Coir, an Alternative to Peat—Effects on Plant Growth, Phytochemical Accumulation, and Antioxidant Power of Spinach
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Substrates
2.2. Measurements
3. Results and Discussion
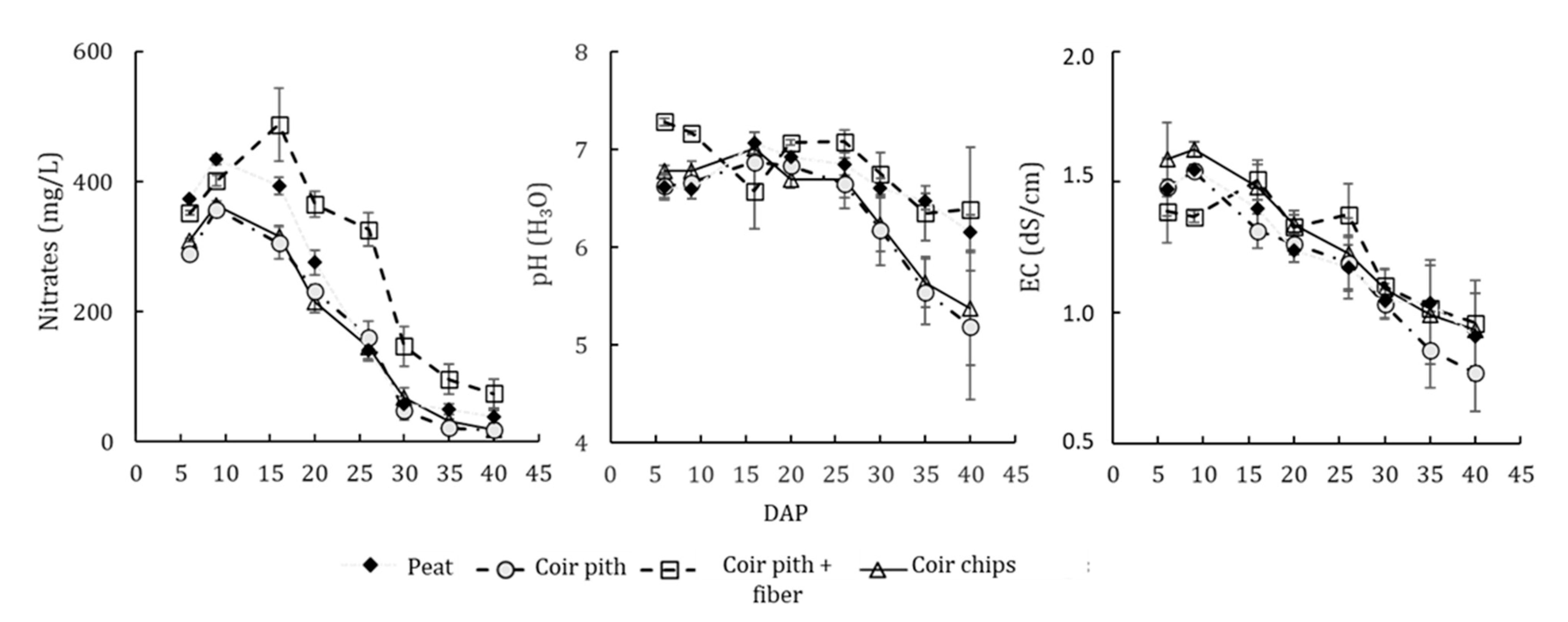
3.1. Drainage Water
3.2. Plant Growth and Yield
3.3. Leaf Nutrients
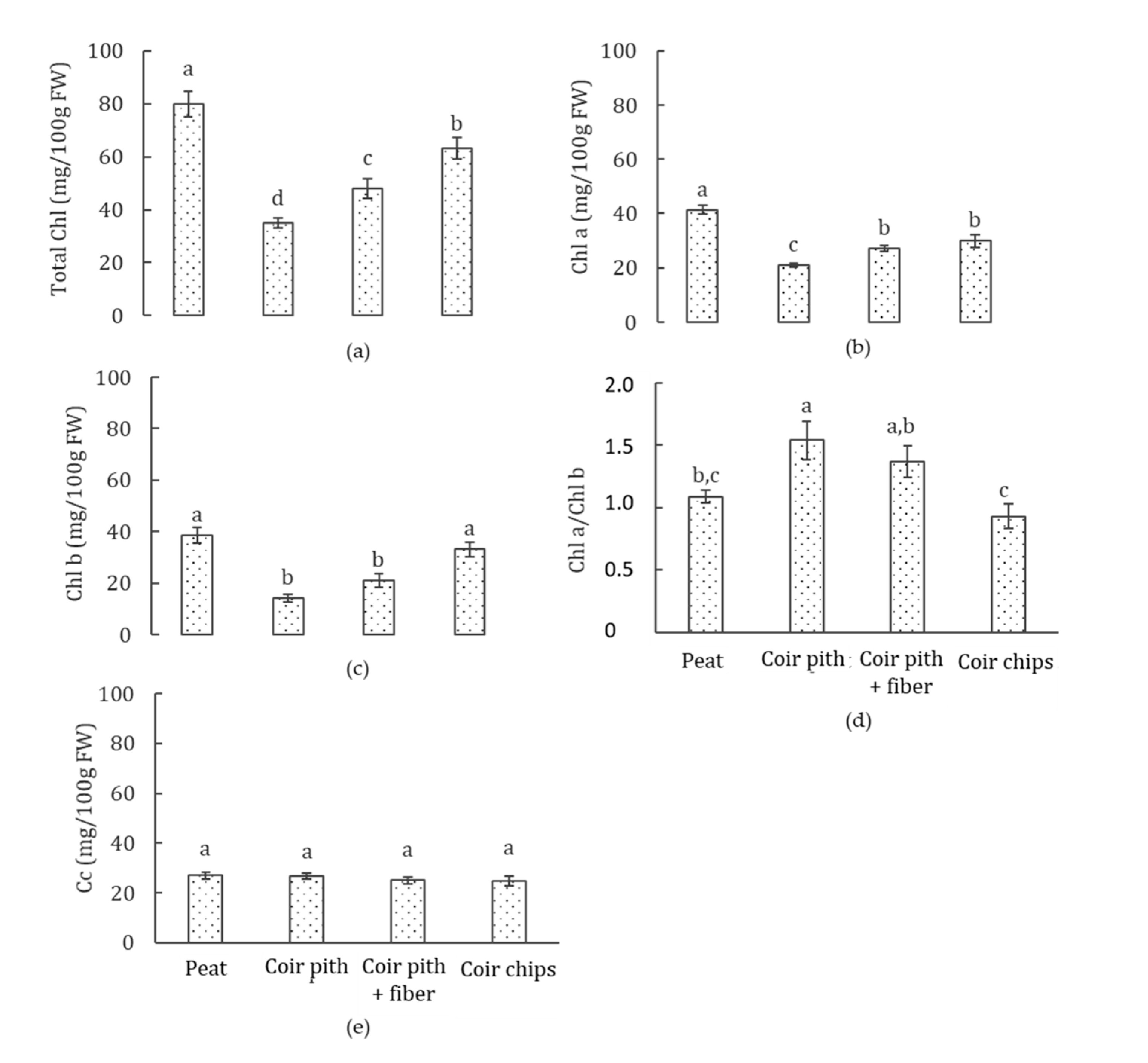
3.4. Photosynthetic Pigments
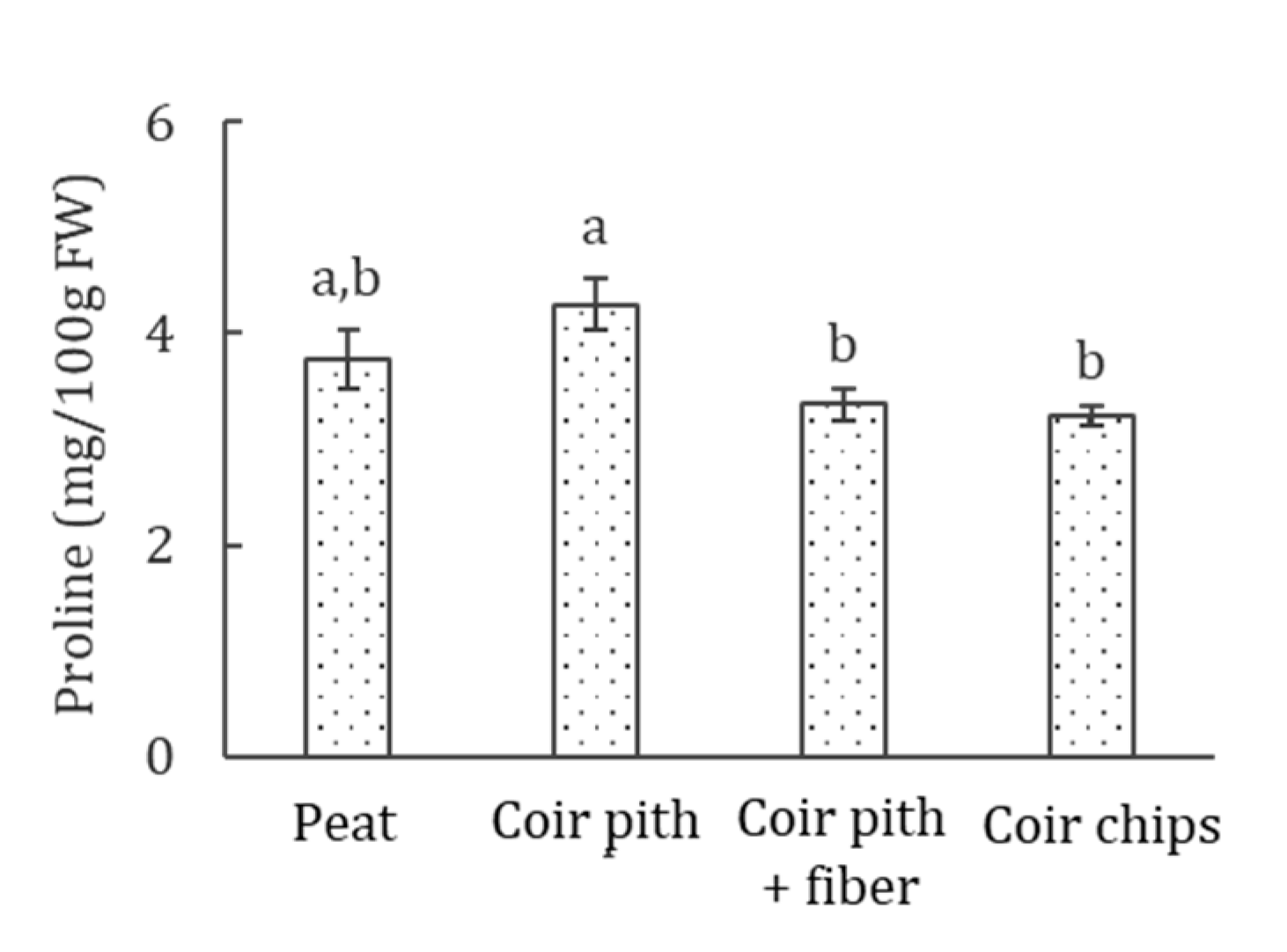
3.5. Proline Accumulation
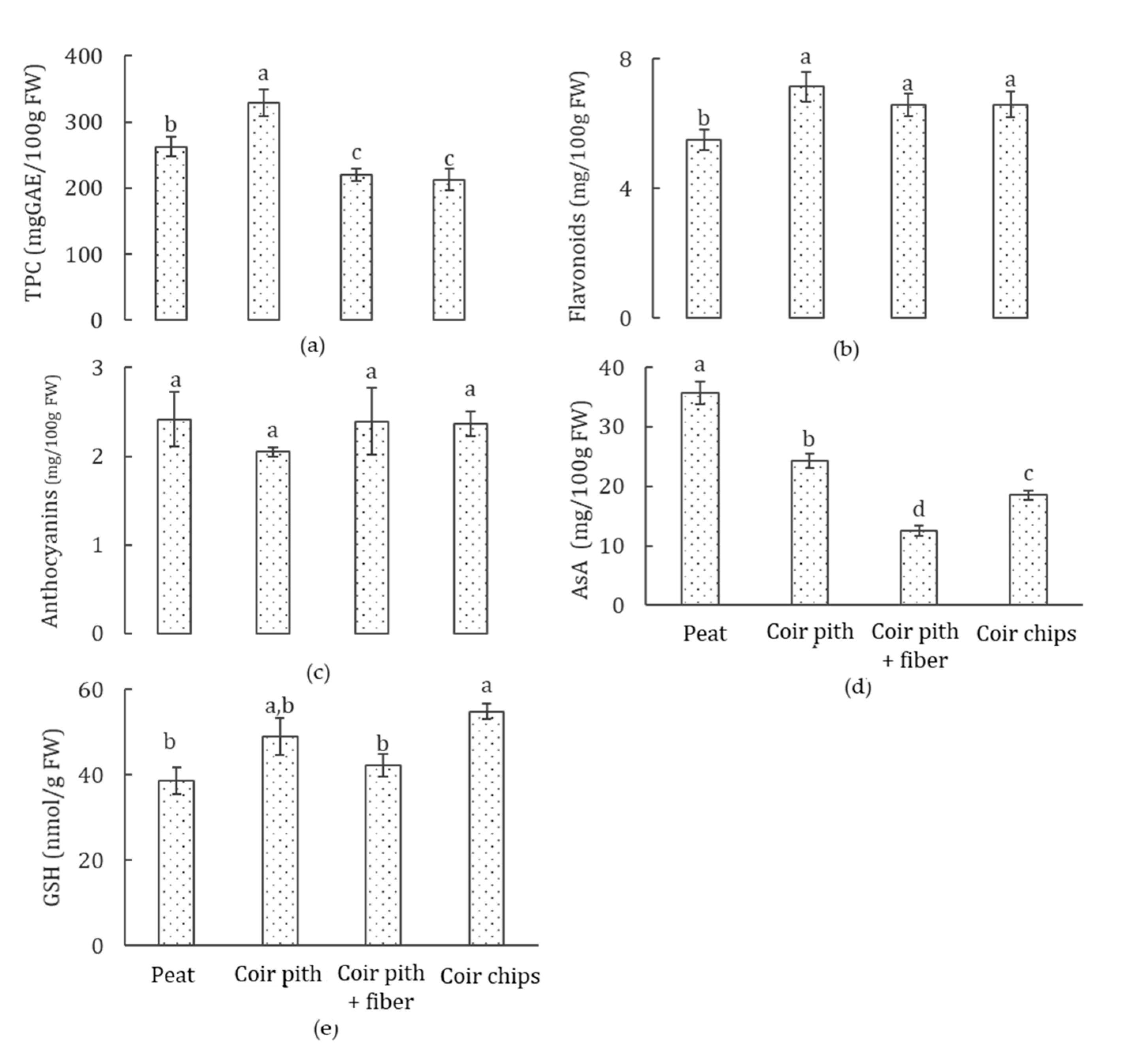
3.6. Phytochemical Accumulation
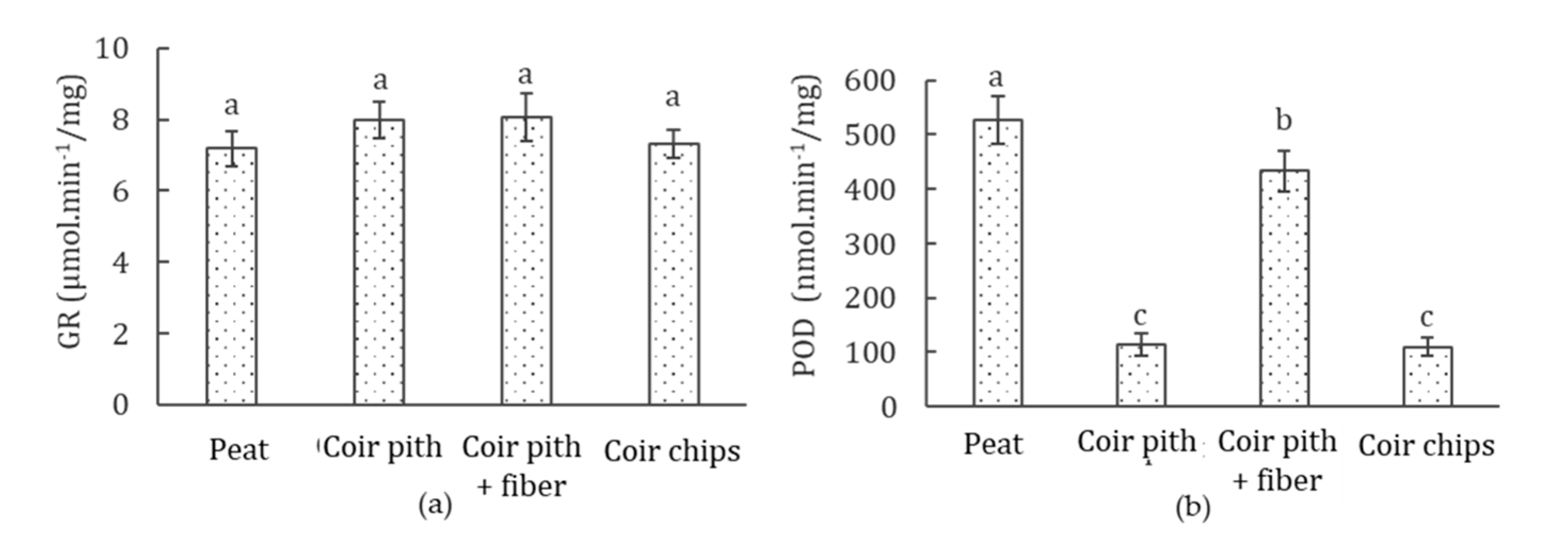
3.7. Antioxidant Enzyme (GR and POD) Activities
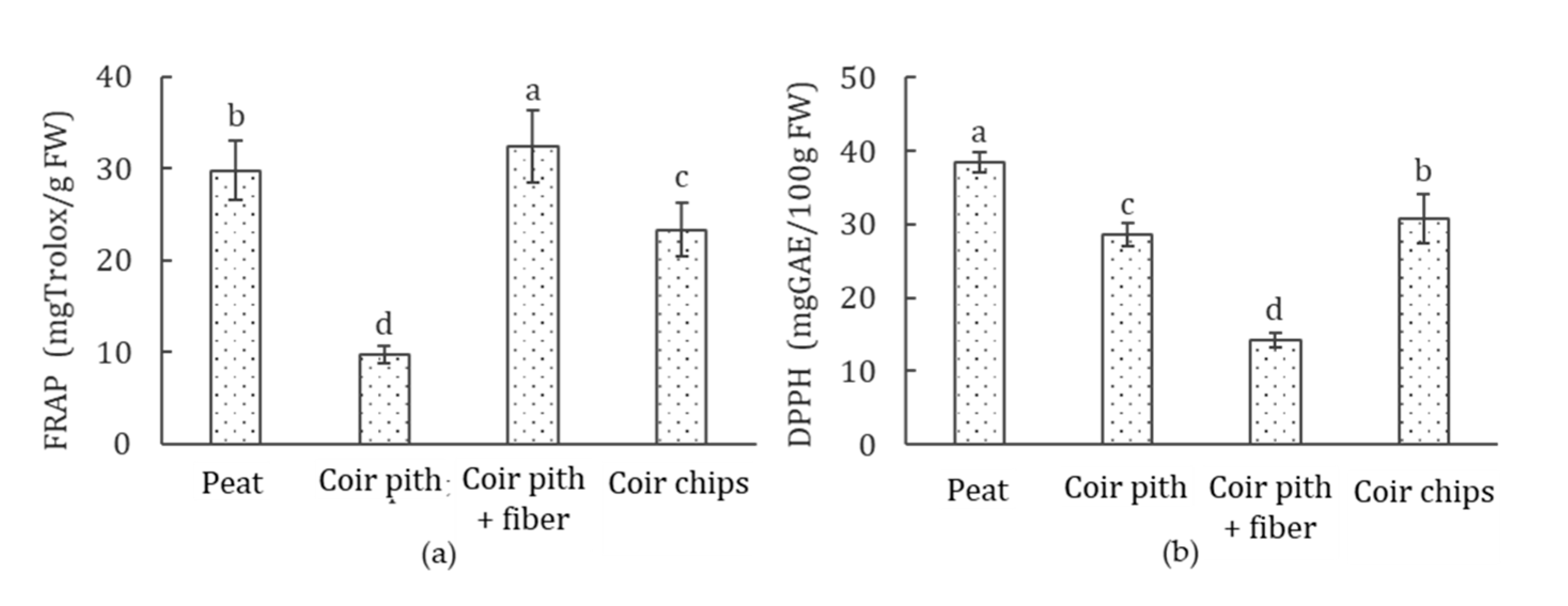
3.8. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production—A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Gruda, N. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- QUANTIS. Comparative Life Cycle Assessment of Horticultural Growing Media Based on Peat and Other Growing Media Constituents; EPAGMA Final Report: Lausanne, Switzerland, 2012; pp. 1–156. [Google Scholar]
