Iodine Biofortification Counters Micronutrient Deficiency and Improve Functional Quality of Open Field Grown Curly Endive
Abstract
1. Introduction
2. Materials and Methods
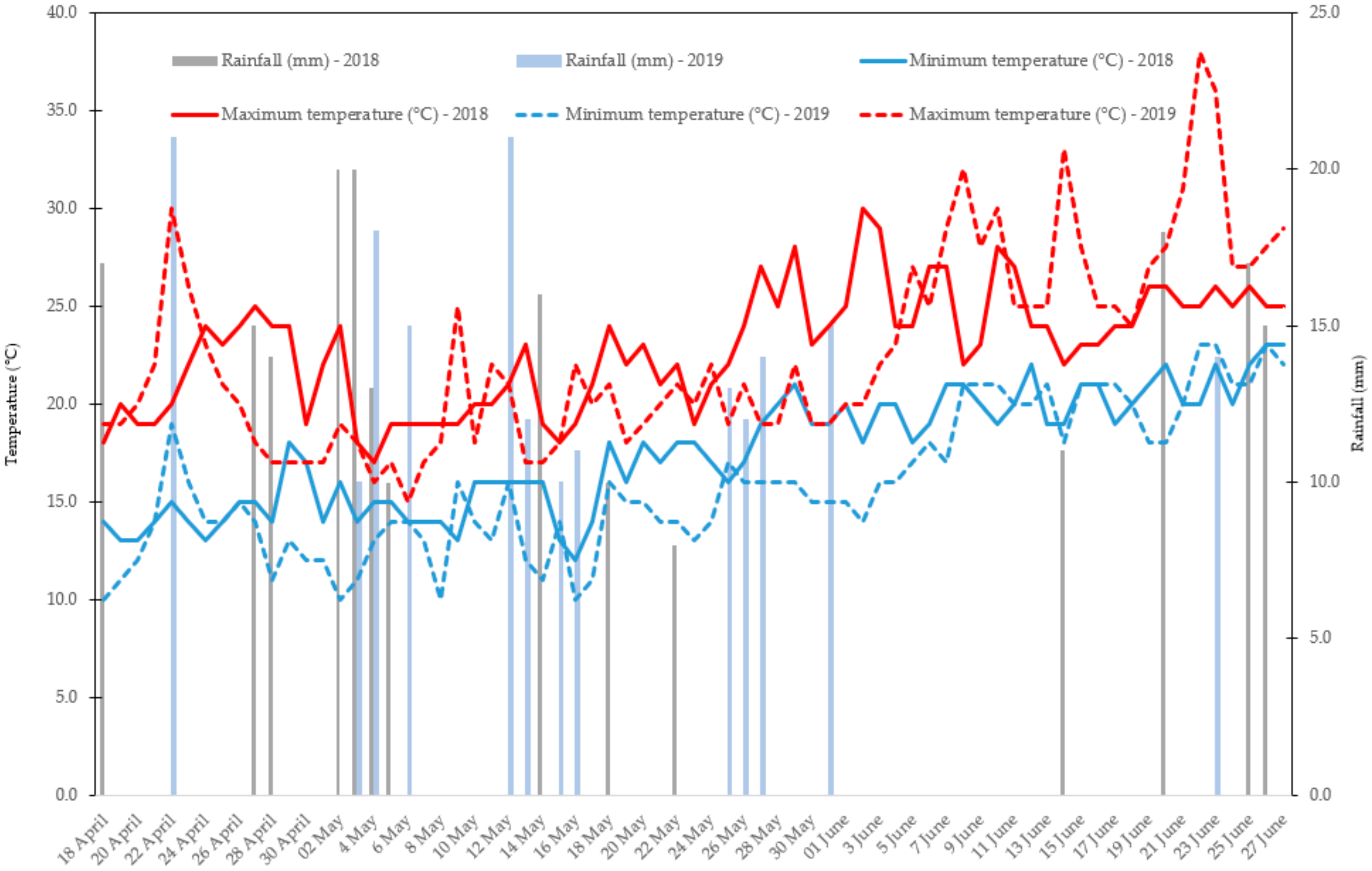
2.1. Trial Setup, Plant Materials, and Crop Management
2.2. Yield and Biometric Parameters
2.3. Nutraceutical Features
2.4. Sugars Assessment
2.5. Mineral Profile
2.6. Experimental Design and Statistics
3. Results
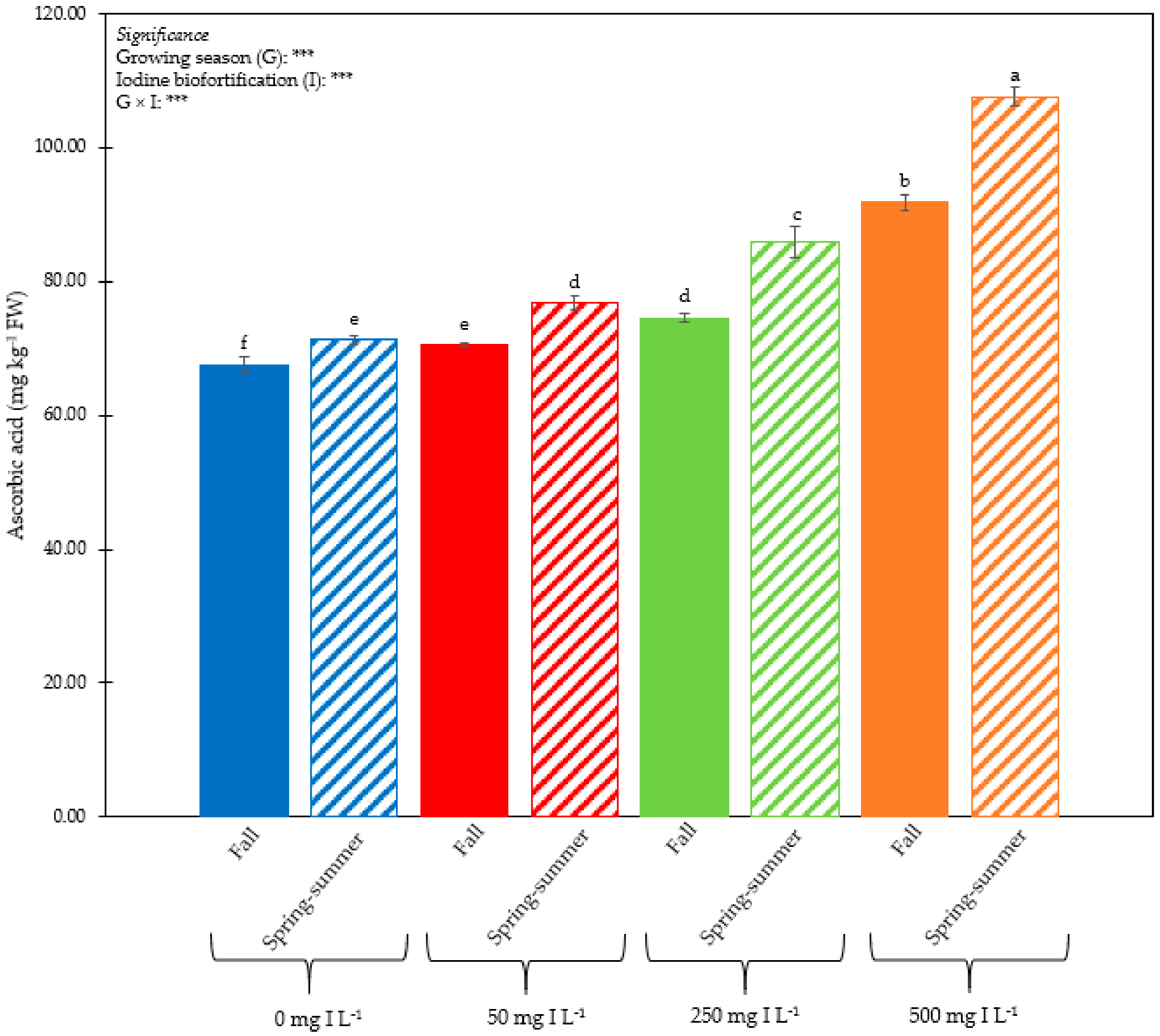
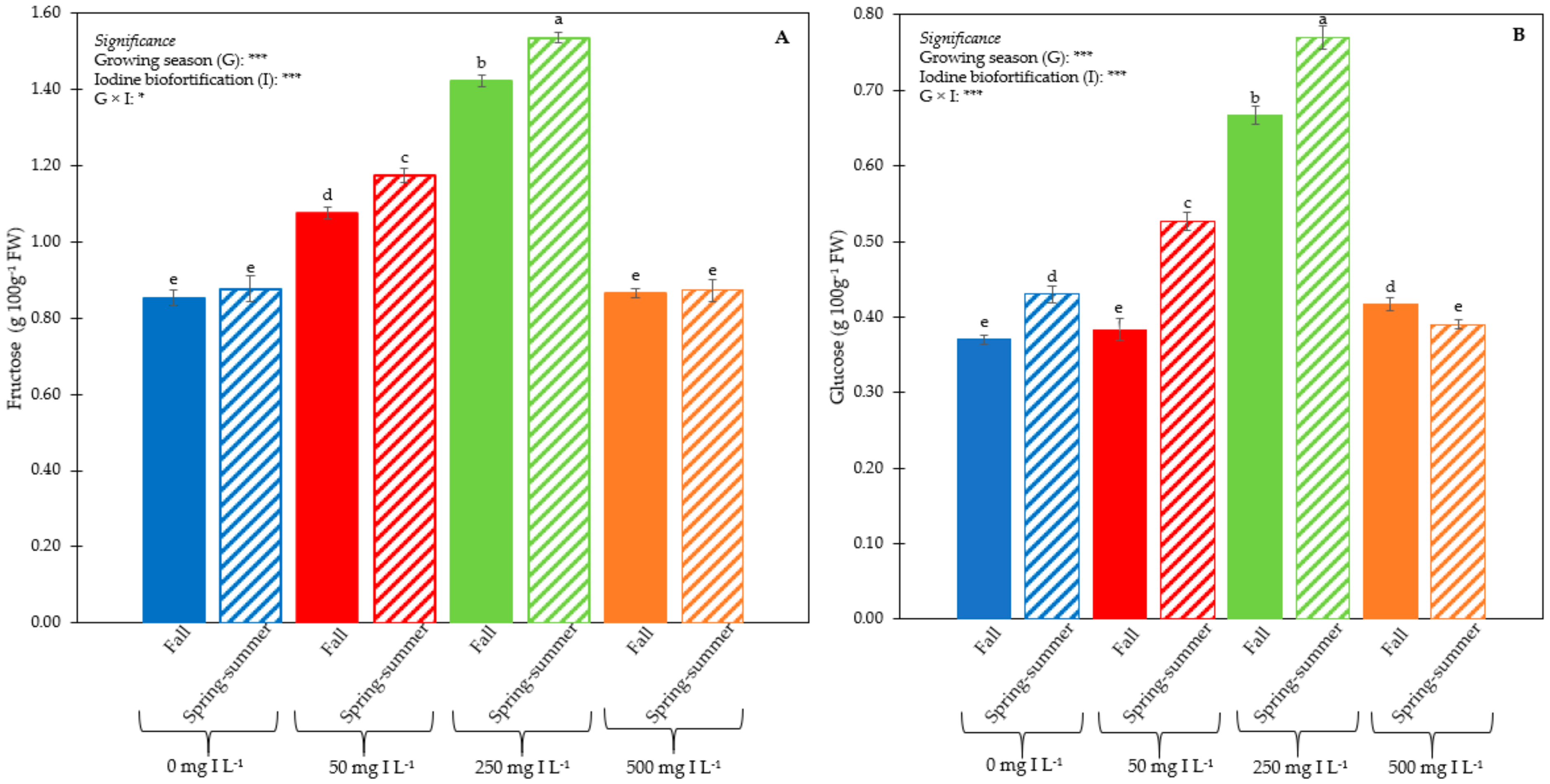
3.1. Plant Performance and Quality
3.2. Iodine Concentration in Leaf Tissues
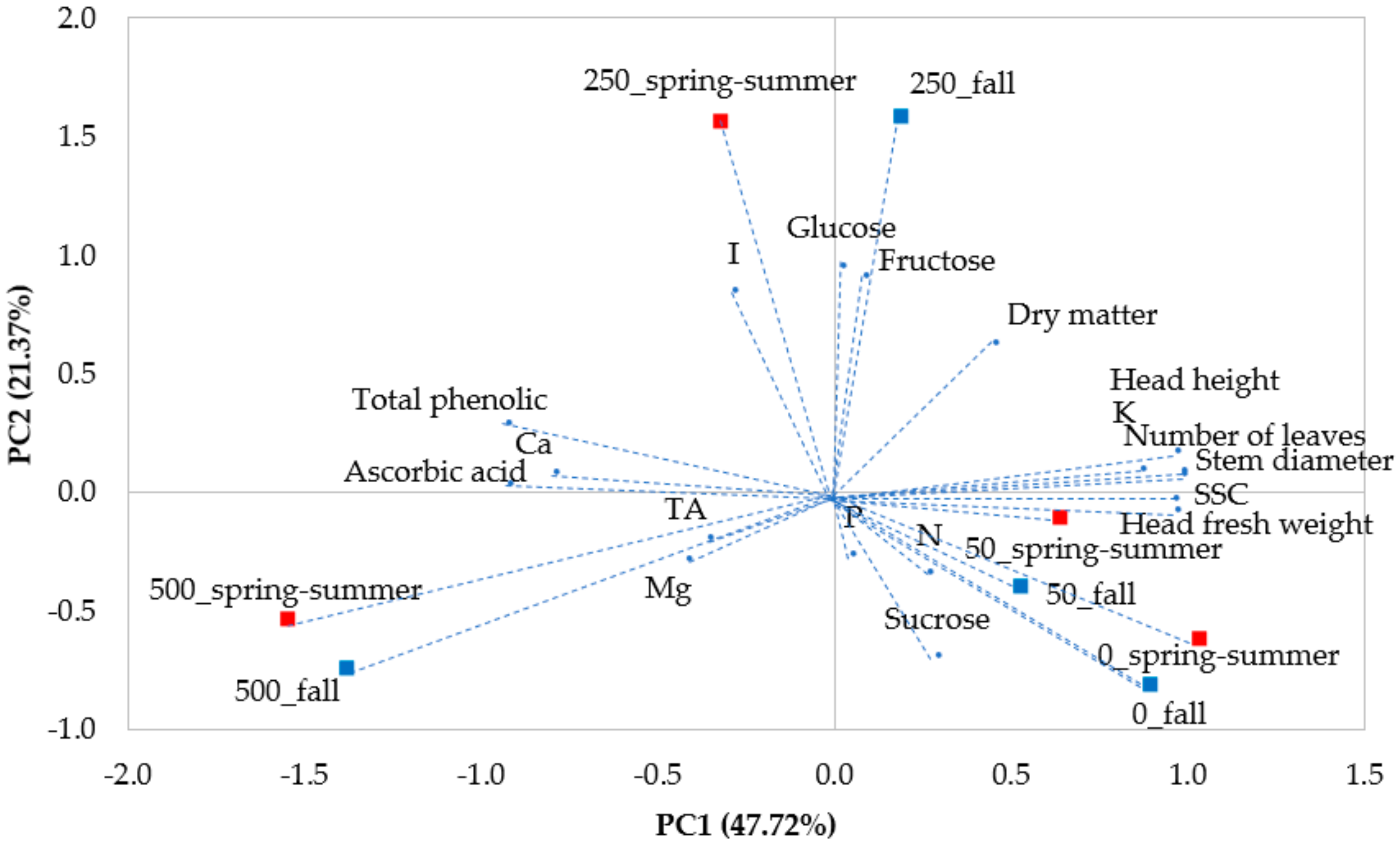
3.3. Principal Component Analysis of all Plant Traits (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grasberger, H.; Refetoff, S. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr. Opin. Pediatr. 2011, 23, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Takkouche, B.; Egli, I.; Allen, H.E.; de Benoist, B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull. World Health Organ. 2005, 83, 518–525. [Google Scholar]
- Delange, F. The role of iodine in brain development. Proc. Nutr. Soc. 2000, 59, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.C.; Milliez, J. Reproductive failure in women living in iodine deficient areas of West Africa. Br. J. Obstet. Gynaecol. 2000, 107, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. UNICEF, International Council for the Control of Iodine Deficiency Disorders. In Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for iodine. EFSA J. 2014, 12, 3660. [Google Scholar]
- Zimmermann, M.B. Iodine. In Nutrition and Health in a Developing World; de Pee, D., Taren, M., Bloem, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 287–295. [Google Scholar]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef]
- Mottiar, Y.; Altosaar, I. Iodine sequestration by amy lose to combat iodine deficiency disorders. Trends Food Sci. Technol. 2011, 22, 335–340. [Google Scholar] [CrossRef]
