Abstract
The objective of this study was to compare the morphology of M. spicata and R. officinalis plants, and the relative abundance quantification, colony-forming units, ribotypes, and biofilm former bacteria under an inorganic fertilizer and the use of vermicompost leachate in the rhizosphere under a closed hydroponic system. In mint (Mentha spicata) plants treated with the vermicompost leachate, growth increase was determined mainly in root length from an average of 38 cm in plants under inorganic fertilizer to 74 cm under vermicompost leachate. In rosemary (Rosmarinus officinalis), no changes were determined between the two treatments. There were differences in the compositions of microbial communities: For R. officinalis, eight ribotypes were identified, seven for inorganic fertilizer and four for vermicompost leachate. For M. spicata, eight ribotypes were identified, three of them exclusive to vermicompost leachate. However, no changes were observed in microbial communities between the two treatments. Otherwise, some changes were observed in the compositions of these communities over time. In both cases, the main found phylum was Firmicutes, with 60% for R. officinalis and 80% for M. spicata represented by the Bacillus genus. In conclusion, the use of vermicompost leachate under the hydroponic system is a viable alternative to achieve an increase in the production of M. spicata, and for both plants (mint and rosemary), the quality of the product and the microbial communities that inhabited them remained unaltered.
1. Introduction
At present, the growing global population has put pressure on agriculture in different ways: the increase in demand for food and the need to meet this demand in an environmentally friendly manner. Although the use of chemical fertilizers has led to an enhancement in crop production, several major health- and environment-related concerns are associated with their use [1,2]. Pollution and the increase in global temperature are predicted to have negative consequences for agriculture in the coming decades [3]. Likewise, future climate-change scenarios predict a more frequent occurrence of extreme conditions [4]. In this sense, hydroponic systems have emerged as an alternative to improve yield, product quality, water management, land saving, nutrient recycling, and environmental and pathogen control. Hydroponic systems are cultivation technologies that use nutrient solutions rather than soil substrates, and can use natural or artificial media to provide physical support to plants [5,6,7]. However, there is an intense debate about which hydroponic practices align or do not align with the Organic Foods Production Act (OFPA) and USDA organic regulations [8]. Furthermore, hydroponic systems are a form of soilless food production, and one of the points of conflictive in points in organic agriculture is the use of inorganic nutrition in water solutions, which many people strongly believe should not be allowed [8]. Hydroponic production has increased in recent years due to its multiple benefits. Thus, it is convenient to understand the role of microorganisms and natural sources of nutrients to improve hydroponic systems for the production of healthy food beyond reaching certification in organic agriculture. At present, the use of vermicompost leachate coupled with hydroponic systems seems to be a viable alternative. Vermicompost is the resulting product from the processing of organic waste in the digestive tract of earthworms [9,10]. This process involves the bio-oxidation and stabilization of organic compounds by the joint action of earthworms and microorganisms [11]. Consequently, the obtained vermicompost is a fertilizer with available nutrients for plants and a strong charge of beneficial bacteria [12,13]. Likewise, vermicompost is an effective technique to reduce the toxicity of waste material [14]. Vermicompost leachate is a subproduct of the vermicompost process with nutrients, microorganisms, and biologically active substances, such as fulvic acids and humic acids, and the released water during the decomposition of the organic material [15,16]. One of the positive effects of the use of vermicompost leachate is an increase in the population of plant-growth-promoting bacteria (PGPB) [17]. PGPB can promote plant growth by both direct and indirect mechanisms. Direct mechanisms include the production of auxin, ACC deaminase activity, cytokinin, gibberellin, the nitrogen fixation process, phosphorus solubilization, and the sequestration of iron by bacterial siderophores. Indirect mechanisms refer to the bacterial capability to inhibit the proliferation of plant pathogenic organisms, such as fungi and bacteria [2,18]. Most studies on hydroponic systems reported the role of indigenous bacteria and the effects of bacterial addition, and indirect bacterial mechanisms for biological pathogen control, but scarce data are available about the existence of differences between the bacteria content and plant growth when applying vermicompost leachate to a hydroponic system [13,19]. The influence of agricultural management practices on plant microbial communities is not completely clear [20]. Opportune microorganism identification in hydroponic systems which uses vermicompost leachate as a low-cost organic fertilizer is essential to select the most adequate microorganisms for an efficient pathogen biocontrol program, also to define a fertilization protocol for this system environmentally friendly and accessible to any producer [21]. Mint (Mentha spicata L.) and rosemary (Rosmarinus officinalis L.) are two plants of agronomic importance belonging to the Lamiaceae family [19,22], a family with many wild and cultivated officinal species, rich in essential oils and antioxidant compounds that are useful to humans [23,24]. The leaves of M. spicata are dried and used for tea infusions, and cultivated for the production of essential oils that are widely used in the pharmaceutical and cosmetic industries [19]. R. officinalis, besides its culinary uses due to its characteristic aroma, is also widely employed by indigenous populations in areas where it spontaneously grows. Rosemary extracts are used as a natural antioxidant, improving the shelf life of perishable foods [22,25]. This study assessed the effect of two types of fertilizer (inorganic versus organic fertilizer) on the growth of mint (M. spicata) and rosemary (R. officinalis) plants under a hydroponic production system, as an alternative agronomic method contributing to a reduction in pollution, water use, and fertilizer consumption, and low-cost production.
2. Materials and Methods
2.1. Study Area
The experiment was conducted in a shade-enclosure environment that served as a greenhouse facility in La Paz, located in a Bw (h’) hw (e) climate, which is considered to be semiarid and sustains the xerophytic vegetation of Baja California Sur, northwest Mexico, at 7 m above sea level. Mean, maximal, and minimal temperature in the shade-enclosure facility were 21.4, 31.8, and 8.9 °C, respectively, with a mean of 70% relative humidity. Meteorological records were obtained during the study from an automated weather station located inside the shade-enclosure facility.
