The Effects of Nutrient Solution Feeding Regime on Yield, Mineral Profile, and Phytochemical Composition of Spinach Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genetic Material, Growth Chamber Settings, and Nutrient Feeding
2.2. CIELAB Color Space Parameters Measurement of Spinach Microgreens’ Canopy, Sampling, and Yield Assessment
2.3. Determination of Minerals, Nitrates, and Total Ascorbic Acid
2.4. Chlorophyll Pigments, ABTS Antioxidant Activity, Carotenoid Extraction, and Quantification by HPLC-DAD
2.5. Phenolic Compound Extraction and Conditions of UHPLC-HRMS Analysis
2.6. Statistics
3. Results and Discussion
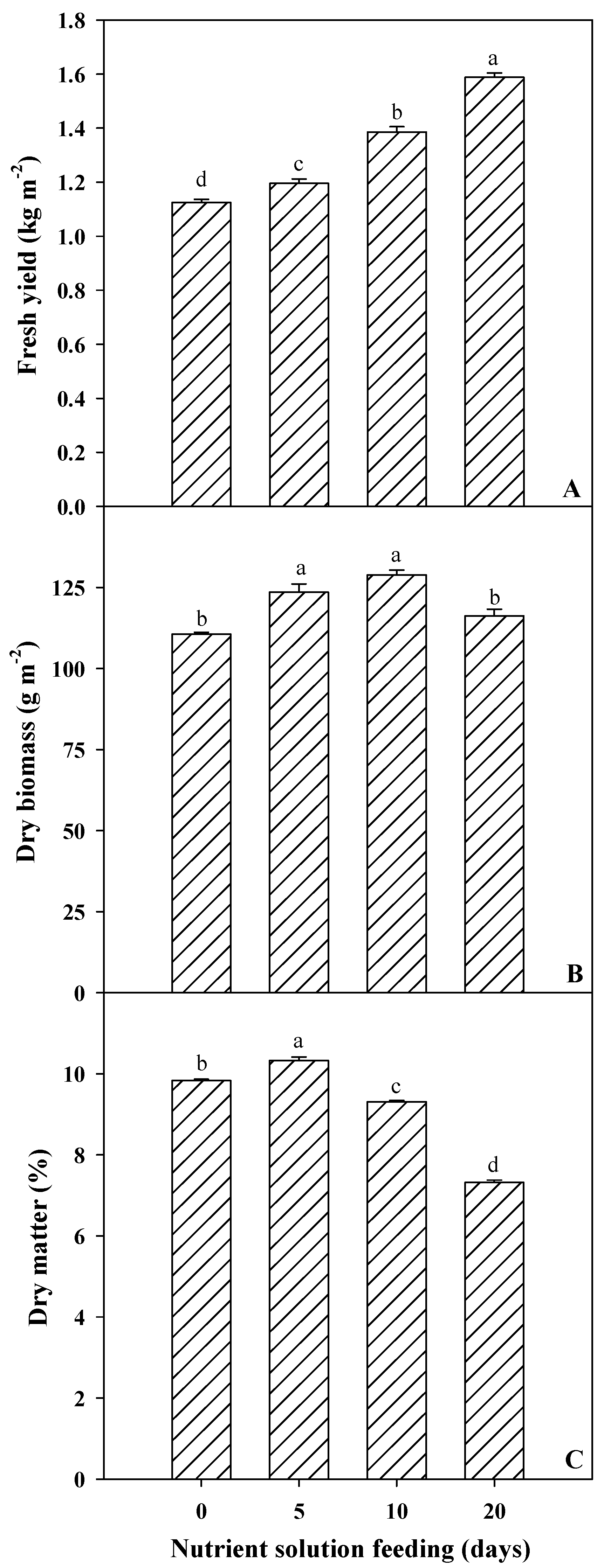
3.1. Spinach Microgreens’ Biometric and Colorimetric Parameters
3.2. Spinach Microgreens’ Nitrate and Macromineral Concentrations
3.3. Spinach Microgreens’ Pigments, Total Ascorbic Acid, and ABTS Antioxidant Activity
3.4. Spinach Microgreens’ Polyphenol Profiles and Total Polyphenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Renna, M.; Crupi, P.; Lovece, A.; Corbo, F.; Santamaria, P. Yield and Quality Characteristics of Brassica Microgreens as A ff ected by the NH4:NO3 Molar Ratio and Strength of the Nutrient Solution. Foods 2020, 9, 677. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Minimally Processed Refrigerated Fruits and Vegetableså; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 403–432. ISBN 978-1-4939-7016-2. [Google Scholar]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and iron agronomic biofortification of Brassicaceae microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Iron biofortification of red and green pigmented lettuce in closed soilless cultivation impacts crop performance and modulates mineral and bioactive composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- Bergquist, S.Å.; Gertsson, U.E.; Knuthsen, P.; Olsson, M.E. Flavonoids in baby spinach (Spinacia oleracea L.): Changes during plant growth and storage. J. Agric. Food Chem. 2005, 53, 9459–9464. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Renna, M.; Paradiso, V.M. Ongoing research on microgreens: Nutritional properties, shelf-life, sustainable production, innovative growing and processing approaches. Foods 2020, 9, 862. [Google Scholar] [CrossRef]
- Ghoora, M.; Srividya, N. Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus sativus L. and Hibiscus sabdariffa L. Microgreens. Foods 2020, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rasane, P.; Dey, A.; Singh, J.; Kaur, S.; Dhawan, K.; Kumar, A.; Joshi, H.S. Optimization of a Process for a Microgreen and Fruit Based Ready to Serve Beverage. Int. J. Food Stud. 2021, 10, SI41–SI56. [Google Scholar] [CrossRef]
- Schlering, C.; Zinkernagel, J.; Dietrich, H.; Frisch, M.; Schweiggert, R. Alterations in the chemical composition of spinach (Spinacia oleracea L.) as provoked by season and moderately limited water supply in open field cultivation. Horticulturae 2020, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-Specific Modulatory Effects of Select Spectral Bandwidths on the Nutritive and Phytochemical Composition of Microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef]