- Tonacchera, M.; Dimida, A.; De Servi, M.; Frigeri, M.; Ferrarini, E.; De Marco, G.; Grasso, L.; Agretti, P.; Piaggi, P.; Alghino-Lombardi, F.; et al. Iodine fortification of vegetables improves human iodine nutrition: In vivo evidence for a new model of iodine prophylaxis. J. Clin. Endocrinol. Metab. 2013, 98, E694–E697. [Google Scholar] [CrossRef]
- Kiferle, C.; Gonzali, S.; Holwerda, H.T.; Real Ibaceta, R.; Perata, P. Tomato fruits: A good target for iodine biofortification. Front. Plant Sci. 2013, 4, 205. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Stein, A.J. Global impacts of human mineral nutrition. Plant Soil 2010, 335, 133–154. [Google Scholar] [CrossRef]
- Tschiersch, J.; Shinonaga, T.; Heuberger, H. Dry deposition of gaseous radioiodine and particulate radiocaesium onto leafy vegetables. Sci. Total Environ. 2009, 407, 5685–5693. [Google Scholar] [CrossRef] [PubMed]
- Lawson, P.G.; Daum, D.; Czauderna, R.; Meuser, H.; Härtling, J.W. Soil versus foliar iodine fertilization as a biofortification strategy for field-grown vegetables. Front. Plant Sci. 2015, 6, 450. [Google Scholar] [CrossRef] [PubMed]
- Duborská, E.; Urík, M.; Kubová, J. Interaction with soil enhances the toxic effect of iodide and iodate on barley (Hordeum vulgare L.) compared to artificial culture media during initial growth stage. Arch. Agron. Soil Sci. 2018, 64, 46–57. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez- Maldonado, A.; Benavides Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Smoleń, S.; Sady, W. Influence of iodine form and application method on the effectiveness of iodine biofortification, nitrogen metabolism as well as the content of mineral nutrient sand heavy metals in spinach plants (Spinacia oleracea L.). Sci. Hortic. 2012, 143, 176–183. [Google Scholar] [CrossRef]