2.2. Plant Cultivation Conditions and Hydroponic System
The experiment was carried out from September to November. M. spicata and R. officinalis cuttings were obtained from mother plants within their regional cultivars and were placed in pots with vermiculite until they developed enough roots to be able to absorb nutrients from fertilizers after applying the treatments. The pots were placed in 30 propylene containers of 20 L (24.5 × 16 × 10 cm (length × width × height)) filled with water. Oxygen supplementation in containers was provided with a Blogger Sweetwater pump (model SST20, 50 Hz). The water volume was maintained constant to build a closed hydroponic system; there was no recirculating water because the study was on the early vegetative stage (September to November).
2.3. Treatments and Experimental Design
The experimental design consisted of two treatments: one applying vermicompost leachate (L) and the other applying inorganic fertilizer (SS; control group) [26]. Vermicompost leachate (L) was produced at the CIBNOR experimental field according to recommendations by Gunadi et al. [27]. The vermicomposting process was carried out in 200 L containers cut in half, to which 5 holes were made in its base. Subsequently, a 5 cm thick layer of gravel and an antiaphid mesh were placed to separate the gravel from the bed where the earthworms developed. Kitchen waste and manure were used as food for the earthworms in a ratio of 1:1 volume:volume. Both kitchen waste and manure were precomposted for 21 days before being used as food for the earthworms. The feeding process was carried out using 5 cm thick layers of precomposted food every week for 12 weeks. The vermicomposting process was considered to have ended when a homogeneous material was observed without the presence of remnants of the original material. The vermicompost was separated to be laid and sheltered in a dry place and away from light for 90 days for its mineralization. Vermicompost leachate was obtained according to the methodology described by García-Galindo et al. [28], where 5 kg of vermicompost was placed in a container. Three liters of distilled water was poured into the container, and the leachate was collected. Information of the nutrient content of both inorganic fertilizer and vermicompost leachate is shown in Table 1. The experiment was established under a completely randomized design with 15 replicates for each treatment (vermicompost leachate and inorganic fertilizer). Each replicate consisted in a container before descripted with 12 pots, each pot with one plan. Treatments were applied once at five days after sowing (DAS), for inorganic fertilizer a commercial fertilizer of 17% NPK was used to prepared 10 mL that contained 0.0079, 0.000087, 0.070 (parts per million of N, P K, respectively) diluted in 40 L of top water (the capacity of pot container). For the vermicompost-leachate treatment, 140 mL that contained 0.00709, 0.000259, and 0.074 (parts per million of N, P K, respectively) was diluted in 40 L of tap water. The nutrient doses of N–P–K corresponded to the minimum established for these crops in the region to examine if any differences could be detected in microbial and morphological traits in the use of an organic versus inorganic fertilizer. Plants were analyzed in early-stage growth at 35 days after fertilizer application.

Table 1.
Solution-component analyses of nutritional source for M. spicata and R. officinalis in hydroponic system.
2.4. Morphological Traits and Relative-Growth Analysis
Stem length (SL, cm), fresh stem weight (FSW), dry stem weight (DSW), foliar area (FA), fresh foliar weight (FFW), dry foliar weight (DFW), root length (RL), fresh root weight (FRW), and dry root weight (DRW) were evaluated in five M. spicata plants and five R. officinalis rosemary plants before treatment application and at the end of the experiment (35 DAS). Stem and root weights (g) were obtained using an analytical scale (Mettler Toledo, AG204); for dry weights, an oven was used with forced air circulation at 70 °C (Shel-Lab®, FX-5, series 1000203) until constant weight. Data of initial and final dry weights were used to calculate total relative growth rate (TGR), foliar growth rate (FGR), root growth rate (RGR), and stem growth rate (SGR) in grams per day, according to Hunt [29], following Formula (1):
where DW2 and DW1 are the total plant (TGR), foliar (FGR), root (RGR) and stem (SGR) dry weight (g), recorded at times t2 (time of sampling) and t1 (beginning of the experiment), respectively. The difference (t2 − t1) is expressed in days. TGR, FGR, RGR, and SGR are expressed in g−1 day−1.
TGR = ((lnDW2) × (lnDW1))/(t2 − t1),
2.5. Photosynthetic Pigments
For M. spicata and R. officinalis plants under organic and inorganic treatments, we determined chlorophyll with seven plants (one leaf per plant) per treatment. M. spicata SPAD values [30,31] were recorded for 20 consecutive days after the beginning of both organic and inorganic treatments application. In R. officinalis plants, chlorophyll was evaluated two times: before any treatment application, and 20 days after both treatment applications. For R. officinalis, the chlorophyll was extracted following the acetone extraction methodology from leaf tissue, and the absorbance measure was carried out with a UV/visible spectrophotometer (model HELIOS OMEGA, Thermo Scientific, Vantaa, Finland). Chlorophyll a and b concentrations were estimated by applying the following functions [32]:
where A663 and A645 correspond for the absorbance values at wavelengths (λ) of 663 and 645 nm, respectively.
Chlorophyll a (mg mL−1) = 11.64 (A663) − 2.16 (A645)
Chlorophyll b (mg mL−1) = 20.97 (A645) − 3.94 (A663),
2.6. Sampling for Bacterial-Community Characterization
To determine the influence of organic and inorganic fertilizers on rhizobial microbial communities from the plant rhizosphere, samples of the root rhizosphere were taken in the hydroponic system as follows: a water sample of 50 mL with the roots (0–0.5 cm) from three different reservoirs at three times (1, 7, and 35 DAS). The collected samples were processed immediately for: (i) total DNA isolation from water (rhizosphere) samples, and (ii) bacterial isolation from R. officinalis and M. spicata root samples with the methodology that follows below. Vermicompost was free of pathogens.
2.7. Colony-Forming Units (CFU) Quantification and Isolation of Bacteria from M. spicata and R. officinalis Cultivated by Hydroponic System
The water and root samples were vorticed for 30 s. Then, 25 mL of the sample was transferred to a new tube for DNA isolation. The remaining 25 mL was used to determine the colony-forming units (CFU). One milliliter of the remaining sample was used to perform serial dilutions in saline solution 0.85% (w/v) (from 10-2 to 10-7). Lastly, 100 µL for each dilution (from 10-2 to 10-7) was plated on nutrient agar (NA) and incubated for 24 h at 30 °C. After 24 h, the CFU count was performed.
