Rootstock–Scion Hydraulic Balance Influenced Scion Vigor and Yield Efficiency of Malus domestica cv. Honeycrisp on Eight Rootstocks
Abstract
1. Introduction
2. Materials and Methods
2.1. Planting Materials and Trial Conditions
2.2. Tree Growth, Yield, and Nutrient Status
2.3. Fruit Skin Chlorophyll Content, Dry Matter, and Tissue Water Potential
2.4. Tree–Water Relations
2.5. Xylem Vessel Elements
2.6. Data Analysis
3. Results
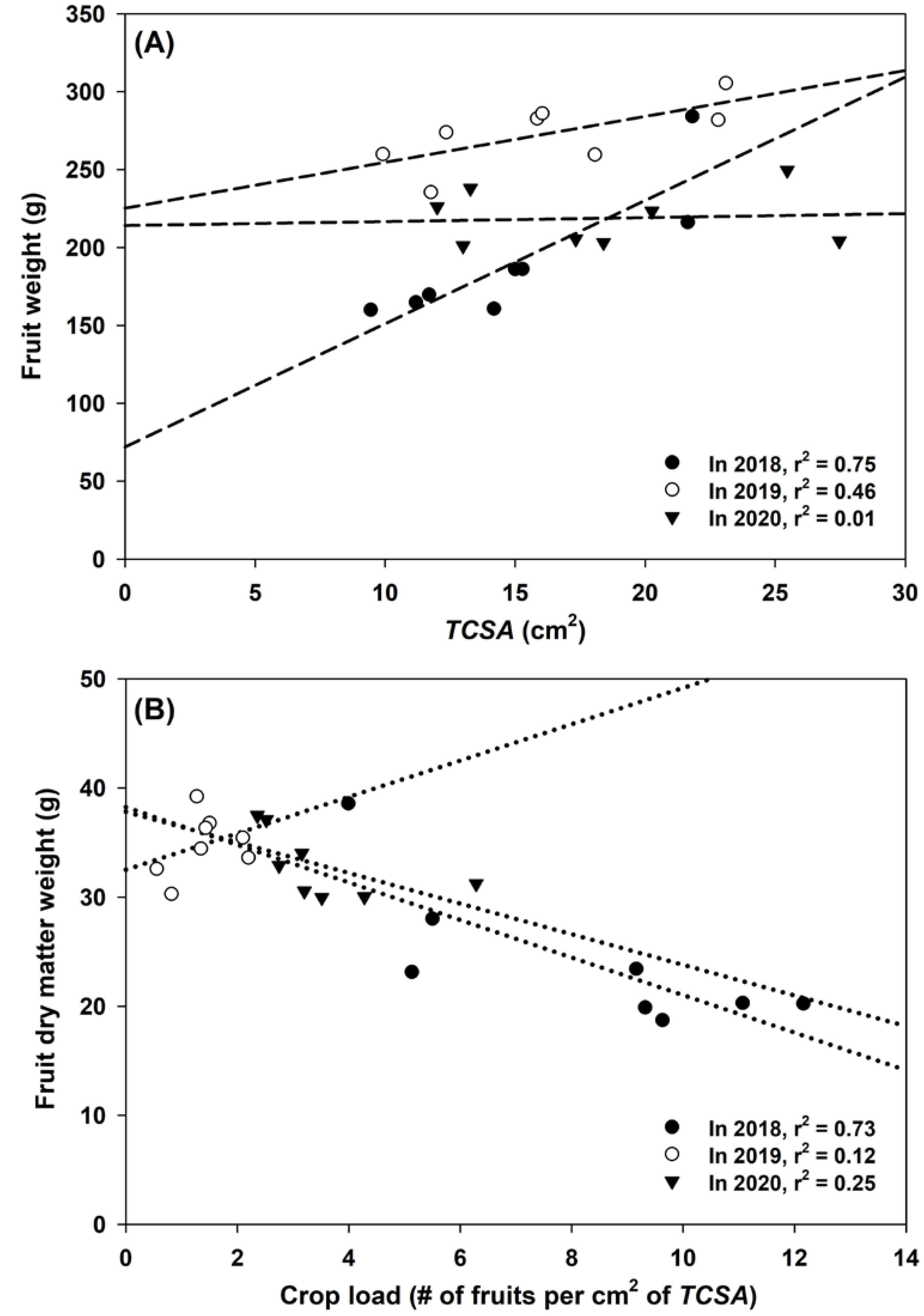
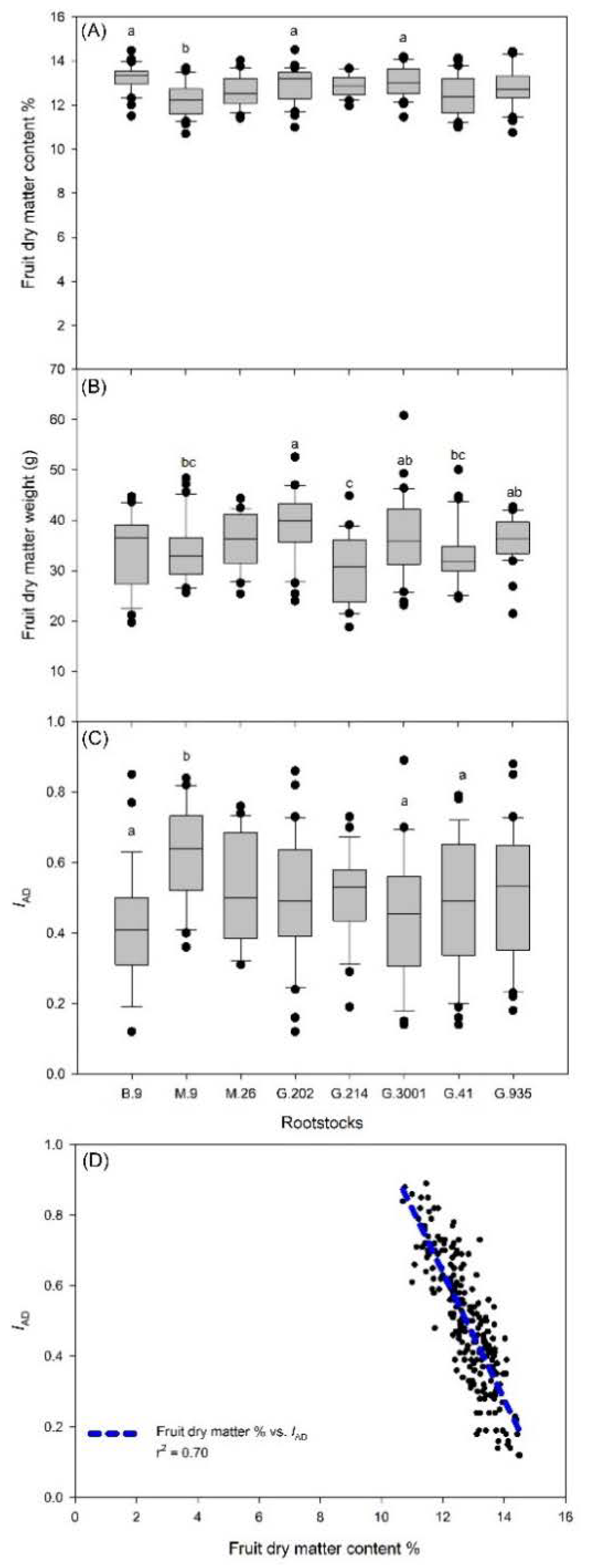
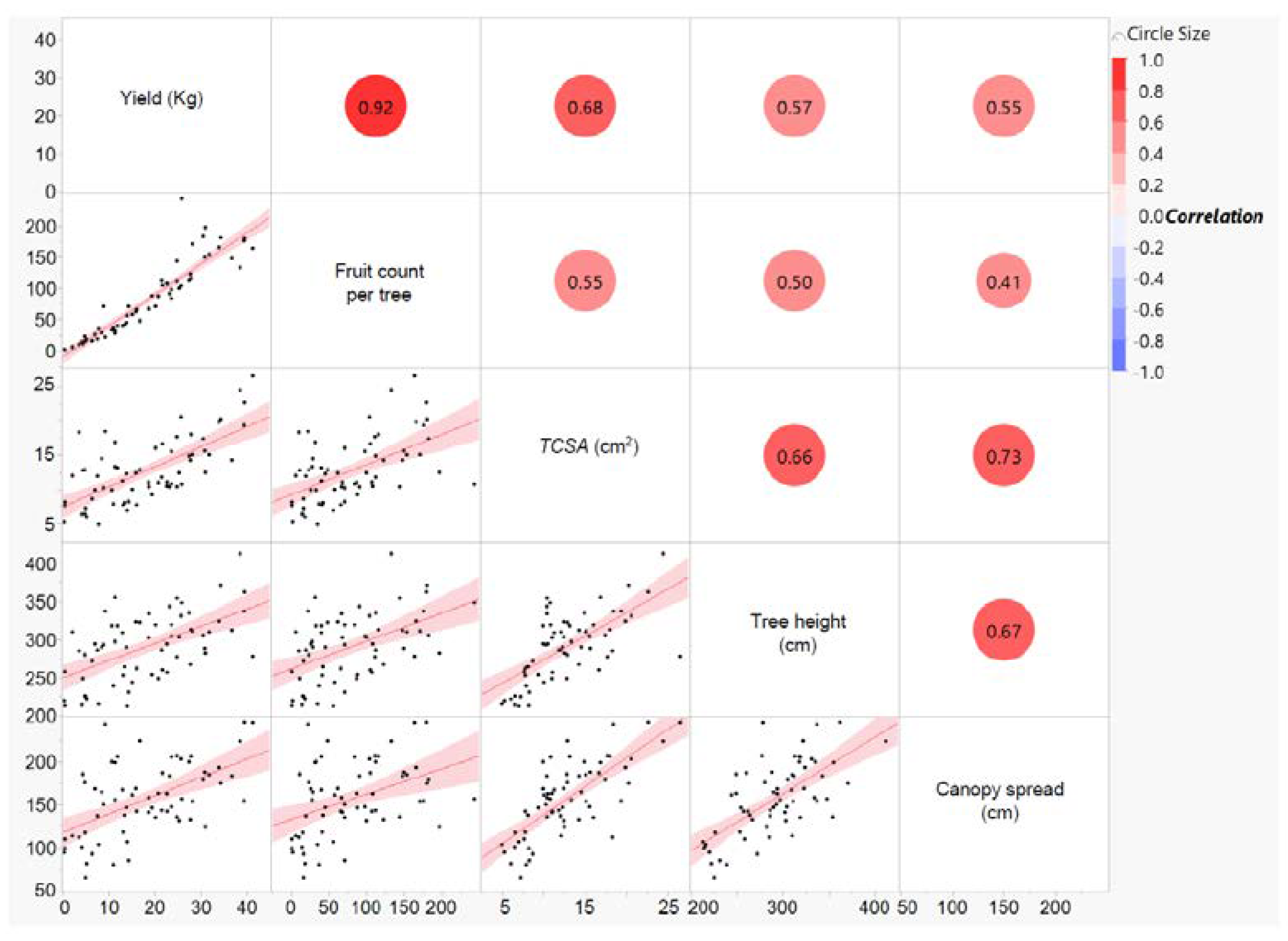