- Caffagni, A.; Arru, L.; Meriggi, P.; Milc, J.; Perata, P.; Pecchioni, N. Iodine fortification plant screening process and accumulation in tomato fruits and potato tubers. Commun. Soil Sci. Plant Anal. 2011, 42, 706–718. [Google Scholar] [CrossRef]
- Smoleń, S.; Sady, W.; Kołton, A.; Wiszniewska, A.; Liszka-Skoczylas, M. Iodine biofortification with additional application of salicylic acid affects yield and selected parameters of chemical composition of tomato fruits (Solanum lycopersicum L.). Sci. Hortic. 2015, 188, 89–96. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M.; D’Imperio, M.; Santamaria, P.; Serio, F. Iodine Biofortification of Four Brassica Genotypes is Effective Already at Low Rates of Potassium Iodate. Nutrients 2019, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Koudela, M.; Petříková, K. Nutritional composition and yield of endive cultivars—Cichorium endivia L. Hort. Sci. 2007, 34, 6–10. [Google Scholar] [CrossRef]
- D’Antuono, L.F.; Ferioli, F.; Manco, M.A. The impact of sesquiterpene lactones and phenolics on sensory attributes: An investigation of a curly endive and escarole germplasm collection. Food Chem. 2016, 199, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of selenium enrichment and type of application on yield, functional quality and mineral composition of curly endive grown in a hydroponic system. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; La Bella, S.; Tuttolomondo, T.; D’Anna, F.; Cardarelli, M.; Rouphael, Y. An Appraisal of Calcium Cyanamide as Alternative N Source for Spring-Summer and Fall Season Curly Endive Crops: Effects on Crop Performance, NUE and Functional Quality components. Agronomy 2020, 10, 1357. [Google Scholar] [CrossRef]
- Pardossi, A.; Prosdocimi Gianquinto, G.; Santamaria, P.; Incrocci, L. Orticoltura Principi e Pratica; Edagricole: Milano, Italy, 2018. [Google Scholar]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2008, 33, 67–78. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Garcia, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Serna, M.; Hernández, F.; Coll, F.; Coll, Y.; Amorós, A. Effects of brassinosteroid analogues on total phenols, antioxidant activity, sugars, organic acids and yield of field grown endive (Cichorium endivia L.). J. Sci. Food Agric. 2013, 93, 1765–1771. [Google Scholar] [CrossRef]