After the CFU count, bacterial colonies were isolated on the basis of their morphology. A representative colony of the five most abundant colonial morphologies was reseeded by streak dilution in a new plate of NA and incubated at 30 °C overnight. This step was repeated until a pure isolate in each case (a single bacterial morphology per isolate) was obtained. The obtained pure isolates were stored in glycerol 30% (v/v) at −80 °C until their use.
2.8. DNA Isolation
The total DNA isolation of the water samples and bacterial isolates was carried out according to the protocol with slight modifications [33]. For water samples, 25 mL was centrifuged at 5000× g for 10 min, and the supernatant was discarded. For bacterial isolates, 3 mL of liquid culture was placed in nutrient broth (NB) at 30 °C overnight and centrifuged at 5000× g for 5 min, and the supernatant was discarded. Both the pellet from water samples and the bacterial isolate pellets were processed in the same way. The resulting pellet was resuspended in 1 mL of a lysis buffer (15% sucrose, 0.3 mg/mL lysozyme, 0.05 M EDTA and 1 M Tris, pH 8) and incubated for 30 min at 37 °C. Then, 100 μL of 10% SDS (w/v), 100 μL of 5 M NaCl, and 5 μL of proteinase K (0.4 mg/mL) were added and incubated under agitation for 1 h at 50 °C. After incubation, 200 μL of phenol–chloroform–isoamyl alcohol (25:24:1) was added to 500 μL of the solution, briefly vorticed, and then centrifuged at 12,000× g for 5 min. The aqueous phase was recovered, and 200 μL of ammonium acetate (7.5 M) and 500 μL (1 volume) of absolute ethanol were added to be mixed by inversion and precipitate at 4 °C overnight to centrifuge at 4 °C at 12,000× g for 15 min. The supernatant was discarded, and the pellet was washed twice with 100 μL of ethanol 70% (v/v). The DNA was dried at room temperature, resuspended in molecular-biology-grade water, and stored at −20 °C until use.
2.9. Relative-Abundance Quantification by qPCR
The relative abundance of the bacterial population was assessed through qPCR to determine the effect of treatments. The qPCR was performed on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) according to the instructions of the iTaq™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA). The relative abundance of the total bacteria in the rhizosphere samples for each treatment was assessed according to the methodology described by López-Gutiérrez et al. [33] with slight modifications.
2.10. Characterization of Bacterial Communities by Ribotype Assay Analysis (16S rRNA Gene)
Ribotype assay analysis was conducted according to the Bogino et al. [34] methodology. A total DNA of 36 water samples (3 samples × 3 times × 2 treatments × 2 species of plants = 36 samples in total) and 60 bacterial isolate strains (30 isolate strains for each plant for both organic and inorganic fertilization treatments) were characterized by amplified ribosomal DNA restriction analysis (ARDRA). Bacterial genomic DNA was extracted from each isolate as mentioned previously. For 16S rRNA gene amplification, we used primers fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rD1 (5′- AAGGAGGTGATCCAGCC-3′). PCR amplification products (~1500 bp) were processed by a restriction endonuclease assay with HaeIII (Thermo Fisher Scientific), and the resulting fragments were electrophoretically separated on a 2% (w/v) agarose gel, stained with ethidium bromide to visualize them with UV radiation, and the corresponding image was photographed. Ribotype identification is directly associated with a specific restriction fragment fingerprint. The community structure dendrogram was constructed on the basis of ribotypes of the bacterial isolates with GelCompar II software. Bacterial isolate strains belonging to either unique majority ribotypes or common ribotypes were selected for further identification through 16S rRNA gene nucleotide sequence analysis with primers COM 1 (5′-CAGCAGCCGCGGTAATAC-3′) and COM 2 (5′-CCGTCAATTCCTTTGAGTTT-3′) with the methodology described by Stach et al. [35]. The 16S rRNA gene sequences were analyzed using the BLAST (blastn) search program (National Center for Biotechnology Information (NCBI)).
2.11. Biofilm-Formation Assay
Biofilms are microbial communities that adhere to surfaces and are enclosed in a protective matrix; this is also the primary structure from which bacteria interact with plants and other eukaryotes. Thus, to characterize the bacterial capability of the rhizosphere (water samples) isolate strains from M. spicata and R. officinalis to form biofilms, we carried out the crystal violet (CV) staining quantitative assay of Labrie et al. [36] with slight modifications. CV staining absorbance was measured at 590 nm using a spectrophotometer (Multiskan Spectrum, Thermo Scientific, Wilmington, DE, USA).
2.12. Statistical Analysis
Data were analyzed using univariate and multivariate analysis of variance (ANOVA and MANOVA) for one-way classification, and the nutrition source was the study factor. For chlorophyll content, multiple analysis of variance (MANOVA) and significant differences between means for each recorded date were determined by two-way analysis of variance (ANOVA). Least significant differences (LSD) in Tukey’s HSD test (p = 0.05) were estimated for one-way ANOVA. For all cases, significant differences between means were considered to be significant at p < 0.05. All statistical analyses were performed with Statistica software program v10.0 and GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Plant Morphology and Photosynthetic Pigments
3.1.1. M. spicata
Stem height (SL), dry foliar weight (DFW), fresh foliar weight (FFW), foliar area (FA), and root length (RL) showed a significant increase in the vermicompost leachate treatment compared with the inorganic treatment for M. spicata (Table 2). There was no difference between the vermicompost leachate treatment and the inorganic treatment for relative growth rates of leaves (FGR), stems (RGS), total growth rate (TGR), and roots (RGR), which was lower for vermicompost leachate than inorganic fertilizer was (Table 3). Chlorophyll a and b, and total content did not show any differences between plants with vermicompost leachate or inorganic treatment (Table 4 and Figure 1).

Table 2.
Morphometric parameters in M. spicata and R. officinalis plants under fertilization treatments.

Table 3.
Total growth rate (TGR), foliar growth rate (FGR), root growth rate (RGR), and stem growth rate (SGR) expressed in grams per day of M. spicata and R. officinalis plants.

Table 4.