- Gruda, N.; Brag, N. Developments in alternative organic materials as growing media in soilless culture systems. Chapter 3. In Advances in Horticultural Soilless Culture; Gruda, N., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2021; ISBN 13 9781786764355. [Google Scholar]
- Barcelos, C.; Machado, R.M.; Alves Pereira, I.; Ferreira, R.; Bryla, D.R. Effects of substrate type on plant growth and nitrogen and nitrate concentration in spinach. Int. J. Plant Biol. 2016, 7, 44–47. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Alves-Pereira, I.; Ferreira, R.M.A. Plant growth, phytochemical accumulation and antioxidant activity of substrate-grown spinach. Heliyon 2018, 4, e00751. [Google Scholar] [CrossRef]
- Lester, G.E.; Makus, D.J.; Hodges, D.M.; Jifon, J.L. Summer (subarctic) versus winter (subtropic) production affects spinach (Spinacia oleracea L.) leaf bionutrients: Vitamins (C, E, Folate, 425 K1, provitamin A), lutein, phenolics, and antioxidants. J. Agric. Food Chem. 2013, 61, 7019–7027. [Google Scholar] [CrossRef]
- Dias, J.S. Nutritional quality and effect on disease prevention of vegetables. Food Nutr. Sci. 2019, 10, 369. [Google Scholar] [CrossRef]
- Barker, A.V.; Stratton, M.L. Nutrient density of fruit crops as a function of soil fertility. In Fruit Crops. Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherland, 2020; pp. 13–31. [Google Scholar]
- Gruda, N. Do soilless culture systems have an influence on product quality of vegetables? J. Appl. Bot. Food Qual. 2009, 82, 141–147. [Google Scholar]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Antioxidant defences in olive trees during drought stress: Changes in activity of some antioxidant enzymes. Funct. Plant Biol. 2005, 32, 45–53. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of genetic material and current technologies on product quality of selected greenhouse vegetables—A review. Eur. J. Hortic. Sci. 2018, 83, 319–328. Available online: https://www.pubhort.org/ejhs/83/5/5/index.htm (accessed on 5 March 