- Morand, P.; Gullo, J.L. Mineralisation des tissus vegetaux en vue du dosage de P, Ca, Mg, Na, K. Ann. Agron. 1970, 21, 229–236. [Google Scholar]
- Fogg, D.N.; Wilkinson, N.T. The colorimetric determination of phosphorus. Analist 1958, 83, 406–414. [Google Scholar] [CrossRef]
- Puccinelli, M.; Landi, M.; Maggini, R.; Pardossi, A.; Incrocci, L. Iodine biofortification of sweet basil and lettuce grown in two hydroponic systems. Sci. Hortic. 2021, 276, 109783. [Google Scholar] [CrossRef]
- Sabatino, L.; D’Anna, F.; Iapichino, G.; Moncada, A.; D’Anna, E.; De Pasquale, C. Interactive effects of genotype and molybdenum supply on yield and overall fruit quality of tomato. Front. Plant Sci. 2019, 9, 1922. [Google Scholar] [CrossRef]
- Blasco, B.; Ríos, J.J.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Leyva, R.; Romero, L.; Ruiz, J.M. Study of the interactions between iodine and mineral nutrients in lettuce plants. J. Plant Nutr. 2012, 35, 1958–1969. [Google Scholar] [CrossRef]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Pardossi, A. Iodine accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves grown in floating system technique. Front. Plant Sci. 2019, 10, 1494. [Google Scholar] [CrossRef]
- Borst Pauwels, G.W.F.H. Iodine as a micronutrient for plants. Plant Soil 1961, 14, 377–392. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Huang, Y.-Z.; Hu, Y.; Liu, Y.-X. Iodine uptake by spinach (Spinacia oleracea L.) plants grown in solution culture: Effects of iodine species and solution concentrations. Environ. Int. 2003, 29, 33–37. [Google Scholar] [CrossRef]