3.1. Yield, Crop Load, and Fruit Dry Matter
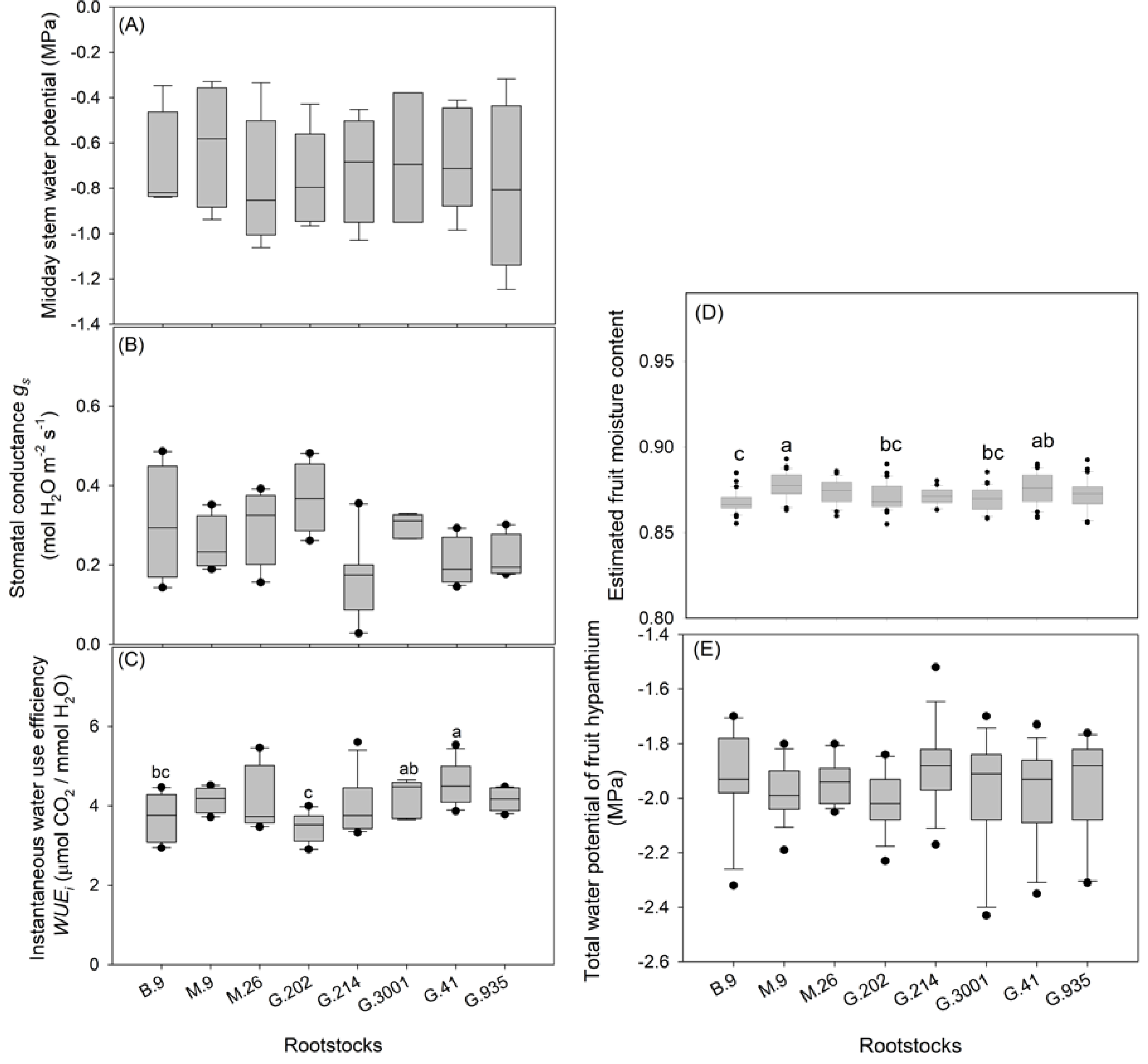
3.2. Tree Water and Nutrient Status
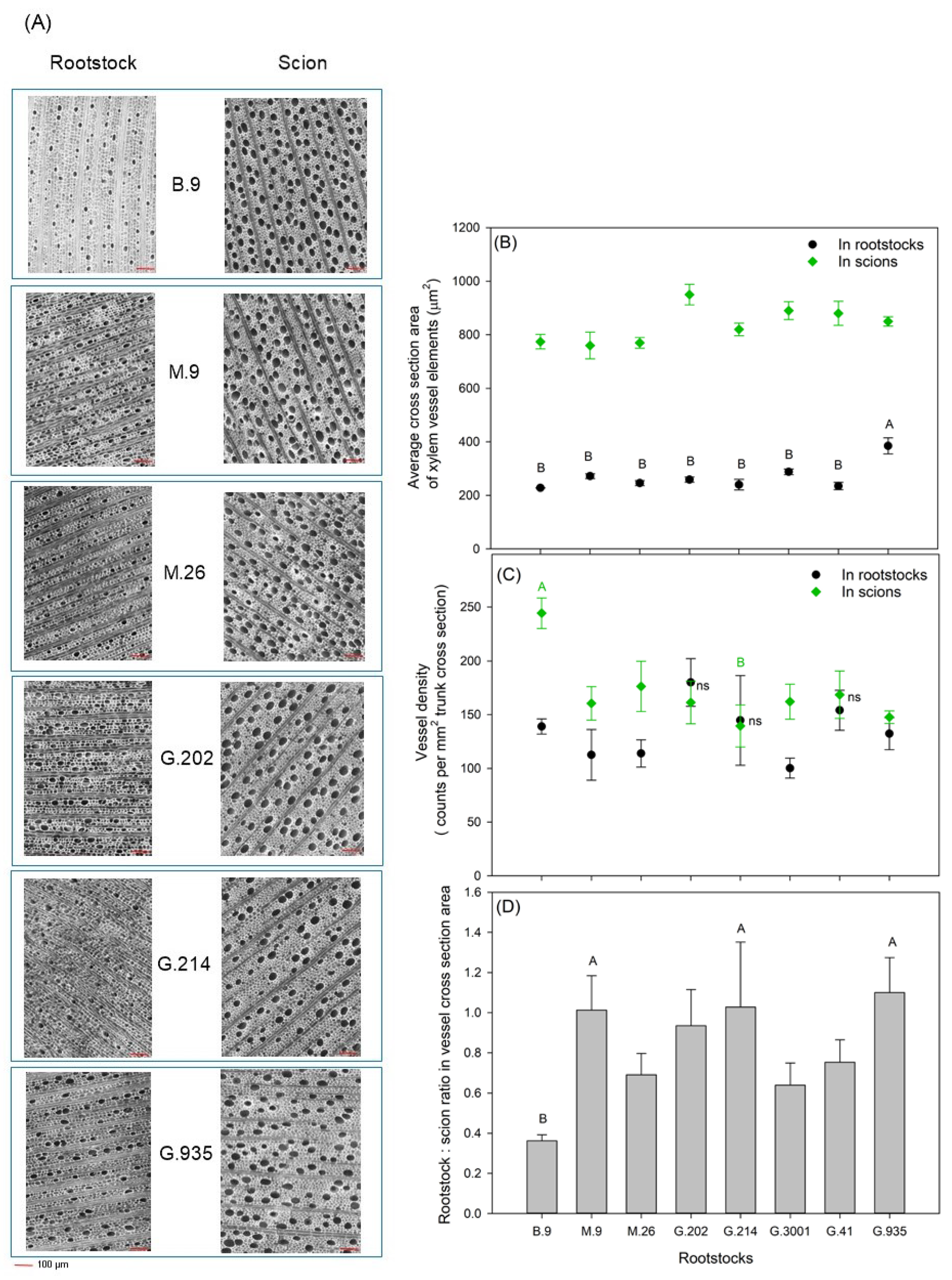
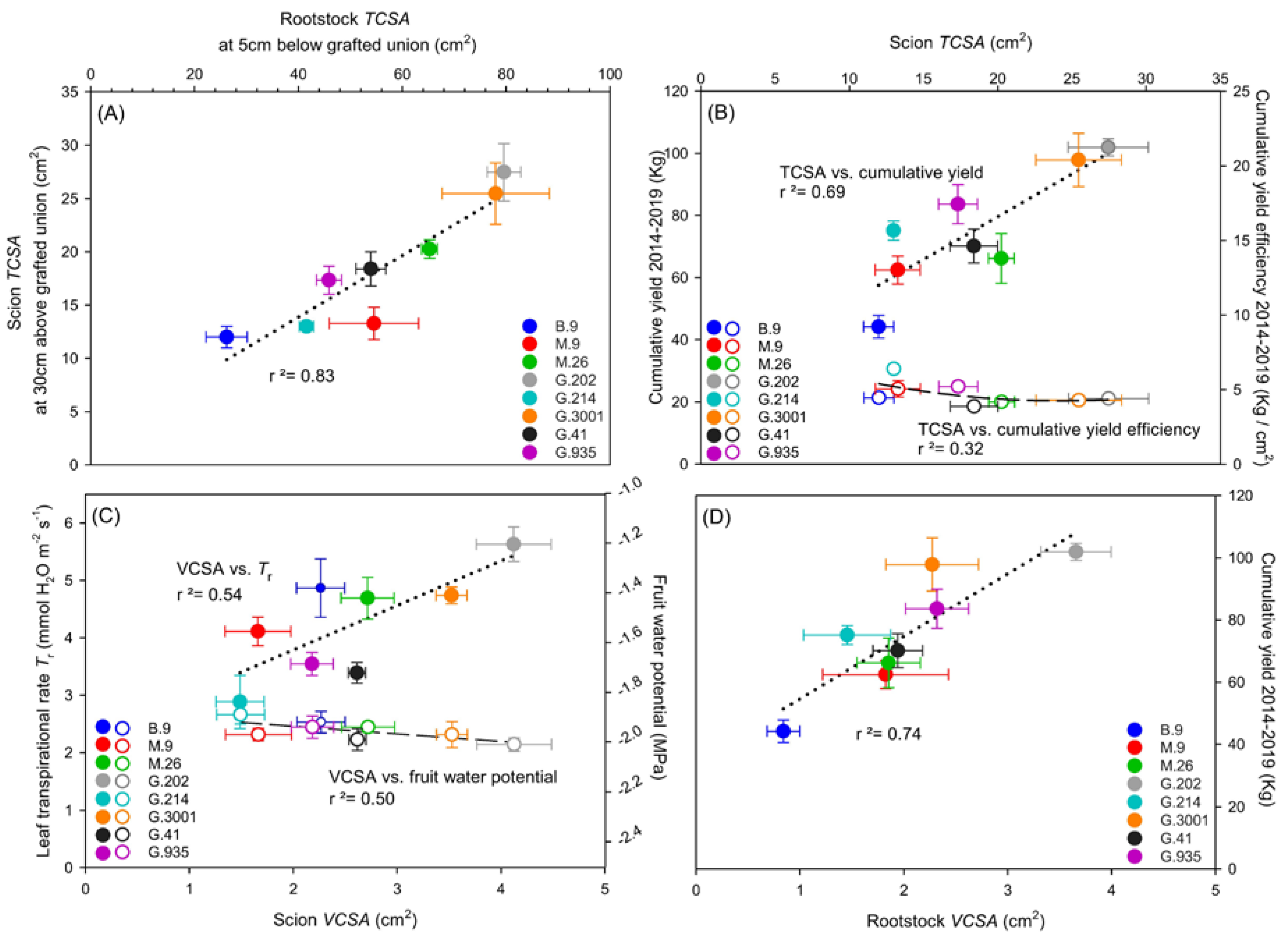
3.3. Xylem Vessel Elements in Relation to Tree Vigor and Yield Efficiency
4. Discussion
4.1. Tree Vigor, Yield, and Water Use Strategies
4.2. Influence of Rootstock–Scion Hydraulic Balance on Scion Vigor and Yield Efficiency
5. Conclusions
- (1)
- The rootstocks with higher vigor demonstrated stronger annual fluctuations in yield. The least vigorous rootstocks showed significant fluctuations in crop load in 2017, 2018, and 2019, as well as more tree-to-tree variations in crop load each year. Therefore, in the most productive rootstocks and the least vigorous ones, managing crop load was particularly important for alleviating biennial bearing and sustaining fruit quality.