- Tomasi, N.; Pinton, R.; Dalla Costa, L.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. New “solutions” for floating cultivation system of ready-to-eat salad: A review. Trends Food Sci. Technol. 2014, 46, 267–276. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef]
- Courbet, G.; D’Oria, A.; Lornac, A.; Diquélou, S.; Pluchon, S.; Arkoun, M.; Koprivova, A.; Kopriva, S.; Etienne, P.; Ourry, A. Specificity and Plasticity of the Functional Ionome of Brassica napus and Triticum aestivum Subjected to Macronutrient Deprivation. Front. Plant Sci. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Sardare, M.D.; Admane, S. A Review on Plant without Soil—Hydroponics. Int. J. Res. Eng. Technol. 2013, 2, 299–304. [Google Scholar] [CrossRef]
- Hajnos, M.; Hajnos, M. Buffer Capacity of Soils BT. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 94–95. ISBN 978-90-481-3585-1. [Google Scholar]
- Yadav, L.P.; Koley, T.K.; Tripathi, A.; Singh, S. Antioxidant Potentiality and Mineral Content of Summer Season Leafy Greens: Comparison at Mature and Microgreen Stages Using Chemometric. Agric. Res. 2019, 8, 165–175. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Lester, G.; Hallman, G.; Pérez, J.A. γ -Irradiation Dose: Effects on Baby-Leaf Spinach Ascorbic Acid, Carotenoids, Folate, r -Tocopherol, and Phylloquinone Concentrations. J. Agric. Food Chem. 2010, 58, 4901–4906. [Google Scholar] [CrossRef]
- Elia, A.; Santamaria, P.; Serio, F. Nitrogen nutrition, yield and quality of spinach. J. Sci. Food Agric. 1998, 76, 341–346. [Google Scholar] [CrossRef]
- Tamme, T.; Reinik, M.; Roasto, M. Chapter 21-Nitrates and Nitrites in Vegetables: Occurrence and Health Risks. In Bioactive Foods in Promoting Health Fruits and Vegetables; Watson, R.R., Preedy, V., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 307–321. ISBN 978-0-12-374628-3. [Google Scholar]
- Erfani, F.; Hassandokht, M.R.; Jabbari, A.; Barzegar, M. Effect of cultivar on chemical composition of some Iranian spinach. Pak. J. Biol. Sci. 2007, 10, 602–606. [Google Scholar]
- Bergquist, S.Å.M.; Gertsson, U.E.; Olsson, M.E. Influence of growth stage and postharvest storage on ascorbic acid and carotenoid content and visual quality of baby spinach (Spinacia oleracea L.). J. Sci. Food Agric. 2006, 86, 346–355. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Fellegrini, N.; Ke, R.; Yang, M.; Rice-Evans, C.B.T.-M. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azinobis(3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. In Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 379–389. ISBN 0076-6879. [Google Scholar]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Pham, Q.; Sun, J.