Effect of Spaceflight on Tomato Seed Quality and Biochemical Characteristics of Mature Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions and Experimental Protocol
2.2. Resistance to Phytophtorosis
2.3. Sample Preparation
2.4. Dry Matter
2.5. Ascorbic Acid
2.6. Preparation of Ethanolic Extracts
2.7. Total Polyphenols (TP)
2.8. Antioxidant Activity (AOA)
2.9. Total Dissolved Solids (TDS)
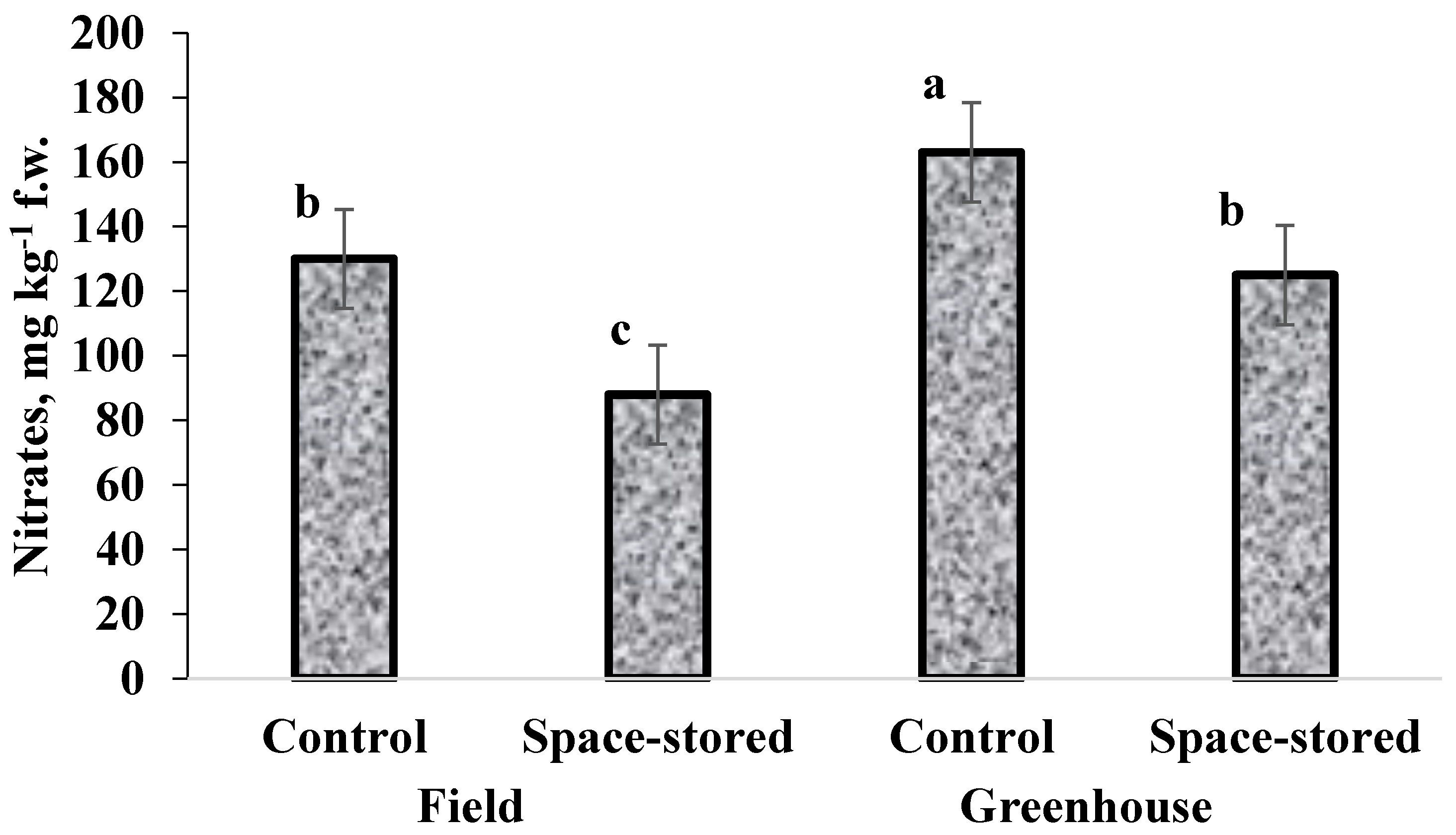
2.10. Nitrates
2.11. Monosaccharides (SS)
2.12. Titratable Acidity (TA)
2.13. Taste Index (TI)
2.14. Carotenoid Content
2.15. Statistical Analysis
3. Results and Discussion
3.1. Yield, Dry Matter Content, TDS and Nitrates
3.2. Antioxidant Status
3.3. Monosaccharides, Organic Acids, Taste
3.4. Elemental Composition
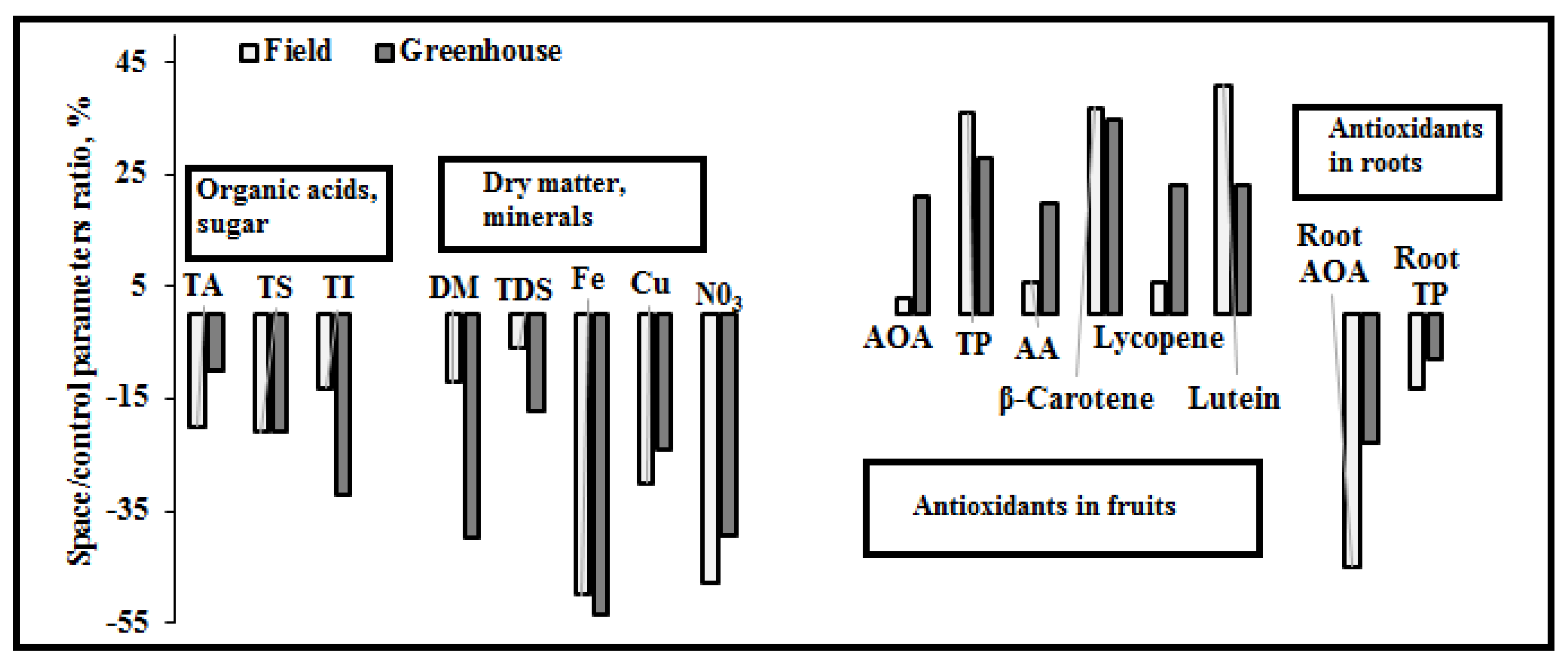
3.5. Relationships between the Analyzed Parameters
4. Conclusions and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Visscher, A.M.; Seal, C.E.; Newton, R.J.; Frances, A.L.; Pritchard, H.W. Dry seeds and environmental extremes: Consequences for seed lifespan and germination. Funct. Plant Biol. 2016, 43, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Vandenbrink, J.P.; Kiss, J.Z. Space, the final frontier: A critical review of recent experiments performed in microgravity. Plant Sci. 2016, 243, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.L.; Wheeler, R.M.; Levine, H.G.; Ferl, R.J. Fundamental plant biology enabled by the space shuttle. Am. J. Bot. 2013, 100, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kordyum, E.; Chapman, D.; Brykova, V. Plant cell development and aging may accelerate in microgravity. Acta Astronaut. 2019, 157, 157–161. [Google Scholar] [CrossRef]
- Musgrave, M.E. Seeds in space. Seed Sci. Res. 2007, 12, 1–17. [Google Scholar] [CrossRef]
- Cannon, K.M.; Britt, D.T. Feeding one million people on Mars. New Space 2019, 7, 245–254. [Google Scholar] [CrossRef]
- Wheeler, R.M. Agriculture for space: People and places paving the way. Open Agric. 2017, 2, 14–32. [Google Scholar] [CrossRef]
- Paul, A.L.; Sng, N.J.; Zupanska, A.K.; Krishnamurthy, A.; Schultz, E.R.; Ferl, R.J. Genetic dissection of the Arabidopsis spaceflight transcriptome: Are some responses dispensable for the physiological adaptation of plants to spaceflight? PLoS ONE 2017, 12, e0180186. [Google Scholar] [CrossRef] [PubMed]
- Poulet, L.; Fontaine, J.-P.; Dussap, C.-G. Plant’s response to space environment: A comprehensive review including mechanistic modelling for future space gardeners. Bot. Lett. 2016, 163, 337–347. [Google Scholar] [CrossRef]
