim, Y.-H.; Khan, M.; Lee, I.-J. Effect of salt stress on growth attributes and endogenous growth hormones of soybean cultivar Hwangkeumkong. Abstr. Pap. 2010, 42, 3103–3112. [Google Scholar]
- Hamayun, M.; Sohn, E.-Y.; Afzal Khan, S.; Shinwari, Z.; Khan, A.; Lee, I.-J. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Abstr. Pap. 2010, 42, 1713–1722. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shin, J.H.; Ahmad, B.; Shin, D.H.; Lee, I.J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 2010, 58, 7226–7232. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.-Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins Producing Endophytic Fungus Porostereum spadiceum AGH786 Rescues Growth of Salt Affected Soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.L.; Waqas, M.; Hamayun, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Co-synergism of endophyte Penicillium resedanum LK6 with salicylic acid helped Capsicum annuumin biomass recovery and osmotic stress mitigation. BMC Microbiol. 2013, 13, 51. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-M.; Khan, A.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant Growth Promoting Rhizobacterias reduces adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Berg, G.; Lindström, K.; Räsänen, L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing Pseudomonas. Plant Soil 2013, 369, 453–465. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Yoo, S.-J.; Weon, H.-Y.; Song, J.; Sang, M.K. Induced tolerance to salinity stress by halotolerant bacteria Bacillus aryabhattai H19-1 and B. mesonae H20-5 in tomato plants. J. Microbiol. Biotechnol. 2019, 29, 1124–1136. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Glick, B.R.; Chauhan, P.S.; jong Yim, W.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 2011, 49, 427–434. [Google Scholar] [CrossRef]
- Hidri, R.; Barea, J.M.; Mahmoud, O.M.-B.; Abdelly, C.; Azcón, R. Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions. J. Plant Physiol. 2016, 201, 28–41. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na(+) and K(+) Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Aldubise, A.; Egamberdieva, D. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 2015, 10, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Shilev, S. Plant-Growth-Promoting Bacteria Mitigating Soil Salinity Stress in Plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Nayyar, H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mungbean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol. Mol. Biol. Plants 2016, 22, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lin, Q.; Feng, Y.; Fan, X.; Zou, F.; Yuan, D.-Y.; Zeng, X.; Cao, H. Transcriptomic Identification and Expression of Starch and Sucrose Metabolism Genes in the Seeds of Chinese Chestnut (Castanea mollissima). J. Agric. Food Chem. 2015, 63, 929–942. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Liu, W. Sugar accumulation and characterization of metabolizing enzyme genes in leafy head of Chinese cabbage (Brassica campestris L. ssp. pekinensis). Hortic. Environ. Biotechnol. 2021, 62, 17–29. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; Lee, S.-M.; Park, Y.-G.; Kim, A.-Y.; Seo, C.-W.; Lee, I.-J. Enterobacter sp. SE992-induced regulation of amino acids, sugars, and hormones in cucumber plants improves salt tolerance. Acta Physiol. Plant. 2015, 37, 149. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Song, L.; Li, K.; Zhang, X.; Liu, S.; Qin, Y.; Li, P. Polysaccharides from Grateloupia filicina enhance tolerance of rice seeds (Oryza sativa L.) under salt stress. Int. J. Biol. Macromol. 2019, 124, 1197–1204. [Google Scholar] [CrossRef]
- Chinsamy, M.; Kulkarni, M.G.; Van Staden, J. Garden-waste-vermicompost leachate alleviates salinity stress in tomato seedlings by mobilizing salt tolerance mechanisms. Plant Growth Regul. 2013, 71, 41–47. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, Y.; Guo, L.; Li, C. Root colonization of encapsulated Klebsiella oxytoca Rs-5 on cotton plants and its promoting growth performance under salinity stress. Eur. J. Soil Biol. 2014, 60, 81–87. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of Detrimental Effects of Salt Stress on Date Palm (Phoenix dactylifera L.) by the Application of Arbuscular Mycorrhizal Fungi and/or Compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Zahra, S.; Amin, B.; Ali, V.S.M.; Ali, Y.; Mehdi, Y. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) sugar, protein and proline contents under salinity stress (NaCl). J. Biophys. Struct. Biol. 2011, 2, 35–41. [Google Scholar]
- Ahanger, M.A.; Agarwal, R. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; IntechOpen: London, UK, 2016; pp. 463–480. [Google Scholar]
- Laouane, R.B.; Meddich, A.; Bechtaoui, N.; Oufdou, K.; Wahbi, S. Effects of arbuscular mycorrhizal fungi and rhizobia symbiosis on the tolerance of Medicago sativa to salt stress. Gesunde Pflanz. 2019, 71, 135–146. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).