- Li, R.; Liu, H.P.; Hong, C.L.; Dai, Z.X.; Liu, J.W.; Zhou, J.; Hu, C.Q.; Weng, H.X. Iodide and iodate effects on the growth and fruit quality of strawberry. J. Sci. Food Agric. 2017, 97, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Signore, A.; Renna, M.; D’Imperio, M.; Serio, F.; Santamaria, P. Preliminary evidences of biofortification with iodine of “Carota di Polignano”, an Italian carrot landrace. Front. Plant Sci. 2018, 9, 170. [Google Scholar] [CrossRef]
- Tesi, R. Orticoltura Mediterranea Sostenibile; Pàtron Editore: Bologna, Italy, 2010. [Google Scholar]
- Islam, M.Z.; Ho-Min, K.A.N.G. Iron, iodine and selenium effects on quality, shelf life and microbial activity of cherry tomatoes. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 388–392. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, quality and antioxidant properties of Indian mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef]
- Kiferle, C.; Ascrizzi, R.; Martinelli, M.; Gonzali, S.; Mariotti, L.; Pistelli, L.; Flamini, G.; Perata, P. Effect of Iodine treatments on Ocimum basilicum L.: Biofortification, phenolics production and essential oil composition. PLoS ONE 2020, 15, e0229016. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Moglia, A.; Lanteri, S.; Comino, C.; Acquadro, A.; de Vos, R.; Beekwilder, J. Stress induced biosynthesis of dicaffeoylquinic acids in globe artichoke. J. Agric. Food Chem. 2008, 56, 8641–8649. [Google Scholar] [CrossRef]
- Gisbert, C.; Prohens, J.; Raigón, M.D.; Stommel, J.R.; Nuez, F. Eggplant relatives as sources of variation for developing new rootstocks: Effects of grafting on eggplant yield and fruit apparent quality and composition. Sci. Hortic. 2011, 128, 14–22. [Google Scholar] [CrossRef]