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S.; et al. Red Cabbage Microgreens Lower Circulating Low-Density Lipoprotein (LDL), Liver Cholesterol, and Inflammatory Cytokines in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef] [PubMed]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Gaspari, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Nutrient Supplementation Configures the Bioactive Profile and Production Characteristics of Three Brassica L. Microgreens Species Grown in Peat-Based Media. Agronomy 2021, 11, 346. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Murphy, C.J.; Pill, W.G. Cultural practices to speed the growth of microgreen arugula (roquette; Eruca vesicaria subsp. sativa). J. Hortic. Sci. Biotechnol. 2010, 85, 171–176. [Google Scholar] [CrossRef]
- Wieth, A.R.; Pinheiro, W.D.; Duarte, T.D.S. Purple cabbage microgreens grown in different substrates and nutritive solution concentrations. Rev. Caatinga 2019, 32, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Pannico, A.; Graziani, G.; El-Nakhel, C.; Giordano, M.; Ritieni, A.; Kyriacou, M.C.; Rouphael, Y. Nutritional stress suppresses nitrate content and positively impacts ascorbic acid concentration and phenolic acids profile of lettuce microgreens. Italus Hortus 2020, 27, 41–52. [Google Scholar] [CrossRef]
- Chen, X.G.; Gastaldi, C.; Siddiqi, M.Y.; Glass, A.D.M. Growth of a lettuce crop at low ambient nutrient concentrations: A strategy designed to limit the potential for eutrophication. J. Plant Nutr. 1997, 20, 1403–1417. [Google Scholar] [CrossRef]
- Van Iersel, M. Fertilizer Concentration Affects Growth and Nutrient Composition of Subirrigated Pansies. HortScience 1999, 34, 660–663. [Google Scholar] [CrossRef] [Green Version]
- Blom-Zandstra, M.; Lampe, J.A.N.E.M. The Role of Nitrate in the Osmoregulation of Lettuce (Lactuca sativa L.) Grown at Different Light Intensities. J. Exp. Bot. 1985, 36, 1043–1052. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Borah, K.D.; Bhuyan, J. Magnesium porphyrins with relevance to chlorophylls. Dalt. Trans. 2017, 46, 6497–6509. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Walczyk, T.; Leisibach, S.; Hurrell, R. Chlorophyll-bound Magnesium in Commonly Consumed Vegetables and Fruits: Relevance to Magnesium Nutrition. J. Food Sci. 2004, 69, 347–350. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Timilsina, R.; Khadka, D. Profile of chlorophylls and carotenoids of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) microgreens. J. Food Sci. Technol. 2019, 56, 2758–2763. [Google Scholar] [CrossRef]
- Pannico, A.; El-nakhel, C.; Kyriakou, M.C.; Giordano, M.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Combating Micronutrient Deficiency and Enhancing Food Functional Quality through Selenium Fortification of Select Lettuce Genotypes Grown in a Closed Soilless System. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, C.; Urli, B.; Juki, M.; Kläring, H. Nitrogen Limited Red and Green Leaf Lettuce Accumulate Flavonoid Glycosides, Caffeic Acid Derivatives and Sucrose while Losing. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.; Gioia, F.D.