- Lu, J.; Xue, H.; Pan, Y.; Kan, S.; Liu, M.; Nechitailo, G.S. Effect of spaceflight duration of subcellular morphologies and defense enzyme activities in earth-grown tomato seedlings propagated from space-flown seeds. Russ. J. Phys. Chem. B 2009, 3, 981–986. [Google Scholar] [CrossRef]
- Jiyuan, L.; Zhenye, Q.; Yongcheng, S.; Tianjun, Q.; Jun, H. Seed growth experiments after space flight: The Chinese experience. In Selected Papers on Remote Sensing, Space Science and Information Technology; Seminars of the United Nations Programmer on Space Applications; United Nations: New York, NY, USA, 1999; Volume 10, p. 71. [Google Scholar]
- Chandler, J.O.; Haas, F.B.; Khan, S.; Bowden, L.; Ignatz, M.; Enfissi, E.M.A.; Gawthrop, F.; Griffiths, A.; Fraser, P.D.; Rensing, S.A.; et al. Rocket Science: The effect of spaceflight on germination physiology, ageing, and transcriptome of Eruca sativa seeds. Life 2020, 10, 49. [Google Scholar] [CrossRef]
- Nechitailo, G.; Jinying, L.; Huai, X.; Yi, P.R.; Chongqin, T.; Liu, M. Influence of long term exposure to space flight on tomato seeds. Adv. Space Res. 2005, 36, 1329–1333. [Google Scholar] [CrossRef]
- Mishchenko, L.T.; Dunich, A.A.; Danilova, O.I. Impact of a real microgravity on the productivity of tomato plants and resistance to viruses. In Proceedings of the Life in Space for Life on Earth, Aberdeen, UK, 18–22 June 2012. ESA SP-706. [Google Scholar]
- Kahn, B.A.; Stoffella, P.J. No evidence of adverse effects on germination, emergence, and fruit yield due to space exposure of tomato seeds. J. Am. Soc. Hortic. Sci. 1996, 121, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.B.; Zhang, Y.; Deng, B.; Guo, H.; Cheng, L.; Liu, Y. Effect of space flight factors on alfalfa seeds. Afr. J. Biotechnol. 2010, 9, 7273–7279. [Google Scholar] [CrossRef]
- De Micco, V.; Arena, C.; Pignalosa, D.; Durante, M. Effects of Sparsely and Densely Ionizing Radiation on Plants. Radiat. Environ. Biophys. 2011, 50, 1–19. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; De Maio, A. Growth alteration and leaf biochemical responses in Phaseolus Vulgaris exposed to different doses of ionizing radiation. Plant Biol. 2014, 16, 194–202. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; Aronne, G.; Pugliese, M.G.; Virzo, A.; DeMaio, A. Response of Phaseolus vulgaris L. plants to low-LET ionizing radiation: Growth and oxidative stress. Acta Astronaut. 2014, 91, 107–114. [Google Scholar] [CrossRef]