- Maršič, N.K.; Mikulič-Petkovšek, M.; Štampar, F. Grafting influences phenolic profile and carpometric traits of fruits of greenhouse-grown eggplant (Solanum melongena L.). J. Agric. Food Chem. 2014, 62, 10504–10514. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Rotino, G.L.; Palazzolo, E.; Mennella, G.; D’Anna, F. Solanum aethiopicum gr. gilo and its interspecific hybrid with S. melongena as alternative rootstocks for eggplant: Effects on vigor, yield, and fruit physicochemical properties of cultivar ‘Scarlatti’. Agronomy 2019, 9, 223. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Romero, L. Photorespiration process and nitrogen metabolism in lettuce plants (Lactuca sativa L.): Induced changes in response to iodine biofortification. J. Plant Growth Regul. 2010, 29, 477–486. [Google Scholar] [CrossRef]
- Lester, G.E. Environmental regulation of human health nutrients (ascorbic acid, β-carotene, and folic acid) in fruits and vegetables. HortScience 2006, 41, 59–64. [Google Scholar] [CrossRef]
- Eskling, M.; Akerlund, H.E. Changes in the quantities of violaxanthin de-epoxidase, xanthophylls and ascorbate in spinach upon shift from low to high light. Photosynth. Res. 1998, 57, 41–50. [Google Scholar] [CrossRef]
- Schonhof, I.; Kläring, H.P.; Krumbein, A.; Claußen, W.; Schreiner, M. Effect of temperature increase under low radiation conditions on phytochemicals and ascorbic acid in greenhouse grown broccoli. Agric. Ecosyst. Environ. 2007, 119, 103–111. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Ruiz, J.M. Iodine application affects nitrogen-use efficiency of lettuce plants (Lactuca sativa L.). Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 378–383. [Google Scholar]
- Weston, L.A.; Barth, M.M. Preharvest factors affecting postharvest quality of vegetables. HortScience 1997, 32, 812–816. [Google Scholar] [CrossRef]
- Caruso, G.; Villari, A.; Villari, G. Quality characteristics of Fragaria vesca L. fruits influenced by NFT solution EC and shading. In South Pacific Soilless Culture Conference-SPSCC 648; ISHS: Belgium, Leuven, February 2003; pp. 167–175. [Google Scholar]