Chlorophyll (Chl) a and b, and total (mg·mL−1) content in M. spicata and R. officinalis plants under different nutrient sources in two times before (BT) and after (AT) application of vermicompost leachate and inorganic treatments.
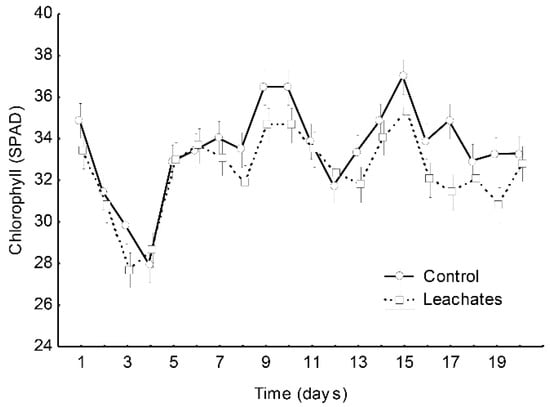
Figure 1.
Chlorophyll SPAD readings in M. spicata plants under leachates of inorganic and vermicompost leachate fertilizers. Vertical bars represented mean ± standard error.
3.1.2. R. officinalis
For all morphological traits, there were no differences between the vermicompost leachate and inorganic treatments (Table 2 and Table 3) except for rosemary under treatment with leachate in RGR, which showed lower growth (Table 3). Organic treatment did not affect chlorophyll a and b, and total content did not undergo alterations in either organic or inorganic treatment, and the only variable that exerted an effect was the time (date) of chlorophyll sampling (Table 4).
3.2. CFU Quantification and Relative Abundance of Bacterial Communities
The relative abundance of total bacterial communities due to the effect of treatments was assessed by CFU estimation and by a qPCR-based assay. For both M. spicata and R. officinalis, no differences were determined between the vermicompost leachate and inorganic treatments regarding the abundance of bacterial populations; however, an increase in relative abundance in time was more evident for the vermicompost leachate (Figure 2).
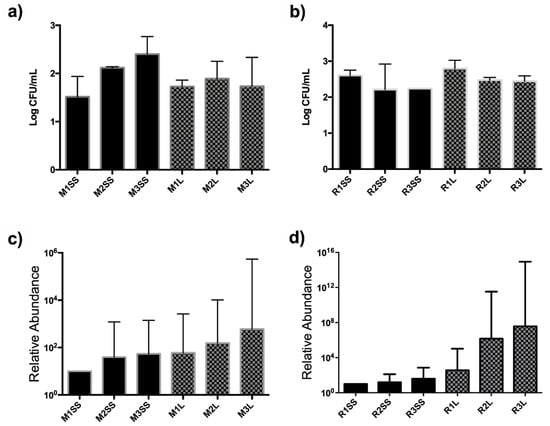
Figure 2.
Colony-forming unit (CFU) quantification and relative abundance (qPCR) of bacterial communities in M. spicata and R. officinalis. CFU quantification in (a) M. spicata and (b) R. officinalis; relative abundance (qPCR) of bacterial communities in (c) M. spicata and (d) R. officinalis (M1SS: mint composed sample, time 1, inorganic fertilizer; M2SS: M. spicata composed sample, time 2, inorganic fertilizer; M3SS: M. spicata composed sample, time 3, inorganic fertilizer; M1L: M. spicata composed sample, time 1, vermicompost leachate; M2L: M. spicata composed sample, time 2, vermicompost leachate; M3L: M. spicata composed sample, time 3, vermicompost leachate; R1SS: R. officinalis composed sample, time 1, inorganic fertilizer; R2SS: R. officinalis composed sample, time 2, inorganic fertilizer; R3SS: R. officinalis composed sample, time 3, inorganic fertilizer; R1L: R. officinalis composed sample, time 1, vermicompost leachate; R2L: R. officinalis composed sample, time 2, vermicompost leachate; R3L: R. officinalis composed sample, time 3, vermicompost leachate.
Bacterial community structure kinetics between both vermicompost leachate and inorganic treatments was analyzed. Thirty-six total DNA water samples were analyzed by amplified ribosomal DNA restriction analysis (ARDRA). As this test showed for M. spicata and R. officinalis, bacterial community structures underwent changes through time without a significant effect between treatments (Figure 3a,b). Thus, these results highlight the feasibility of replacing inorganic fertilizer with the vermicompost leachate without significant impact on the bacterial abundance or bacterial community structures of M. spicata and R. officinalis in hydroponic systems.
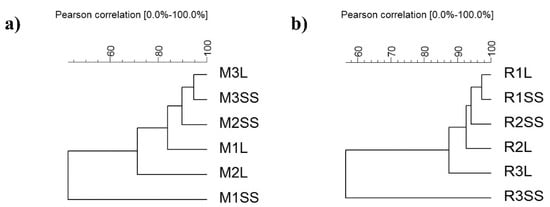
Figure 3.
Dendrogram of general distribution of bacterial composition of communities between treatments in (a) M. spicata and (b) R. officinalis (M1SS: M. spicata composed sample, time 1, inorganic fertilizer; M2SS: M. spicata composed sample, time 2, inorganic fertilizer; M3SS: M. spicata composed sample, time 3, inorganic fertilizer; M1L: M. spicata composed sample, time 1, vermicompost leachate; M2L: M. spicata composed sample, time 2, vermicompost leachate; M3L: M. spicata composed sample, time 3, vermicompost leachate; R1SS: R. officinalis composed sample, time 1, inorganic fertilizer; R2SS: R. officinalis composed sample, time 2, inorganic fertilizer; R3SS: R. officinalis composed sample, time 3, inorganic fertilizer; R1L: R. officinalis composed sample, time 1, vermicompost leachate; R2L: R. officinalis composed sample, time 2, vermicompost leachate; R3L: R. officinalis composed sample, time 3, vermicompost leachate).