2021). [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional value. J. Am. Soc. Hortic. Sci. 2016, 141, 12–21. [Google Scholar] [CrossRef]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Shimomachi, Y.; Kawahara, C.; Kobashigawa, E.; Omoda, K.; Hamabe, K.T. Effect of residual salinity on spinach growth and nutrient contents in polder soil. Acta Hortic. 2008, 797, 419–424. [Google Scholar] [CrossRef]
- Machado, R.; Alves-Pereira, I.; Robalo, M.; Ferreira, R. Effects of municipal solid waste compost supplemented with inorganic nitrogen on physicochemical soil characteristics, plant growth, nitrate content, and antioxidant activity in Spinach. Horticulturae 2021, 7, 53. [Google Scholar] [CrossRef]
- Telesiñski, A.; Nowak, J.; Smolik, B.; Dubowska, A.; Skrzypiec, N. Effect of soil salinity on activity of antioxidant enzymes and content of ascorbic acid and phenols in bean [Phaseolus vulgaris L.] plants. J. Elem. 2008, 13, 401–409. [Google Scholar]
- Ferreira, J.F.; Sandhu, D.; Liu, X.; Halvorson, J.J. Spinach (Spinacea oleracea L.) response to salinity: Nutritional value, physiological parameters, antioxidant capacity, and gene expression. Agriculture 2018, 10, 163. [Google Scholar]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef]
- Fonteno, W.C.; Harden, C.T. Procedures for determining physical properties of horticultural substrates using the NCS-Porometer. In Horticultural Substrates Laboratory; North Carolina State University: Raleigh, NC, USA, 2003; Available online: https://www.ncsu.edu/project/hortsublab/pdf/porometer_manual.pdf (accessed on 21 March 2020).
- Prazeres, A.O. Comparação de Metodologias Laboratoriais para Determinação de Azoto Nítrico e Amoniacal em Solos e Águas; Programa e Livro de Resumos do 1° Congresso Nacional e Rega e Drenagem: Beja, Portugal, 2005; pp. 59–60. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 1987, 4. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties. Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. Available online: http://www.academicjournals.org/AJB50 (accessed on 21 January 2021).