- Kato, S.; Wachi, T.; Yoshihira, K.; Nakagawa, T.; Ishikawa, A.; Takagi, D.; Takahashi, M. Rice (Oryza sativa L.) roots have iodate reduction activity in response to iodine. Front. Plant Sci. 2013, 4, 227. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements of ten lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium, and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Voogt, W.; Steenhuizen, J.W.; Eveleens, B.A. Uptake and Distribution of Iodine in Cucumber, Sweet Pepper, Round, and Cherry Tomato; Wageningen UR Greenhouse Horticulture: Bleiswijk, The Netherlands, 2014; pp. 1–69. [Google Scholar]




| Treatments | Head Fresh Weight (g) | Stem Diameter (mm) | Head Height (cm) | |||
|---|---|---|---|---|---|---|
| Growing season | ||||||
| Fall | 734.07 | a | 22.94 | a | 31.00 | a |
| Spring–summer | 797.81 | a | 23.02 | a | 32.08 | a |
| Iodine Biofortification (mg L−1) | ||||||
| 0 | 1125.45 | a | 26.28 | a | 36.78 | a |
| 50 | 853.02 | b | 25.30 | a | 32.53 | b |
| 250 | 697.25 | c | 23.15 | b | 32.53 | b |
| 500 | 388.05 | d | 17.18 | c | 24.32 | c |
| Significance | ||||||
| Growing season (G) | NS | NS | NS | |||
| Iodine biofortification (I) | *** | *** | *** | |||
| G × I | NS | NS | NS | |||
| Treatments | TA (%) | SSC (°Brix) | Total Phenolics (mg of Caffeic Acid g−1 FW) | |||
|---|---|---|---|---|---|---|
| Growing season | ||||||
| Fall | 0.667 | 2.71 | 0.701 | b | ||
| Spring–summer | 0.667 | 2.64 | 0.786 | a | ||
| Iodine Biofortification (mg L−1) | ||||||
| 0 | 0.683 | 2.90 | a | 0.580 | d | |
| 50 | 0.683 | 2.87 | ab | 0.700 | c | |
| 250 | 0.663 | 2.62 | b | 0.790 | b | |
| 500 | 0.667 | 2.32 | c | 0.910 | a | |
| Significance | ||||||
| Growing season (G) | NS | NS | *** | |||
| Iodine biofortification (I) | NS | *** | *** | |||
| G × I | NS | NS | NS | |||
| Treatments | N (mg g−1 DW) | P (mg g−1 DW) | K (mg g−1 DW) | Mg (mg g−1 DW) | ||||
|---|---|---|---|---|---|---|---|---|
| Growing season | ||||||||
| Fall | 5.77 | a | 0.61 | a | 3.23 | 0.34 | ||