3.3. Composition and Diversity of Bacterial Communities
A total of 60 bacterial isolate strains (30 isolate strains for each plant for both vermicompost leachate and inorganic fertilization treatments) were characterized by ARDRA. From ARDRA, 15 ribotypes were identified in M. spicata and R. officinalis according to the yielded fingerprint after the restriction assay with the HaeIII restriction enzyme (Table 5). In the case of R. officinalis, eight different ribotypes were identified (Figure 4). Of these eight ribotypes, seven were present in inorganic treatment, and four in the vermicompost leachate. Of the ribotypes present in the inorganic treatment, four were exclusively present in this treatment, while only one ribotype was exclusive of the vermicompost leachate. In the case of M. spicata, there were also eight different ribotypes for both the vermicompost leachate and the inorganic treatment. For the inorganic treatment, there were five ribotypes, and none was exclusive to this treatment. For the vermicompost leachate treatment, eight ribotypes were present, and three ribotypes were exclusive of this treatment. However, it was not possible to characterize the ribotype to which three bacterial isolates from M. spicata belonged (two from inorganic treatment and one from organic treatment).

Table 5.
Ribotypes of bacteria isolated from hydroponic system in M. spicata and R. officinalis plants.
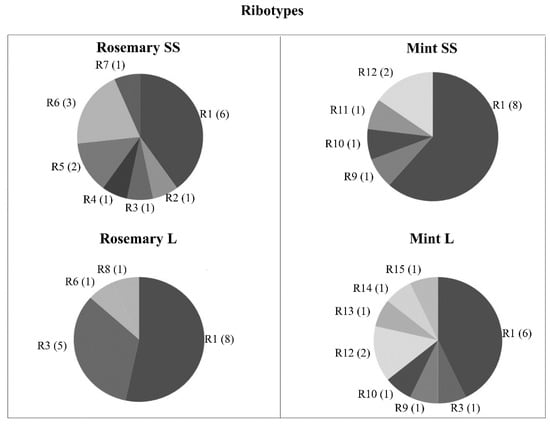
Figure 4.
Ribotypes present in M. spicata and R. officinalis obtained by amplified ribosomal DNA restriction analysis (ARDRA; R: ribotype, number: number of ribotypes, and number in parentheses: number of isolates corresponding to each ribotype).
Representative bacterial strains were identified by 16S rRNA gene sequencing. Bacterial isolate strains were selected according to ribotype ARDRA profiles (Table 6). Most bacterial isolate strains belonged to the Firmicutes phylum, which was mainly composed of the Bacilli class, the Bacillaceae family, and the Bacillus genus. Bacterial isolate strains belonging to Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria classes from the Proteobacteria phylum were found (Table 6).

Table 6.
Identities of bacterial strains isolated from hydroponic system in M. spicata and R. officinalis plants.
Ribotypes found in rosemary bacterial isolate strains belonged to Firmicutes (60%), mainly composed of the Bacillus genus. Comparing the vermicompost leachate and inorganic treatments, we determined that the Firmicutes phylum was the most abundant between treatments, and the Alphaproteobacteria and Betaproteobacteria classes, and Gammaproteobacteria showed greater abundance in inorganic treatment than in the vermicompost leachate treatment (Figure 4, Table 6). The ribotypes found in M. spicata bacterial isolate strains belonged to Firmicutes (80% and were mainly composed of the Bacillus genus. Interestingly, 10% of the bacterial isolate strains were unclassified. Comparing the vermicompost leachate and inorganic treatments, the most abundant phylum was Firmicutes, followed by the Gammaproteobacteria class (Table 5 and Table 6). For the vermicompost leachate, the Betaproteobacteria class showed greater abundance in the vermicompost leachate treatment than in inorganic treatment (Table 5 and Table 6). Therefore, the Firmicutes phylum was the most abundant in both R. officinalis and M. spicata plants, and in both the vermicompost leachate and the inorganic treatment.
3.4. Biofilm-Forming Ability of Bacterial Communities
All bacterial isolate strains from R. officinalis (30 isolates) and M. spicata (30 isolates) were assessed for adhesion and biofilm-establishment capability with a CV assay. The CV assay showed that all bacterial isolates were able to adhere to the surface and establish biofilms (Figure 5). Differences were found in biofilm formation that were categorized according to the capability to retain CV measured by the OD at 595 nm (CV-OD595) [28], for all bacterial isolate strains as follows: weak (<0.6), moderate (0.6–1.2), and strong (>1.2). R. officinalis bacterial isolate strains with the vermicompost leachate treatment showed that 3 bacterial isolates formed a moderate biofilm, 2 a strong biofilm, and the remaining 10 a weak biofilm. For the bacterial isolate strains from the inorganic treatment, 4 bacterial isolates formed a moderate biofilm, 1 a strong biofilm, and the remaining 10 a weak biofilm. The M. spicata bacterial isolate strains with the vermicompost leachate treatment showed that 1 bacterial isolate formed a strong biofilm, 2 a moderate biofilm, and the remaining 12 formed a weak biofilm. For the inorganic treatment, 2 bacterial isolates were able to form a strong biofilm, 1 a moderate biofilm, and the remaining 12 a weak biofilm. Altogether, for the R. officinalis and M. spicata plants and both the vermicompost leachate and the inorganic treatment, most bacterial isolates were able to form weak biofilms in the conditions assessed in this study.
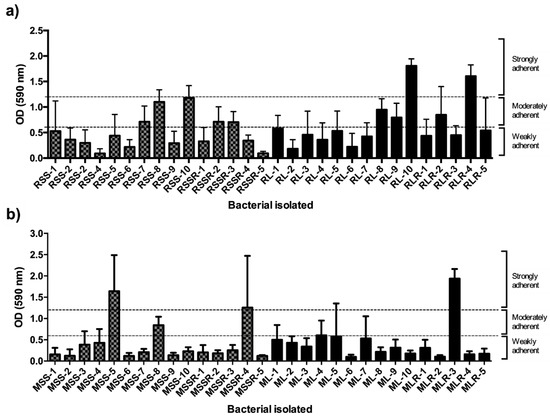
Figure 5.
Biofilm formation quantified by staining with crystal violet of isolates from (a) R. officinalis and (b) M. spicata (RSS-number or RSSR-number: isolates from R. officinalis inorganic fertilizer, RL-number or RLR-number: isolates from R. officinalis vermicompost leachate, MSS-number or MSSR-number: isolates from M. spicata inorganic fertilizer, and ML-number or MLR-number: isolates from M. spicata vermicompost leachate.