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.; Barros, L.; et al. Wild and cultivated Centaurea raphanina subsp. mixta: A valuable source of bioactive compounds. Antioxidants 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Liberal, Â.; Fernandes, Â.; Polyzos, N.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Bioactive Properties and Phenolic Compound Profiles of Turnip-Rooted, Plain-Leafed and Curly-Leafed Parsley Cultivars. Molecules 2020, 25, 5606. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The effects of biostimulants, biofertilizers and water-stress on nutritional value and chemical composition of two spinach genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Chrysargyris, A.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.D.; Barros, L.; et al. Chemical composition and plant growth of Centaurea raphanina subsp. mixta plants cultivated under saline conditions. Molecules 2020, 25, 2204. [Google Scholar] [CrossRef] [PubMed]
- Senizza, B.; Zhang, L.; Miras-Moreno, B.; Righetti, L.; Zengin, G.; Ak, G.; Bruni, R.; Lucini, L.; Sifola, M.I.; El-Nakhel, C.; et al. The strength of the nutrient solution modulates the functional profile of hydroponically grown lettuce in a genotype-dependent manner. Foods 2020, 9, 1156. [Google Scholar] [CrossRef]
| Nutrient Solution Feeding (Days) | L * | a * | b * | Chroma | Hue Angle |
|---|---|---|---|---|---|
| 0 | 38.52 ± 0.35 a | −7.61 ± 0.42 a | 31.21 ± 0.32 a | 16.05 ± 0.16 a | 103.7 ± 0.61 c |
| 5 | 38.04 ± 0.71 a | −7.51 ± 0.04 a | 30.60 ± 0.22 a | 15.81 ± 0.09 a | 103.8 ± 0.08 c |
| 10 | 35.31 ± 0.61 b | −7.42 ± 0.35 a | 25.67 ± 0.57 b | 13.87 ± 0.22 b | 106.2 ± 0.96 b |
| 20 | 38.66 ± 0.95 a | −8.71 ± 0.02 b | 22.41 ± 0.75 c | 12.73 ± 0.29 c | 111.3 ± 0.71 a |
| * | * | *** | *** | *** |
| Nutrient Solution Feeding (Days) | NO3 | P | K | Ca | Mg |
|---|---|---|---|---|---|
| (mg kg−1 fw) | (mg kg−1 fw) | (mg kg−1 fw) | (mg kg−1 fw) | (mg kg−1 fw) | |
| 0 | 159 ± 14 c | 789 ± 19 a | 8225 ± 72 c | 147 ± 12 a | 1038 ± 18 b |
| 5 | 240 ± 8.3 c | 775 ± 12 a | 8897 ± 84 a | 115 ± 8.4 b | 1110 ± 2.8 a |
| 10 | 498 ± 43 b | 817 ± 15 a | 8568 ± 86 b | 95.6 ± 1.5 b | 893 ± 28 c |
| 20 | 1698 ± 24 a | 694 ± 7.6 b | 6115 ± 89 d | 53.5 ± 3.9 c | 618 ± 18 d |
| *** | *** | *** | *** | *** |
| Nutrient Solution Feeding (Days) | ABTS | Total Chlorophylls | Lutein | β-Carotene | Total Ascorbic Acid |
|---|---|---|---|---|---|
| mol Trolox eq. 100g−1 fw | mg 100g−1 fw | µg g−1 fw | µg g−1 fw | mg 100g−1 fw | |
| 0 | 663.7 ± 22.2 b | 43.0 ± 1.19 c | 33.9 ± 0.59 c | 19.3 ± 0.86 c | 130.5 ± 1.61 d |
| 5 | 725.8 ± 26.2 b | 41.3 ± 0.87 c | 37.4 ± 0.46 c | 21.4 ± 0.87 c | 145.1 ± 2.47 c |
| 10 | 751.6 ± 15.9 b | 56.9 ± 2.07 b | 48.8 ± 1.04 b | 27.2 ± 2.34 b | 167.3 ± 5.03 a |
| 20 | 994.8 ± 36.2 a | 82.8 ± 5.36 a | 54.2 ± 2.33 a | 44.0 ± 0.96 a | 156.4 ± 3.04 b |
| *** | *** | *** | *** | *** |
| Nutrient Solution Feeding (Days) | Sig. | |||||
|---|---|---|---|---|---|---|
| Peak No | Phenolic Compounds | 0 | 5 | 10 | 20 | |