| Spring–summer | 5.59 | b | 0.57 | b | 3.23 | 0.33 | ||
| Iodine Biofortification (mg L−1) | ||||||||
| 0 | 5.73 | 0.58 | 3.25 | 0.30 | ||||
| 50 | 5.73 | 0.60 | 3.25 | 0.35 | ||||
| 250 | 5.68 | 0.59 | 3.23 | 0.33 | ||||
| 500 | 5.68 | 0.59 | 3.19 | 0.34 | ||||
| Significance | ||||||||
| Growing season (G) | * | *** | NS | NS | ||||
| Iodine biofortification (I) | NS | NS | NS | NS | ||||
| G × I | NS | NS | NS | NS | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatino, L.; Di Gaudio, F.; Consentino, B.B.; Rouphael, Y.; El-Nakhel, C.; La Bella, S.; Vasto, S.; Mauro, R.P.; D’Anna, F.; Iapichino, G.; et al. Iodine Biofortification Counters Micronutrient Deficiency and Improve Functional Quality of Open Field Grown Curly Endive. Horticulturae 2021, 7, 58. https://doi.org/10.3390/horticulturae7030058
Sabatino L, Di Gaudio F, Consentino BB, Rouphael Y, El-Nakhel C, La Bella S, Vasto S, Mauro RP, D’Anna F, Iapichino G, et al. Iodine Biofortification Counters Micronutrient Deficiency and Improve Functional Quality of Open Field Grown Curly Endive. Horticulturae. 2021; 7(3):58. https://doi.org/10.3390/horticulturae7030058
Chicago/Turabian StyleSabatino, Leo, Francesca Di Gaudio, Beppe Benedetto Consentino, Youssef Rouphael, Christophe El-Nakhel, Salvatore La Bella, Sonya Vasto, Rosario Paolo Mauro, Fabio D’Anna, Giovanni Iapichino, and et al. 2021. "Iodine Biofortification Counters Micronutrient Deficiency and Improve Functional Quality of Open Field Grown Curly Endive" Horticulturae 7, no. 3: 58. https://doi.org/10.3390/horticulturae7030058
APA StyleSabatino, L., Di Gaudio, F., Consentino, B. B., Rouphael, Y., El-Nakhel, C., La Bella, S., Vasto, S., Mauro, R. P., D’Anna, F., Iapichino, G., Caldarella, R., & De Pasquale, C. (2021). Iodine Biofortification Counters Micronutrient Deficiency and Improve Functional Quality of Open Field Grown Curly Endive. Horticulturae, 7(3), 58. https://doi.org/10.3390/horticulturae7030058