4. Discussion
The vermicompost leachate treatment for both M. spicata (mint) and R. officinalis (rosemary) plants did not affect their growth; even for M. spicata plants, we were able to determine a growth increase for several morphometric parameters. Moreover, for R. officinalis plant growth, for all morphometric parameters, there were only differences for root growth, which was lower for vermicompost than for inorganic leachate; similar results were found by Peng et al. [37]. This is important since the aim of healthy food production is avoiding the application of inorganic fertilizer [25,38,39,40,41]. Furthermore, vermicompost leachate contains a high amount of plant hormones, such as auxins, gibberellins, and cytokinins from microbial origin, giving rise to plant-growth enhancement, and acting as a liquid fertilizer [15,42,43,44,45]. Emperor and Kumar [45] determined that organic matter processed in the earthworm gut and then excreted as vermicast undergoes an increased level of microbial population, microbial respiration, microbial enzyme activity, and N, P, and K enrichment, bacterial exopolysaccharide production, lignocellulolytic activity establishment, nitrifying, and nitrogen-fixing microorganism proliferation. The above allow for us to conclude that the use of vermicompost to replace inorganic fertilizers is a viable option under the use of hydroponic systems [43,46,47,48,49].
The bacterial communities’ relative abundance showed no differences between the vermicompost leachate and inorganic treatments for both R. officinalis and M. spicata plants, showing time-related differences, as expected, in accordance with previous works, where the analyzed bacterial communities underwent the same behavior [50,51]. The bacterial-community structure for the R. officinalis and M. spicata plants and for both treatment types were mainly composed by the Firmicutes phylum, followed by the Proteobacteria phylum, which was represented by the Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria classes; we were also able to determine the presence of beneficial bacteria from the Bacillus (Firmicutes phylum) and Pseudomonas (Proteobacteria phylum) genera. Those bacteria are designated as beneficial or plant-growth-promoting (PGPB), and the characterization of the bacterial-community structures of the rhizosphere for other plant members (Thymus vulgaris, T. citriodorus, T. zygis, Santolina chamaecyparissus, Lavandula dentata, and Salvia miltiorrhiza) of the Lamiaceae family showed that Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, Acidobacteria, and Gemmatimonadetes were among the most abundant bacterial phyla [5,52,53,54,55,56].
Lastly, the capability to establish biofilms was assessed for all 60 bacterial isolate strains from the M. spicata and R. officinalis plants and both treatments, with no differences highlighting the essential role of biofilm development in bacterial survival and physiology [36]. We determined that most of the isolates (66.67% in R. officinalis and 80% in M. spicata) had weak capacity (CV-OD595) to form a biofilm; a smaller proportion were able to produce a strong biofilm for both M plants and both treatments. In an aqueous environment, such as a hydroponic system, biofilm establishment follows other mechanisms that are not yet characterized. Authors should discuss the results and how they can be interpreted from the perspective of previous studies and working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
In this study, we showed that the substitution of inorganic fertilizer by vermicompost leachate in a hydroponic system allows for us to maintain or increase the production of two crop plants with agricultural importance (M. spicata (mint), and R. officinalis (rosemary)). Furthermore, we determined that this fertilizer substitution modifies neither the bacterial communities for both plants nor their ability to form biofilms. Through time, the vermicompost leachate tendency showed an increase in relative abundance, which is important to consider for future studies. Therefore, we propose the use of vermicompost leachate fertilizer as a feasible replacement for inorganic fertilizer in hydroponic systems to achieve sustainable and ecofriendly agricultural production, in agreement with our results and recent research conducted on open-field cultures, to face the challenge of a growing population and pollution derived from the use of inorganic fertilizers.
Author Contributions
A.L.-M.: conceptualization, methodology, formal analysis, investigation, writing—original draft. A.B.: writing—review and editing. G.C.-C.: writing—review and editing. E.T.-D.: supervision and writing-review. B.M.-A.: Funding acquisition. A.N.-G.: conceptualization, formal analysis, writing—review and editing, funding acquisition, project administration. G.L.-V.: methodology. All authors have read and agreed to the published version of the manuscript.
Funding
The current investigation was supported by CONACYT/Mexico through the Ciencia Básica SEP-CONACYT grant No. 236240, and funds provided to Centro de Investigaciones Biologicas del Noroeste S.C. (CIBNOR) and Consejo Sudcaliforniano de Ciencia y Tecnología grant 20403.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available for transparency at www.cibnor.gob.mx (anieto04@cibnor.mx, aloera04@cibnor.mx) (accessed on 27 April 2021).