| 1 | Km 3-hydroxyferuloylsophorotrioside-7-glucoside | 46.36 ± 3.41 a | 35.65 ± 1.12 b | 34.98 ± 1.58 b | 28.20 ± 0.57 c | *** |
| 2 | Qn 3-sophoroside-7-glucoside | 539.8 ± 27.0 c | 529.5 ± 42.1 c | 690.1 ± 15.5 b | 861.9 ± 35.4 a | *** |
| 3 | Kaempferol-3-diglucoside | 1.34 ± 0.13 c | 2.50 ± 0.04 ab | 2.40 ± 0.17 b | 2.91 ± 0.18 a | *** |
| 4 | Km 3-sinapoylsophoroside-7-glucoside | 464.8 ± 16.1 | 464.8 ± 9.51 | 489.7 ± 29.7 | 556.8 ± 30.8 | ns |
| 5 | Km 3-sinapoylsophorotrioside-7-glucoside | 36.99 ± 0.15 a | 31.25 ± 2.78 b | 29.00 ± 0.92 b | 19.78 ± 1.11 c | *** |
| 6 | Qn 3-sinapoyltriglucoside | 113.7 ± 5.95 a | 101.0 ± 2.34 b | 87.68 ± 1.72 c | 90.85 ± 1.81 bc | ** |
| 7 | Synapoyl-hexose | 86.54 ± 2.88 ab | 90.78 ± 0.74 a | 81.98 ± 3.35 b | 34.63 ± 1.38 c | *** |
| 8 | Caffeoylquinic acid | 9.81 ± 0.33 c | 14.08 ± 0.46 b | 33.05 ± 1.18 a | 5.37 ± 0.21 d | *** |
| 9 | Rutin | 1.35 ± 0.08 c | 1.94 ± 0.15 bc | 3.13 ± 0.58 a | 2.86 ± 0.30 ab | * |
| 10 | Coumaroyl-diglucoside | 9.71 ± 0.22 b | 11.87 ± 1.01 a | 6.73 ± 0.55 c | 4.35 ± 0.17 d | *** |
| 11 | Ferulic acid | 141.9 ± 5.28 b | 189.3 ± 7.81 a | 182.0 ± 3.99 a | 154.1 ± 8.40 b | ** |
| 12 | Km 3-p-coumaroylsophoroside-7-glucoside | 1.90 ± 0.06 b | 1.30 ± 0.03 c | 2.17 ± 0.10 b | 2.95 ± 0.16 a | *** |
| 13 | Isorhamnetin-3-gentiobioside | 5.56 ± 0.20 c | 14.31 ± 0.40 b | 25.13 ± 1.06 a | 13.93 ± 1.01 b | *** |
| 14 | Spinacetin derivative | 368.6 ± 5.66 | 369.9 ± 15.6 | 410.5 ± 34.5 | 436.2 ± 23.3 | ns |
| 15 | 5,3′,4′-trihydroxy-3 methoxy-6,7-methylendioxyflavone 4′ glucuronide | 2015 ± 77.9 b | 2314 ± 43.8 a | 2335 ± 44.6 a | 1933 ± 32.2 b | *** |
| 16 | Patuletin derivative | 2479 ± 221 c | 3005 ± 232 bc | 3114 ± 136 b | 3874 ± 102 a | ** |
| Total phenols | 6323 ± 321 c | 7178 ± 247 b | 7528 ± 78.2 ab | 8021 ± 146 a | ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petropoulos, S.A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Rouphael, Y. The Effects of Nutrient Solution Feeding Regime on Yield, Mineral Profile, and Phytochemical Composition of Spinach Microgreens. Horticulturae 2021, 7, 162. https://doi.org/10.3390/horticulturae7070162
Petropoulos SA, El-Nakhel C, Graziani G, Kyriacou MC, Rouphael Y. The Effects of Nutrient Solution Feeding Regime on Yield, Mineral Profile, and Phytochemical Composition of Spinach Microgreens. Horticulturae. 2021; 7(7):162. https://doi.org/10.3390/horticulturae7070162
Chicago/Turabian StylePetropoulos, Spyridon A., Christophe El-Nakhel, Giulia Graziani, Marios C. Kyriacou, and Youssef Rouphael. 2021. "The Effects of Nutrient Solution Feeding Regime on Yield, Mineral Profile, and Phytochemical Composition of Spinach Microgreens" Horticulturae 7, no. 7: 162. https://doi.org/10.3390/horticulturae7070162
APA StylePetropoulos, S. A., El-Nakhel, C., Graziani, G., Kyriacou, M. C., & Rouphael, Y. (2021). The Effects of Nutrient Solution Feeding Regime on Yield, Mineral Profile, and Phytochemical Composition of Spinach Microgreens. Horticulturae, 7(7), 162. https://doi.org/10.3390/horticulturae7070162