- Paiva, E.A.S.; Isaias, R.M.D.S.; Vale, F.H.A.; Queiroz, C.G.D.S. The influence of light intensity on anatomical structure and pigment contents of Tradescantia pallida (Rose) Hunt. cv. purpurea Boom (Commelinaceae) leaves. Braz. Arch. Biol. Technol. 2003, 46, 617–624. [Google Scholar] [CrossRef]
- Cai, W.M.; Tang, Z.C. Plant tolerance physiology. In Experimental Guide for Modern Plant Physiology, 1st ed.; Tang, Z.C., Ed.; Science Press: Beijing, China, 1999; pp. 315–316. [Google Scholar] [CrossRef][Green Version]
- Bates, L.S. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berse, C. Use of a free radical method to evaluate antioxidant activity. Lwt-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lake, B. Preparation and characterization of microsomal fractions for studies of xenobiotic metabolism. In Biochemical Toxicology: A Practical Approach; Snell, K., Mullock, B., Eds.; IRL Press: Oxford, UK, 1987; pp. 183–215. [Google Scholar]
- Hissin, A.; Hilf, P.A. Fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Goldberg, D.; Spooner, R. Gluathione reductase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.-U., Ed.; Academic Press: Weinheim, Germany, 1987; pp. 258–265. [Google Scholar]
- Hernández, A.; Cano, M.P. High-pressure and temperature effects on enzyme inactivation in tomato puree. J. Agric. Food Chem. 1998, 46, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Roseburg, N.J.; Farr, A.L.; Randell, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Silber, A.; Bar-Tal, A. Nutrition of substrate-grown plants. In Soilless Culture: Theory and Practice; Elsevier: San Diego, CA, USA, 2008; pp. 291–339. [Google Scholar]
- Gastal, F.; Lemaire, G. N uptake and distribution in crops: An agronomical and ecophysiological perspective. J. Exp. Bot. 2002, 53, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Z.; Jiao, D.; Tian, X.; Wang, C. Different water and nitrogen fertilizer rates effects on growth and development of spinach. Commun. Soil Sci. Plan. 2018, 49, 1922–1933. [Google Scholar] [CrossRef]
- Russell, R.S.; Clarkson, D.T. Ion transport in root systems. Perspect. Exp. Biol. 1976, 2, 401–411. [Google Scholar]
- Savvas, D.; Passam, H.C.; Olympios, C.; Nasi, E.; Moustaka, E.; Mantzos, N.; Barouchas, P. Effects of ammonium nitrogen on lettuce grown on pumice in a closed hydroponic system. HortScience 2006, 41, 1667–1673. [Google Scholar] [CrossRef]
- Mills, H.A.; Jones, J.R. Plant Analysis Handbook II. A Practical Sampling, Preparation Analysis and Interpretation Guide; Micro Macro International, Inc.: Athens, GA, USA, 1996; ISBN 1878148001. [Google Scholar]
- Campbell, C. Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; Southern Cooperative Series Bulletin #394; Southern Cooperative Series Bulletin: Raleigh, NC, USA, 2000; ISBN 1-58161-394-6. Available online: www.ncagr.gov/agronomi/saaesd/scsb394.pdf (accessed on 10 March 2020).
- Lopez-Mosquera, M.E.; Moiron, C.; Carral, E. Use of dairy-industry sludge as fertiliser for grasslands in northwest Spain: Heavy metal levels in the soil and plants. Resour. Conserv. Recycl. 2000, 30, 95–109. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological limits to zinc biofortification of edible crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Hussain, P.R.; Suradkar, P.; Javaid, S.; Akram, H.; Parvez, S. Influence of postharvest gamma irradiation treatment on the content of bioactive compounds and antioxidant activity of fenugreek (Trigonella foenum-graceum L.) leaves. Innov. Food Sci. Emerg. 2016, 33, 268–281. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Alves-Pereira, I.; Ferreira, R.M.A. Effects of plant density and the number of emitters by styrofoam box on plant growth and nitrate concentration in spinach cultivated in substrate. Acta Hortic. 2019, 1242, 847–853. [Google Scholar] [CrossRef]
- Batziakas, K.G.; Rivard, C.L.; Stanley, H.; Batziakas, A.G.; Pliakoni, E.D. Reducing preharvest food losses in spinach with the implementation of high tunnels. Sci. Hortic. 2020, 265, 109268. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2019, 1–23. [Google Scholar] [CrossRef]
- Delfine, S.; Alvino, A.; Villani, M.C.; Loreto, F. Restrictions to carbon dioxide conductance and photosynthesis in spinach leaves recovering from salt stress. Plant Physiol. 1999, 119, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Ebrahimi, F.; Ahmadizadeh, M. Effect of different substrates on herbaceous pigments and chlorophyll amount of strawberry in hydroponic cultivation system. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 154–158, ISSN 1818-6769. [Google Scholar]
- Borowski, E.; Michalek, S. The effect of foliar nutrition of spinach (Spinacia oleracea L.) with magnesium salts and urea on gas exchange, leaf yield and quality. Acta Agrobot. 2010, 63, 77–85. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Alves-Pereira, I.; Lourenço, D.; Ferreira, R.M.A. Effect of organic compost and inorganic nitrogen fertigation on spinach growth, phytochemical accumulation, and antioxidant activity. Heliyon 2020, 6, e05085. [Google Scholar] [CrossRef]