- (2)
- The size of the vessel cross-section in Honeycrisp scion was larger than that in each rootstock. Vessel density in scion was either higher than or close to that in rootstocks. This represented an imbalance between rootstock water supply and scion water demand. However, the size of vessel cross-section and the vessel density in semi-dwarfing rootstocks was not significantly different from dwarfing rootstocks of less vigor, which indicated that the intensity of the dwarfing effect was not solely determined by the vessel size and density. These findings were consistent with previous statements on the hydraulic restriction being one but not the only important mechanism for conferring dwarfing effects.
- (3)
- Scion vigor and cumulative yield were positively correlated with rootstock TCSA and VCSA, with G.202 being the highest and B.9 being the lowest. Vessel size and density in rootstock xylem could be important indicative traits for predicting yield and yield efficiency, as G.202 had higher vessel density, and G.935 with both high yield and high yield efficiency had larger vessel size in rootstock xylem.
- (4)
- Scion VCSA was dependent on the size and density of vessel elements in scion xylem, and scion TCSA. In turn, it could influence scion water and nutrient use.
- Higher scion VCSA in G.202 was associated with higher production at a cost of higher water use and lower WUEi. In contrast, low water use in G.41 led to high WUEi but low yield efficiency.
- In G.214, leaf chlorosis, low values in leaf gas exchange, leaf nutrients, and fruit dry matter weight, and the previously reported high number of root suckers, were associated with low vessel density and small VCSA of its scion. These disadvantages compromised its high yield efficiency. It suggests the necessity of taking multiple tradeoff traits into consideration while evaluating a rootstock with high yield efficiency.
- B.9 produced the smallest scion TCSA with small xylem vessels in very high density; it demonstrated moderate tree–water status and earlier fruit maturation; such characteristics are worthy of further investigation in relation to its cold hardiness and resilience to water deficits.
- (5)
- In the three rootstocks with the highest cumulative yield efficiency and the moderate tree vigor, i.e., M.9, G.214, and G.935, the VCSArootstock/VCSAscion ratio was equal to or slightly higher than 1, suggesting the importance of this ratio in determining rootstock–scion hydraulic balance and vegetative-reproductive biomass partitioning, and consequently influencing tree size and cumulative yield efficiency.
- (6)
- G.935 was ranked in the top three in both yield and yield efficiency, and it demonstrated moderate water and nutrient status. These traits indicated its great potential as a substitute for standard dwarfing rootstocks in high-density planting of Honeycrisp.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Autio, W.; Robinson, T.; Blatt, S.; Cochran, D.; Francescato, P.; Hoover, E.; Kushad, M.; Lang, G.; Lordan, J.; Miller, D.; et al. Pillnitz and Malling apple rootstocks affect ’Honeycrisp’ performance over eight years in the 2010 NC-140’Honeycrisp’ apple rootstock trial. J. Am. Pomol. Soc. 2020, 74, 182–195. [Google Scholar]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Harrison, N.; Harrison, R.J.; Barber-Perez, N.; Cascant-Lopez, E.; Cobo-Medina, M.; Lipska, M.; Conde-Ruíz, R.; Brain, P.; Gregory, P.J.; Fernández-Fernández, F. A new three-locus model for rootstock-induced dwarfing in apple revealed by genetic mapping of root bark percentage. J. Exp. Bot. 2016, 67, 1871–1881. [Google Scholar] [CrossRef]
- Fazio, G.; Lordan, J.; Francescatto, P.; Robinson, T.L. Breeding apple rootstocks to match cultural and nutrient requirements of scion varieties. FRUIT Q. 2018, 26, 25–30. [Google Scholar]