- Zaka, R.; Vandecasteele, C.M.; Misset, M.T. Effects of low chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipa capillata (Poaceae). J. Exp. Bot. 2002, 53, 1979–1987. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; Macaevac, E.; Quintens, R. Space radiation effects on plant and mammalian cells. Acta Astronaut. 2014, 104, 419–431. [Google Scholar] [CrossRef]
- Fleming, M.B.; Patterson, E.L.; Reeves, P.A.; Richards, C.M.; Gaines, T.A.; Walters, C. Exploring the fate of mRNA in aging seeds: Protection, destruction, or slow decay? J. Exp. Bot. 2018, 69, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.B.; Hill, L.M.; Walters, C. The kinetics of ageing in dry-stored seeds: A comparison of viability loss and RNA degradation in unique legacy seed collections. Ann. Bot. 2019, 123, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Alpatiev, A.V.; Skvortsova, R.V.; Gurkina, L.K. Guidelines for the selection of tomato for phytophtorosis resistance. In Greenhouse and Field Conditions; VNIISSOK: Moscow, Russia, 1986; pp. 36–45. (In Russian) [Google Scholar]
- AOAC Association Official Analytical Chemists. The Official Methods of Analysis of AOAC International; 22 Vitamin C; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020. [Google Scholar] [CrossRef]
- Swamy, P.M. Laboratory Manual on Biotechnology; Rastogi: New Delhi, India, 2008; p. 617. [Google Scholar]
- Navez, B.; Letard, M.; Graselly, D.; Jost, J. Les critéres de qualité de la tomate. Infos-Ctifl 1999, 155, 41–47. [Google Scholar]
- Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Sumalan, R.L. The Antioxidant profile evaluation of some tomato landraces with soil salinity tolerance correlated with high nutraceutical and functional value. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Riccioni, G. Carotenoids and cardiovascular disease. Curr. Atheroscler. Rep. 2009, 11, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Keikel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti- proliferative properties of lycopene. Free Radic. Res. 2011, 45, 925–940. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Del Río, D.; Rodríguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Ayala-Zavala, J.F.; Dhua, R.S. Genotypic variation in tomatoes affecting processing and antioxidant properties. Crit. Rev. Food Sci. Nutr. 2015, 55, 1819–1835. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activities in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Naschimento, V.L.; Medeiros, D.B.; Nunes-Besi, A.; Ribeiro, D.M.; Zsoson, A.; Araujo, W.L. Modification of organic acids profile during fruit development and ripening: Correlation of causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.B.; Lechiheb, B.; Benyahya, L.; Ferchichi, A. Evaluation of fruit quality traits of traditional varieties of tomato (Solanum lycopersicum) grown in Tunisia. Afr. J. Food Sci. 2013, 7, 350–354. [Google Scholar] [CrossRef]