Acknowledgments
Current investigations from the group are supported by CONACYT/México. We thank the technical assistance to all the team of the experimental field of the Agriculture Program in Arid Zones, Saúl Edel Briseño Ruiz, Pedro Luna García, Adrián Jordán Castro, and Raymundo Ceseña Nuñez. We also thank the technical assistance of Lidia Hirales-Lucero of Fitotecnia Laboratory and Angel Edgardo Carrillo García of Microbial Molecular Ecology Laboratory.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gupta, R.; Bisaria, V.S.; Sharma, S. Effect of agricultural amendments on Cajanus cajan (Pigeon pea) and its rhizospheric microbial communities—A comparison between chemical fertilizers and bioinoculants. PLoS ONE 2015, 10, e0132770. [Google Scholar] [CrossRef]
- Hartman, K.; Tringe, S.G. Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kong, F.; Zhang, S.; Meng, X.; Wang, Y.; Meng, Q. A tomato chloroplast-targeted DnaJ protein protects Rubisco activity under heat stress. J. Exp. Bot. 2015, 66, 3027–3040. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Beneficial bacteria and fungi in hydroponic systems: Types and characteristics of hydroponic food production methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 5, 280–293. [Google Scholar] [CrossRef]
- Gruda, N. Advances in Horticultural Soilless Culture; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2021; 442p, ISBN 9781786764355. [Google Scholar]
- NOSB. Hydroponic and Aquaponic Task Force Report; National Organic Standards Board (NOSB): Washington, DC, USA, 2016; 75p. [Google Scholar]
- Kiyasudeen, K.S.; Ibrahim, M.H.; Quaik, S.; Ismail, S.A. Prospects of Organic Waste Management, and the Significance of Earthworms; Springer: Berlin/Heidelberg, Germany, 2016; 254p, pp. 201–230, Chapter 9 Vermicompost, Its Applications and Derivatives. [Google Scholar]
- Garg, V.K.; Suthar, S.; Yadav, A. Management of food industry waste employing vermicomposting technology. Bioresour. Technol. 2012, 126, 437–443. [Google Scholar] [CrossRef]
- Hanc, A.; Boucek, J.; Svehla, P.; Dreslova, M.; Tlustos, P. Properties of vermicompost aqueous extracts prepared under different conditions. Environ. Technol. 2017, 38, 1428–1434. [Google Scholar] [CrossRef]
- Grantina-Ievina, L.; Andersone, U.; Berkolde-Pīre, D.; Nikolajeva, V.; Ievinsh, G. Critical tests for determination of microbiological quality and biological activity in commercial vermicompost samples of different origins. Appl. Microbiol. Biotechnol. 2013, 97, 10541–10554. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, S.N.; Wong, W.S.; Ng, C.Y.L.; Teo, C.H.; Ge, L.; Chen, X.; Yong, J.W.H. Mass spectrometric evidence for the occurrence of plant growth promoting cytokinins in vermicompost tea. Biol. Fertil. Soils 2014, 50, 401–403. [Google Scholar] [CrossRef]
- Wang, L.M.; Zhang, Y.M.; Lian, J.J.; Chao, J.Y.; Gao, Y.X.; Yang, F.; Zhang, L.Y. Impact of fly ash and phosphatic rock on metal stabilization and bioavailability during sewage sludge vermicomposting. Bioresour. Technol. 2013, 136, 281–287. [Google Scholar] [CrossRef]
- Gutiérrez-Miceli, F.A.; García-Gómez, R.C.; Rincón, R.R.; Abud-Archila, M.; Oliva, L.M.A.; Guillen, M.J.; Dendooven, L. Formulation of a liquid fertilizer for sorghum (Sorghum bicolor (L.) Moench) using vermicompost leachate. Bioresour. Technol. 2008, 99, 6174–6180. [Google Scholar] [CrossRef] [PubMed]
- Churilova, E.V.; Midmore, D.J. Vermiliquer (Vermicompost Leachate) as a Complete Liquid Fertilizer for Hydroponically-Grown Pak Choi (Brassica chinensis L.) in the Tropics. Horticulturae 2019, 5, 26. [Google Scholar] [CrossRef]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus 2012, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Karkanis, A.; Lykas, C.; Liava, V.; Bezou, A.; Petropoulos, S.; Tsiropoulos, N. Weed interference with peppermint (Mentha x piperita L.) and spearmint (Mentha spicata L.) crops under different herbicide treatments: Effects on biomass and essential oil yield. J. Sci. Food Agric. 2018, 98, 43–50. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.; Tringe, S.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Koohakan, P.; Ikeda, H.; Jeanaksorn, T.; Tojo, M.; Kusakari, S.I.; Okada, K.; Sato, S. Evaluation of the indigenous microorganisms in soilless culture: Occurrence and quantitative characteristics in the different growing systems. Sci. Hortic. 2004, 101, 179–188. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, Y.; Hossein-Mirjalili, M.; Nazeri, V. Chemical diversity among the essential oils of wild populations of Stachys lavandulifolia Vahl (Lamiaceae) from Iran. Chem. Biodivers. 2013, 10, 262–273. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and plant community implication on essential oils composition in useful wild Officinal species: A pilot case study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- García-Caparrós, P.; Llanderal, A.; Rodríguez, J.C.; Maksimovic, I.; Urrestarazu, M.; Lao, M. Rosemary growth and nutrient balance: Leachate fertigation with leachates versus conventional fertigation. Sci. Hortic. 2018, 242, 62–68. [Google Scholar] [CrossRef]
- Samperio, R.G. Hidroponía Básica; Editorial Diana: Ciudad de México, Mexico, 1997; 176p. [Google Scholar]
- Gunadi, B.; Blount, C.; Edwards, C.A. The growth and fecundity of Eisenia fetida (Savigny) in cattle solids pre-composted for different periods. Pedobiologia 2002, 46, 15–23. [Google Scholar] [CrossRef]
- García-Galindo, E.; Nieto-Garibay, A.; Troyo-Diéguez, E.; Lucero-Vega, G.; Murillo-Amador, B.; Ruiz-Espinoza, F.H.; Fraga-Palomino, H.C. Germination of Salicornia bigelovii (Torr.) under Shrimp Culture Effluents and the Application of Vermicompost Leachate for Mitigating Salt Stress. Agronomy 2021, 11, 424. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Analysis. The Institute of Biology´s Studies in Biology; Edward Arnold: London, UK, 1978; No 96. [Google Scholar]
- Yang, D.Q.; Dong, W.H.; Luo, Y.L.; Song, W.T.; Cai, T.; Li, Y.; Yin, Y.P.; Wang, Z.L. Effects of nitrogen application and supplemental irrigation on canopy temperature and photosynthetic characteristics in winter wheat. J. Agric. Sci. 2018, 156, 13–23. [Google Scholar] [CrossRef]