- Foster, T.M.; Celton, J.-M.; Chagné, D.; Tustin, D.S.; Gardiner, S.E. Two quantitative trait loci, dw1 and dw2, are primarily responsible for rootstock-induced dwarfing in apple. J. Hort. Res. 2015, 2, 15001. [Google Scholar] [CrossRef] [PubMed]
- Olien, W.; Lakso, A.N. Effect of rootstock on apple (Malus domestica) tree water relations. Physiol. Plant. 1986, 67, 421–430. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Webster, A.D.; Vaughan, S.; Lucas, A.S. Effects of root restriction on the physiology of apple tree growth. Acta Hortic. 1997, 451, 587–598. [Google Scholar] [CrossRef]
- Robinson, T.; Fazio, G.; Hoying, S.; Miranda, M.; Iungerman, K. Geneva® rootstocks for weak growing scion cultivars like ‘Honeycrisp.’. N.Y. Fruit. Q. 2011, 19, 10–16. [Google Scholar]
- Jones, H.G. How do rootstocks control shoot water relations? New. Phytol. Found. 2012, 194, 301–303. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Effects of size-controlling apple rootstocks on growth, abscisic acid, and hydraulic conductivity of scion of different vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Serra, S.; Leisso, R.; Giordani, L.; Kalcsits, L.; Musacchi, S. Crop load influences fruit quality, nutritional balance, and return bloom in ‘Honeycrisp’ apple. HortScience 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and Function in conifer tracheids and angiosperm vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef]
- Bauerle, T.L.; Centinari, M.; Bauerle, W.L. Shifts in xylem vessel diameter and embolisms in grafted apple trees of differing rootstock growth potential in response to drought. Planta 2011, 234, 1045–1054. [Google Scholar] [CrossRef]
- Webster, A.D. Vigour mechanisms in dwarfing rootstocks for temperate fruit trees. Acta Hortic. 2004, 658, 29–41. [Google Scholar] [CrossRef]
- Tombesi, S.; Johnson, R.S.; Day, K.R.; DeJong, T.M. Interactions between rootstock, inter-stem and scion xylem vessel characteristics of peach trees growing on rootstocks with contrasting size-controlling characteristics. AoB Plants 2010, 2010, plq013. [Google Scholar] [CrossRef]
- Parry, C.; Blonquist Jr, J.M.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef]
- DeLong, J.; Prange, R.; Harrison, P.; Nichols, D.; Wright, H. Determination of optimal harvest boundaries for Honeycrisp™ fruit using a new chlorophyll meter. Can. J. Plant Sci. 2014, 94, 361–369. [Google Scholar] [CrossRef]
- Xu, H.; Ediger, D. Rootstocks with different vigor influenced scion–water relations and stress responses in AmbrosiaTM apple trees (Malus domestica var. Ambrosia). Plants 2021, 10, 614. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.-M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap pressure in vascular plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ding, N.; Chen, Q.; Zhu, Z.; Peng, L.; Ge, S.; Jiang, Y. Effects of crop load on distribution and utilization of 13C and 15N and fruit quality for dwarf apple trees. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.G.; Oren, R.; Waring, R.H. Fruiting and sink competition. Tree Physiol. 2018, 38, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Lenz, F. Fruit effects on the dry matter-and carbohydrate distribution in apple trees. Acta Hortic. 2009, 835, 21–38. [Google Scholar] [CrossRef]
- Forshey, C.G.; Elfving, D.C. The relationship between vegetative growth and fruiting in apple trees. Hort. Rev. 1989, 11, 229–287. [Google Scholar]
- Adams, S.W. The Effects of Rootstock, Scion, Grafting Method and Plant Growth Regulators on Flexural Strength and Hydraulic Resistance of Apple. Master′s Thesis, Utah State University, Logan, UT, USA, 2016. [Google Scholar]