- Du, S.-T.; Liu, Y.; Zhang, P.; Liu, H.-J.; Zhang, X.-Q.; Zhang, R.-R. Atmospheric application of trace amounts of nitric oxide enhances tolerance to salt stress and improves nutritional quality in spinach (Spinacia oleracea L.). Food Chem. 2015, 173, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Sultana, B.; Anwar, F.; Munir, H. Foliar spray of selected plant growth regulators affected the biochemical and antioxidant attributes of spinach in a field experiment. Turk. J. Agric. 2016, 40, 136–145. Available online: http://journals.tubitak.gov.tr/agriculture/ (accessed on 11 May 2021). [CrossRef]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P. Salinity, Osmolytes and Compatible Solutes. In Salinity: EnvironmentPlants—Molecules; Läuchli, A., Lüttge, U., Eds.; Springer: Dordrecht, The Netherland, 2002; pp. 181–204. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Claussen, W. Growth, water use efficiency, and proline content of hydroponically grown tomato plants as affected by nitrogen source and nutrient concentration. Plant Soil 2002, 247, 199–209. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. The effect of water supply on biomorphological and plant-physiological parameters of tomato transplants cultivated in wood fiber substrate. J. Appl. Bot. 2000, 74, 233–239. [Google Scholar]
- Gruda, N.; Schnitzler, W.H. The effect of water supply of seedlings cultivated in different substrates and raising systems on the bio-morphological and plant-physiological parameters of lettuce. J. Appl. Bot. 2000, 74, 240–247. [Google Scholar]
- Bhagyawant, S.S.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Koul, K.K.; Srivastava, N. Variations in the antioxidant and free radical scavenging under induced heavy metal stress expressed as proline content in chickpea. Physiol. Mol. Biol. Plants 2019, 25, 683–696. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; van Buren, L.; Wagner, E.; Wiseman, S.; van de Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–223. [Google Scholar] [CrossRef]
- Tavarini, S.; Cardelli, R.; Saviozzi, A.; Degl’Innocenti, E.; Guidi, L. Effects of green compost on soil biochemical characteristics and nutritive quality of leafy vegetables. Compos. Sci. Util. 2011, 19, 114–122. [Google Scholar] [CrossRef]
- Mazzucotelli, C.A.; Gonzalez-Aguilar, G.A.; Villegas-Ochoa, M.A.; Domínguez-Avila, A.J.; Ansorena, M.R.; Di Scala, K.C. Chemical characterization and functional properties of selected leafy vegetables for innovative mixed salads. J. Food Biochem. 2018, 42, e12461. [Google Scholar] [CrossRef]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants: Metabolism, Productivity, and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer Science + Business Media: Berlin, Germany, 2012; pp. 159–179. [Google Scholar] [CrossRef]
- Çetinkaya, H.; Kulak, M.; Karaman, M.; Karaman, H.; Koçer, F. Flavonoid accumulation behavior in response to the abiotic stress: Can a uniform mechanism be illustrated for all plants? In Flavonoids—From Biosynthesis to Human Health; Justino, J.G.D., de Campos Justino, D.D., Pinheiro, P.B.P.F., Eds.; Intech Editors: Lisboa, Portugal, 2017; pp. 151–165. [Google Scholar] [CrossRef]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effect of organic and conventional cropping systems on ascorbic acid, vitamin C, flavonoids, nitrate, and oxalate in 27 varieties of spinach (Spinacia oleracea L.). J. Agric. Food Chem. 2012, 60, 3144–3150. [Google Scholar] [CrossRef]
- Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. Effect of postharvest storage and processing on the antioxidant constituents (flavonoids and vitamin C) of fresh-cut spinach. J. Agric. Food Chem. 1999, 47, 2213–2217. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Morelock, T. Flavonoid content and antioxidant capacity of spinach genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2008, 88, 1099–1106. [Google Scholar] [CrossRef]
- Kidmose, U.; Knuthsen, P.; Edelenbos, M.; Justesen, U.; Hegelund, E. Carotenoids and flavonoids in organically grown spinach (Spinacia oleracea L) genotypes after deep frozen storage. J. Sci. Food Agric. 2001, 81, 918–923. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Colla, G.; Battistelli, Y. The effect of growing spinach (Spinacia oleracea L.) at two light intensities on the amounts of oxalate, ascorbate and nitrate in their leaves. J. Hortic. Sci. Biotechnol. 2004, 79, 606–609. [Google Scholar] [CrossRef]
- Cocetta, G.; Baldassarre, V.; Spinardi, A.; Ferrante, A. Effect of cutting on ascorbic acid oxidation and recycling in fresh-cut baby spinach (Spinacia oleracea L.) leaves. Postharvest Biol. Technol. 2014, 88, 8–16. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Technology. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–209. [Google Scholar] [CrossRef]
- Salomez, J.; Hofman, G. Nitrogen nutrition effects on nitrate accumulation of soil grown greenhouse butterhead lettuce. Commun. Soil Sci. Plant Anal. 2009, 40, 620–632. [Google Scholar] [CrossRef]
- Miceli, A.; Miceli, C. Effect of Nitrogen Fertilization on the quality of swiss chard at harvest and during storage as minimally processed produce. J. Food Qual. 2014, 37, 125–134. [Google Scholar] [CrossRef]
- Krezel, J.; Kolota, E. Source of nitrogen affects the yield and quality of spinach cultivars grown for autumn harvest. Acta Agric. Scand Sect. B Soil Plant Sci. 2014, 64, 583–589. [Google Scholar] [CrossRef]