- Strain, H.H.; Svec, W.A. Extraction, separation, estimation, and isolation of the chlorophylls. In The Chlorophylls; Vernon, L.P., Seely, G.R., Eds.; Academic Press: London, UK, 1966; pp. 21–66. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning a Laboratory Manual, 3rd ed.; Science Press: Beijing, China, 2002; pp. 461–471. [Google Scholar]
- López-Gutiérrez, J.C.; Henry, S.; Hallet, S.; Martin-Laurent, F.; Catroux, G.; Philippot, L. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J. Microbiol. Methods 2004, 57, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bogino, P.; Abod, A.; Nievas, F.; Giordano, W. Water-limiting conditions alter the structure and biofilm-forming ability of bacterial multispecies communities in the alfalfa rhizosphere. PLoS ONE 2013, 8, e79614. [Google Scholar] [CrossRef] [PubMed]
- Stach, J.E.; Bathe, S.; Clapp, J.P.; Burns, R.G. PCR-SSCP comparison of 16S rDNA sequence diversity in soil DNA obtained using different isolation and purification methods. FEMS Microbiol. Ecol. 2001, 36, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Labrie, J.; Pelletier-Jacques, G.; Deslandes, V.; Ramjeet, M.; Auger, E.; Nash, J.H.; Jacques, M. Effects of growth conditions on biofilm formation by Actinobacillus pleuropneumoniae. Vet. Res. 2010, 41, 3. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan-zhi, G.; Ling, Q. Effects of ozone-treated domestic sludge on hydroponic lettuce growth and nutrition. J. Integr. Agric. 2018, 17, 593–602. [Google Scholar]
- Van Diepeningen, A.D.; de Vos, O.J.; Korthals, G.W.; van Bruggen, A.H.G. Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Appl. Soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- Mendoza-Hernández, D.; Fornes, F.; Belda, R.M. Compost and vermicompost of horticultural waste as substrates for cutting rooting and growth of rosemary. Sci. Hortic. 2014, 178, 192–202. [Google Scholar] [CrossRef]
- Rinaldi, S.; De Lucia, B.; Salvati, L.; Rea, E. Understanding complexity in the response of ornamental rosemary to different substrates: A multivariate analysis. Sci. Hortic. 2014, 176, 218–224. [Google Scholar] [CrossRef]
- García-Gómez, R.C.; Dendooven, L.; Gutiérrez-Miceli, F.A. Vermicomposting leachate (Worm Tea) as liquid fertilizer for maize (Zea mays L.) forage production. Asian J. Plant Sci. 2008, 7, 360–367. [Google Scholar]
- Singh, R.; Gupta, R.K.; Patil, R.T.; Sharma, R.R.; Asrey, R.; Kumar, A.; Jangra, K.K. Sequential foliar application of vermicompost leachates improves marketable fruit yield and quality of strawberry (Fragaria x ananassa Duch.). Sci. Hortic. 2010, 124, 34–39. [Google Scholar] [CrossRef]
- Lazcano, C.; Revilla, P.; Malvar, R.A.; Domínguez, J. Yield and fruit quality of four sweet corn hybrids (Zea mays) under conventional and integrated fertilization with vermicompost. J. Sci. Food Agric. 2011, 91, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Tharmaraj, K.; Ganesh, P.; Kolanjinathan, K.; Suresh-Kumar, R.; Anandan, A. Influence of vermicompost and vermiwash on physicochemical properties of rice cultivated soil. Curr. Bot. 2011, 2, 18–21. [Google Scholar]
- Emperor, G.N.; Kumar, K. Microbial population and activity on vermicompost of Eudrilus eugeniae and Eisenia fetida in different concentrations of tea waste with cow dung and kitchen waste mixture. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 496–507. [Google Scholar]
- Allard, S.M.; Walsh, C.S.; Wallis, A.E.; Ottesen, A.R.; Brown, E.W.; Micallef, S.A. Solanum lycopersicum (tomato) hosts robust phyllosphere and rhizosphere bacterial communities when grown in soil amended with various organic and synthetic fertilizers. Sci. Total Environ. 2016, 573, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C.; Depuydt, P.; De Ro, M.; Petit, C.; Van Gysegem, E.; Delaere, P.; Dixon, M.; Stasiak, M.; Aciksöz, S.B.; Frossard, E.; et al. Microbial community dynamics and response to Plant Growth-Promoting Microorganisms in the rhizosphere of four common food crops cultivated in hydroponics. Microb. Ecol. 2017, 73, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Neher, D.A.; Weicht, T.R.; Bates, S.T.; Leff, J.W.; Fierer, N. Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS ONE 2013, 8, e79512. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, C.; Gómez-Brandón, M.; Domínguez, J. Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 2008, 72, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Blanco, S.; García-López, M.; García-Salamanca, A.; Bursakov, S.A.; Genilloud, O.; Bills, G.F.; Ramos, J.L.; van Dillewijn, P. Assessing bacterial diversity in the rhizosphere of Thymus zygis growing in the Sierra Nevada National Park (Spain) through culture-dependent and independent approaches. PLoS ONE 2016, 11, e0146558. [Google Scholar] [CrossRef]
- Checcucci, A.; Maida, I.; Bacci, G.; Ninno, C.; Bilia, A.R.; Biffi, S.; Firenzuoli, F.; Flamini, G.; Fani, R.; Mengoni, A. Is the plant-associated microbiota of Thymus spp. adapted to plant essential oil? Res. Microbiol. 2017, 168, 276–282. [Google Scholar] [CrossRef]
- Armada, E.; Leite, M.F.A.; Medina, A.; Azcón, R.; Kuramae, E.E. Native bacteria promote plant growth under drought stress condition without impacting the rhizomicrobiome. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Chen, H.; Wu, H.; Yan, B.; Zhao, H.; Liu, F.; Zhang, H.; Sheng, Q.; Miao, F.; Liang, Z. Core microbiome of medicinal plant Salvia miltiorrhiza seed: A rich reservoir of beneficial microbes for secondary metabolism? Int. J. Mol. Sci. 2018, 19, 672. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Wang, X.; Riaz, M.; Islam, B.; Khan, Z.H.; Shah, F.; Munsif, F.; Ijaz Ul Haq, S. Biochar: An eco-friendly approach to improve wheat yield and associated soil properties on sustainable basis. Pak. J. Bot. 2019, 51, 1255–1261. [Google Scholar] [CrossRef]
- Perrino, E.V.; Ladisa, G.; Calabrese, G. Flora and plant genetic resources of ancient olive groves of Apulia (southern Italy). Genet. Resour. Crop Evol. 2014, 61, 23–53. [Google Scholar] [CrossRef]
- Rani, A.J.; Murugan, P.P. Suggestions for the increase use efficiency of eco-friendly agricultural practices for sustainable paddy cultivation. Agric. Update 2010, 5, 103–105. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).