| Rootstocks | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| B.9 | M.9 | M.26 | G.202 | G.214 | G.3001 | G.41 | G.935 | ||
| Leaves 2019 | N % | 1.51 ± 0.03 bc | 1.55 ± 0.17 b | 1.45 ± 0.02 c | 1.62 ± 0.12 b | 1.40 ± 0.02 c | 1.82 ± 0.02 a | 1.55 ± 0.05 b | 1.43 ± 0.02 c |
| P % | 0.11 | 0.11 | 0.10 ± 0.01 | 0.12 | 0.10 | 0.10 | 0.11 | 0.11 | |
| K % | 1.1 ab | 1.2 ± 0.1 ab | 1.1 ± 0.1 ab | 1.2 ± 0.1 a | 1.2 ± 0.1 a | 0.9 b | 1.2 ± 0.1 a | 1.1 ± 0.1 ab | |
| Mg % | 0.17 ab | 0.17 ± 0.01 ab | 0.16 ab | 0.18 ± 0.01 ab | 0.14 ± 0.01 b | 0.24 ± 0.01 a | 0.17 ab | 0.15 ± 0.01 ab | |
| Ca % | 0.77 ± 0.03 bc | 0.89 ± 0.0 6ab | 0.67 c | 0.92 ± 0.11 ab | 0.79 ± 0.05 b | 1.09 ± 0.02 a | 0.76 ± 0.04 bc | 0.79 ± 0.10 b | |
| B ppm | 24.4 ± 0.9 b | 25.1 ± 1.3 b | 26.1 ± 0.3 b | 27.8 ± 2.1 b | 26.6 ± 0.1 b | 21.9 ± 0.4 c | 34.6 ± 1.6 a | 27.8 ± 1.5 b | |
| Zn ppm | 17.5 ± 6.7 | 9.0 | 10.0 ± 0.7 | 9.0 ± 1.4 | 8.0 ± 0.7 | 7.0 | 9.0 | 8.5 ± 0.4 | |
| Mn ppm | 17.0 ± 0.7 a | 15.5 ± 3.2 ab | 18.5 ± 1.8 a | 12.0 ± 1.4 b | 7.5 ± 0.4 c | 14.5 ± 0.4 ab | 8.0 c | 7 ± 0.7 c | |
| Fe ppm | 70.5 ± 22.3 a | 27.5 ± 1.8 b | 34 ± 4.9 b | 67.5 ± 26.5 a | 20 ± 8.5 b | 30 ± 1.4 b | 26 ± 4.2 b | 17.5 ± 2.5 b | |
| Chlorophyll µmol cm−2 | 371.8 ± 14.0 ab | 368.1 ± 16.8 ab | 371.7 ± 18.1 ab | 403.9 ± 14.2 a | 304.4 ± 20.1 b | 385.4 ± 30.0 ab | 328.3 ± 24.7 ab | 349.9 ± 16.0 ab | |
| Fruits 2019 | N % | 0.41 | 0.47 | 0.36 | 0.44 | 0.43 ± 0.01 | 0.50 ± 0.04 | 0.43 | 0.50 ± 0.02 |
| P % | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.06 ± 0.01 | 0.06 | 0.075 | |
| K % | 0.71 | 0.68 | 0.72 | 0.74 | 0.72 ± 0.02 | 0.77 ± 0.09 | 0.8 | 0.85 ± 0.02 | |
| Mg % | 0.04 | 0.05 | 0.04 | 0.05 | 0.05 | 0.055 | 0.04 | 0.05 | |
| Ca % | 0.06 | 0.07 | 0.06 | 0.07 | 0.07 | 0.08 ± 0.01 | 0.07 | 0.065 | |
| B ppm | 15.72 | 17.07 | 20.21 | 21.47 | 22.15 ± 0.69 | 18.35 ± 1.80 | 25.36 | 28.09 ± 2.09 | |
| Zn ppm | 2 | 1 | 2 | 1 | 1.50 ± 0.35 | 1.50 ± 0.35 | 2 | 1 | |
| Fruit weight g | 259.9 ± 9.2 b | 274.0 ± 7.2 ab | 282.8 ± 9.9 ab | 305.5 ± 9.9 a | 235.5 ± 11.2 c | 282.0 ± 10.6 ab | 259.6 ± 6.3 b | 286.2 ± 7.9 ab | |
| Tree | Cumulative yield 2014–2019 Kg | 44.21 ± 3.63 e | 62.47 ± 4.52 d | 66.17 ± 7.99 cd | 101.89 ± 2.76 a | 75.15 ± 3.08 bc | 97.86 ± 8.56 a | 70.14 ± 5.45 c | 83.63 ± 6.28 b |
| Yield efficiency 2014–2019 Kg cm−2 TCSA | 4.46 ± 0.16 bc | 5.06 ± 0.55 b | 4.18 ± 0.19 c | 4.41 ± 0.27 c | 6.39 ± 0.22 a | 4.29 ± 0.28 c | 3.88 ± 0.16 c | 5.21 ± 0.28 b | |
| TCSA 2019 cm2 | 9.9 ± 0.9 d | 12.4 ± 1.0 c | 15.9 ± 1.2 b | 23.1 ± 1.3 a | 11.8 ± 0.5 cd | 22.8 ± 2.3 a | 18.1 ± 0.9 ab | 16.1 ± 0.9 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Ediger, D.; Singh, A.; Pagliocchini, C. Rootstock–Scion Hydraulic Balance Influenced Scion Vigor and Yield Efficiency of Malus domestica cv. Honeycrisp on Eight Rootstocks. Horticulturae 2021, 7, 99. https://doi.org/10.3390/horticulturae7050099
Xu H, Ediger D, Singh A, Pagliocchini C. Rootstock–Scion Hydraulic Balance Influenced Scion Vigor and Yield Efficiency of Malus domestica cv. Honeycrisp on Eight Rootstocks. Horticulturae. 2021; 7(5):99. https://doi.org/10.3390/horticulturae7050099
Chicago/Turabian StyleXu, Hao, Danielle Ediger, Amritpal Singh, and Christopher Pagliocchini. 2021. "Rootstock–Scion Hydraulic Balance Influenced Scion Vigor and Yield Efficiency of Malus domestica cv. Honeycrisp on Eight Rootstocks" Horticulturae 7, no. 5: 99. https://doi.org/10.3390/horticulturae7050099
APA StyleXu, H., Ediger, D., Singh, A., & Pagliocchini, C. (2021). Rootstock–Scion Hydraulic Balance Influenced Scion Vigor and Yield Efficiency of Malus domestica cv. Honeycrisp on Eight Rootstocks. Horticulturae, 7(5), 99. https://doi.org/10.3390/horticulturae7050099