- Mozafar, A. Nitrogen fertilizers and the amount of vitamins in plants: A review. J. Plant Nutr. 1993, 16, 2479–2506. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance. Plant Physiol. Bioch. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- De Kok, L.J.; Oosterhuis, F.A. Effects of frost-hardening and salinity on glutathione and sulfhydryl levels and on glutathione reductase activity in spinach leaves. Physiol. Plant 1983, 58, 47–51. [Google Scholar] [CrossRef]
- Hodges, D.M.; Forney, C.F. The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J. Exp. Bot. 2000, 51, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Atamanalp, M.; Turan, M.; Alak, G.; Kul, R.; Kitir, N.; Yildirim, E. Integrated use of nitrogen fertilizer and fish manure: Effects on the growth and chemical composition of spinach. Commun. Soil Sci. Plan. 2019, 50, 1580–1590. [Google Scholar] [CrossRef]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic browning of fruit and vegetables: A review. In Enzymes in Food Technology; Kuddus, M., Ed.; Springer: Singapore, 2018; pp. 63–78. [Google Scholar] [CrossRef]
- Maghoumi, M.; Gómez, P.A.; Mostofi, Y.; Zamani, Z.; Artés-Hernández, F.; Artés, F. Combined effect of heat treatment, UV-C and super atmospheric oxygen packing on phenolics and browning related enzymes of fresh-cut pomegranate arils. LWT Food Sci. Technol. 2013, 54, 389–396. [Google Scholar] [CrossRef]
- Apack, R.; Güçlü, K.; Demirata, B.; Ӧzyürek, M.; Çelik, S.; Bektasoğlü, B.; Berker, K.; Ӧzyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Sreeramulu, D.; Reddy, C.V.K.; Chauhan, A.; Balakrishna, N.; Raghunath, M. Natural antioxidant activity of commonly consumed plant foods in India: Effect of domestic processing. Oxid. Med. Cell Longev. 2013, 369479, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salas-Pérez, L.; Fornari-Reale, T.; Preciado-Rangel, P.; García-Hernández, J.L.; Sánchez-Chávez, E.; Troyo-Diéguez, E. Cultivar variety and added potassium influence the nutraceutical and antioxidant content in hydroponically grown basil (Ocimum basilicum, L.). Agronomy 2018, 8, 13. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Drouza, C.; Tzortzakis, N. Optimization of potassium fertilization/nutrition for growth, physiological development, essential oil composition and antioxidant activity of Lavandula angustifolia Mill. J. Soil Sci. Plant Nutr. 2017, 17, 291–306. [Google Scholar] [CrossRef]
- Massantini, F.; Favilli, R.; Magnani, G.; Oggiano, N. Soilless culture, biotechnology for high-quality vegetables. Soil. Cult. Cult. 1988, 4, 27–40. [Google Scholar]

| Substrate | Peat | Coir Pith | Coir Chips | Coir Pith + Fiber |
|---|---|---|---|---|
| Composition | 70% black peat + 30% white peat | 100% | 100% | 93% coir pith + 7% fiber |
| pH * | 5.5–6.0 | 5.5–6.0 | 5.5–6.2 | 5.5–6.2 |
| EC (dS·m−1) * | 1.5–1.8 | <1.9 | ≥1.5 | ≥1.5 |
| CEC (meq/100g)* | 100–190 | 60–120 | 20–40 | 40–80 |
| N (mg L−1) * | 50–300 | |||
| P (mg L−1) * | 35–131 | |||
| K (mg L−1) * | 60–330 | |||
| Total porosity (v/v, %) * | 95 | |||
| Granulometry (mm) * | 0–10 | 10–15 | 2–4 | |
| Air (v/v, %) * | - | 25 | 40 | 30 |
| Water holding capacity (v/v, %) * | - | 70 | 54 | 65 |
| Mass wetness (g water/g substrate) ** | 6.07 † c | 7.84 b | 5.75 d | 8.65 a |
| Moisture content (w/w, %) ** | 82.6 ab | 84.68 a | 71.10 b | 84.63 a |
| Bulk density (g·cm−3) ** | 0.127 a | 0.103 a | 0.070 b | 0.081 a |
| Substrate | Leaf Macronutrients (%) | Leaf Micronutrients (μg·g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Fe | B | Cu | Mn | Zn | |
| Peat | 4.48 a Z | 0.38 | 6.88 a | 0.94 c | 0.70 b | 89.2 | 34.0 | 31.2 | 105.2 a | 198.2 a |
| Coir pith | 4.18 a | 0.36 | 5.96 b | 1.00 b | 0.64 b | 112.6 | 33.2 | 39.4 | 104.8 a | 211.0 a |
| Coir pith + fiber | 3.98 ab | 0.32 | 6.26 ab | 1.04 ab | 0.72 b | 77.0 | 35.2 | 25.4 | 104.8 a | 210.0 a |
| Coir chips | 3.48 b | 0.32 | 5.14 bc | 1.20 a | 0.82 a | 65.0 | 30.0 | 29.6 | 73.0 b | 115.2 b |
| Recommended range | ||||||||||
| [41] | 4.00–6.00 | 0.30–0.60 | 5.00–8.00 | 0.70–1.20 | 0.60–1.00 | 60–200 | 25–60 | 5–25 | 30–250 | 25–100 |
| [42] | 4.00–6.00 | 0.30–0.50 | 3.00–8.00 | 1.00–1.50 | 0.40–1.00 | 50–200 | 25–60 | 5–15 | 25–200 | 20–75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, R.M.A.; Alves-Pereira, I.; Ferreira, R.; Gruda, N.S. Coir, an Alternative to Peat—Effects on Plant Growth, Phytochemical Accumulation, and Antioxidant Power of Spinach. Horticulturae 2021, 7, 127. https://doi.org/10.3390/horticulturae7060127
Machado RMA, Alves-Pereira I, Ferreira R, Gruda NS. Coir, an Alternative to Peat—Effects on Plant Growth, Phytochemical Accumulation, and Antioxidant Power of Spinach. Horticulturae. 2021; 7(6):127. https://doi.org/10.3390/horticulturae7060127
Chicago/Turabian StyleMachado, Rui M. A., Isabel Alves-Pereira, Rui Ferreira, and Nazim S. Gruda. 2021. "Coir, an Alternative to Peat—Effects on Plant Growth, Phytochemical Accumulation, and Antioxidant Power of Spinach" Horticulturae 7, no. 6: 127. https://doi.org/10.3390/horticulturae7060127
APA StyleMachado, R. M. A., Alves-Pereira, I., Ferreira, R., & Gruda, N. S. (2021). Coir, an Alternative to Peat—Effects on Plant Growth, Phytochemical Accumulation, and Antioxidant Power of Spinach. Horticulturae, 7(6), 127. https://doi.org/10.3390/horticulturae7060127