- Davies, J.N.; Hobson, G.E. The constituents of tomato fruit—The influence of environment, nutrition, and genotype. Crit. Rev. Food Sci. Technol. 1981, 15, 205–280. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Fita, A.; García-Martínez, M.D.; Casanova, C.; Borràs, D.; Plazas, M.; Andújar, I.; Soler, S. Characterization of composition traits related to organoleptic and functional quality for the differentiation, selection and enhancement of local varieties of tomato from different cultivar groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef]
- Causse, M.; Friguet, C.; Coiret, C.; Lépicier, M.; Navez, B.; Lee, M.; Holthuysen, L.; Sinesio, F.; Moneta, E.; Grandillo, S. Consumer preferences for fresh tomato at the European scale: A common segmentation on taste and firmness. J. Food Sci. 2010, 75, S531–S541. [Google Scholar] [CrossRef] [PubMed]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Torija, M.E.; Chaya, C.; Galiana-Balaguer, L.; Roselló, S.; Nuez, F. Internal quality characterization of fresh tomato fruits. HortScience 2004, 39, 339–345. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; ENDE, W.V.D.; Cuylers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plants growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Sakya, A.; Sulandjari, T. Foliar iron application on growth and yield of tomato. IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012001. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]


| Control Seeds | Space-Stored Seeds | |||
|---|---|---|---|---|
| Greenhouse | Field | Greenhouse | Field | |
| Two-leaf stage | 13 | 13 | 12 | 12 |
| Flowering | 52 | 57 | 49 | 56 |
| Fruit ripening | 94 | 115 | 90 | 120 |
| Control Seeds | Space-Stored Seeds | |||
|---|---|---|---|---|
| Greenhouse | Field | Greenhouse | Field | |
| Plant height (cm) | 65 b | 57 c | 77 a | 78 a |
| Yield (t ha−1) | 39.0 b | 34.3 c | 42.0 a | 36.9 b |
| Fruit weight (g) | 58 b | 52 c | 63 a | 56 b |
| Number of trusses per plant | 5 a | 4 b | 5 a | 4 b |
| Marketability (%) | 89 ab | 85 c | 92 a | 87 bc |
| Diseases (number of points) | 3.0 a | 2.5 b | 3.0 a | 3.0 a |
| Dry matter (%) | 11.5 ± 1.0 a | 7.1 ± 0.6 bc | 8.2 ± 0.6 b | 6.4 ± 0.5 c |
| Parameter | Control Seeds | Space-Stored Seeds | ||
|---|---|---|---|---|
| Greenhouse | Field | Greenhouse | Field | |
| Fruits | ||||
| AA (mg 100 g−1 d.w.) | 399 ± 30 c | 537 ± 40 ab | 479 ± 35 b | 571 ± 42 a |
| AOA (mg GAE g−1 d.w.) | 18.0 ± 1 b | 22.5 ± 1 a | 21.8 ± 1 a | 22.5 ± 1 a |
| TP (mg GAE g−1 d.w.) | 13.4 ± 1 b | 13.5 ± 0.9 b | 17.1 ± 1 a | 18.4 ± 1 a |
| Roots | ||||
| AOA (mg GAE g−1 d.w.) | 10.7 ± 0.8 a | 12.6 ± 1.0 a | 6.5 ± 0.3 b | 8.7 ± 0.4 c |
| TP (mg GAE g−1 d.w.) | 7.0 ± 0.5 a | 6.8 ± 0.4 a | 6.5 ± 0.3 ab | 6.0 ± 0.3 b |
| Parameter | Control Seeds | Space-Stored Seeds | ||
|---|---|---|---|---|
| Greenhouse | Field | Greenhouse | Field | |
| Brix (% f.w.) | 7.1 ± 0.4 a | 3.8 ± 0.2 b | 4.2 ± 0.2 b | 2.8 ± 0.1 c |
| TS (% f.w.) | 7.1 ± 0.3 a | 3.8 ± 0.2 c | 4.5 ± 0.3 b | 2.8 ± 0.2 d |
| TA (% f.w.) | 0.75 ± 0.04 a | 0.50 ± 0.03 b | 0.50 ± 0.03 b | 0.37 ± 0.02 c |
| TI | 1.22 | 0.88 | 0.92 | 0.78 |
| TM | 9.47 | 7.60 | 9.00 | 7.57 |
| Growing Environment | Seed Origin | Zn | Mn | Fe | Cu |
|---|---|---|---|---|---|
| Greenhouse | control | 7.0 ± 0.5 ab | 5.5 ± 0.4 bc | 45.8 ± 3.7 a | 3.1 ± 0.2 a |
| space-stored | 6.2 ± 0.4 b | 4.8 ± 0.3 c | 29.8 ± 2.0 b | 2.5 ± 0.1 b | |
| Open field | control | 8.7 ± 0.7 a | 6.8 ± 0.5 a | 39.8 ± 3.1 a | 3.0 ± 0.2 a |
| space-stored | 7.8 ± 0.7 a | 6.0 ± 0.5 ab | 28.4 ± 0.2 b | 2.3 ± 0.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhos, E.; Golubkina, N.; Antoshkina, M.; Kondratyeva, I.; Koshevarov, A.; Shkaplerov, A.; Zavarykina, T.; Nechitailo, G.; Caruso, G. Effect of Spaceflight on Tomato Seed Quality and Biochemical Characteristics of Mature Plants. Horticulturae 2021, 7, 89. https://doi.org/10.3390/horticulturae7050089
Dzhos E, Golubkina N, Antoshkina M, Kondratyeva I, Koshevarov A, Shkaplerov A, Zavarykina T, Nechitailo G, Caruso G. Effect of Spaceflight on Tomato Seed Quality and Biochemical Characteristics of Mature Plants. Horticulturae. 2021; 7(5):89. https://doi.org/10.3390/horticulturae7050089
Chicago/Turabian StyleDzhos, Elena, Nadezhda Golubkina, Marina Antoshkina, Irina Kondratyeva, Andrew Koshevarov, Anton Shkaplerov, Tatiana Zavarykina, Galina Nechitailo, and Gianluca Caruso. 2021. "Effect of Spaceflight on Tomato Seed Quality and Biochemical Characteristics of Mature Plants" Horticulturae 7, no. 5: 89. https://doi.org/10.3390/horticulturae7050089
APA StyleDzhos, E., Golubkina, N., Antoshkina, M., Kondratyeva, I., Koshevarov, A., Shkaplerov, A., Zavarykina, T., Nechitailo, G., & Caruso, G. (2021). Effect of Spaceflight on Tomato Seed Quality and Biochemical Characteristics of Mature Plants. Horticulturae, 7(5), 89. https://doi.org/10.3390/horticulturae7050089








