Abstract
The growing population of tropical countries has led to a new awareness of the importance of vegetables as a source of essential foods and nutrients. The success of vegetable cultivation depends to a large extent on high-quality seedlings. This work aimed at evaluating the effects of different substrates and different nutrient solution concentrations on the development of lettuce and Chinese cabbage seedlings in a semi-arid tropical area. Three independent experiments were conducted at the Soil and Water Research Station at Yezin Agriculture University, Myanmar (Myanmar, 19.83° N; 96.27° E). In all experiments a randomized block design was implemented with four treatments and three repetitions. In the first experiment the adaptability of lettuce seedling to two substrates (namely a Hulls Manure mix composed by 50% of mature cattle manure and 50% of carbonized rice husk and a soil based substrate constituted by 70% local soil, 20% burned rice husk, and 10% fresh cattle manure) and two nutrient solutions with different electrical conductivities (ECs) (W0.1, stored rainwater with EC = 0.13 dS m−1 and NS1.2, nutrient solution with EC = 1.20 dS m−1) were tested. In the second and third experiments, two species (lettuce and Chinese cabbage) were assessed for their response to nutrient solution concentrations. In both crops, 4 fertigation treatments (W0.1; NS0.6; NS1.2; and NS1.8) were supplied, by modulating the concentration of a compound mineral fertilizer (15:15:15) in the following ranges: W0.1: 0 g L−1, electrical conductivity (EC) 0.13 dS m−1, NS0.6: 0.3 g L−1, EC of 0.60 dS m−1; NS1.2: 0.6 g L−1, 1.2 dS m−1 EC, and NS1.8: 0.9 g L−1, 1.8 dS m−1 EC. Adopting different substrates and applying different nutrient solutions significantly affected growth (fresh weight and leaf morphology) and some physiological parameters (stomatal conductance, leaf temperature, and leaf chlorophyll content) of lettuce and Chinese cabbage seedling. From the first experiment, the combination of the soil based substrate and NS1.2 treatments allowed us to improve the seedlings’ growth. In the second experiment, highest growth of lettuce and Chinese cabbage seedlings was associated with NS1.2 and NS1.8, respectively. The presented results allow for the optimization of both growing media and nutrient solution management when lettuce and Chinese cabbage seedling are produced in the Central Dry Zone of Myanmar.
1. Introduction
Climate change and the world population increase are leading to a new awareness on the importance of vegetable crops as a source of food. This is particularly relevant, since vegetables can supply essential nutrients (e.g., vitamins and minerals) that are otherwise not available from other foods [1] Moreover, the current sanitary emergency due to the COVID19 pandemic will likely reinforce the importance of getting knowledge and awareness regarding the cultivation of their own vegetables to increase family food security [2,3]. Small scale production of vegetables can improve food security and build greater resilience, mainly in vulnerable families [4]. The Central Dry Zone of Myanmar (CDZ-Myanmar) lies in the central area of the country, crossed by the Ayeyarwady River, between latitudes 19°20″ to 22°50″ and longitudes 93°40″ to 96°30″. The area covers about 13 percent of the country’s total area with a population of roughly 14.5 million, close to a third of the country’s population (WFP 2011). The CDZ-Myanmar population’s livelihood is highly dependent on the south-west monsoon, which provides the region’s annual share of rainfall. Precipitations are mostly confined to the period from June to mid-October [5]. The local horticulture production is basically limited to onion cultivation grown along the beds and banks of the surface streams. Other vegetables (tomato, Chinese cabbage, lettuce, chili pepper, roselle, and pumpkin) are cultivated for home consumption only and solely during the rainy season but actually, no particular care is paid to horticultural production [6]. People living in the CDZ-Myanmar generally face a food insecurity gap varying from 4 to 6 months each year and have to deal with irregular incomes due to limited job opportunities. As emerged by the World Food Programme’s survey of 2013 [6], food insecurity in the CDZ-Myanmar is mainly associated with poor access to food and markets. The limited alternative sources of income makes the picture even more gloomy. Furthermore, in the CDZ-Myanmar no farmer can produce vegetables regularly due to a limited shortage of natural resources, limited budget, and know-how, and the scarce access to innovative technologies for vegetable production. Accordingly, the local villagers’ diet has been found unbalanced and extremely poor in vitamins and micronutrients, at least for half a year [7]. Fostering people to grow their own vegetables to reduce the food insecurity gap is therefore crucial. Nevertheless, the starting point to ensure final vegetable cultivation success depends mostly on high-quality seedlings [1]. Chinese cabbage (Brassica juncea L.) and lettuce (Lactuca sativa L.) are two extensively grown vegetables in Myanmar. In the CDZ-Myanmar, the production of Chinese cabbage is elevate, reaching 763 MT year−1 while lettuce production is more limited, accounting for around 90 MT year−1 [8]. However, the increase of leaf fresh vegetable production is one of the priorities identified by national and international agencies to stimulate farm diversification and improve the diet of people living in CDZ-Myanmar [9]. From a nutritional perspective, both crops belong to the so-called green leafy vegetables, whose relevance in the diet is associated with their contributions in fibers, vitamins, and minerals (including calcium, iron, and phosphorous), carotenoids, and other antioxidants [10,11,12]. People living in CDZ-Myanmar consume minimal amounts of fresh vegetables while consuming mainly cooked vegetables [13]. Chinese cabbage is consumed fried, resulting in some nutritional value loss because vitamins and antioxidants are lost through oxidation [14]. Generally, lettuce and Chinese cabbage are transplanted, therefore appropriate seedling development is required. In countries with more developed economies (e.g., Europe or North America), seedlings are usually supplied by professional nurseries, who adopt commercial substrates in standard trays [15], which allow for saving both substrate and space. On the other hand, in South-East Asia, vegetable seeds are usually sown directly in the field, resulting in unequal distribution of seeds and subsequently uneven emergence and growth of seedlings [16]. Good quality seedlings guarantee a high rooting rate after transplanting, besides requiring less phytosanitary treatments [17]. Furthermore, seedlings uniformity growing rate allows to save water, while also reducing damage to plants’ roots at the time of transplanting [18]. A main challenge for the vegetable production sector in the CDZ-Myanmar is represented by the scarce availability of good-quality seedlings [19]. This study aims to assess the efficiency of simplified methodologies for the local production of lettuce and Chinese cabbage seedlings in CDZ-Myanmar by comparing two locally available substrates and different concentrations of a nutrient solution obtained by using a local compound fertilizer. The assumption is that the adoption of improved vegetable seedling production methodologies can ensure better quality seedlings, as compared to current production techniques. The study integrates figures from different crop features and management strategies to elaborate specific recommendations on the optimal management of seedling production.
2. Materials and Methods
2.1. Location
Three experiments were conducted in open field conditions at the Soil and Water Research Station of Yezin Agriculture University located in the University Campus, CDZ-Myanmar, 16 km from the Capital Naypyidaw (19°83′ North and 96°27′ East, 122 m a.s.l.) (Figure 1a,b). According to Köppen’s classification, the local climate is the Aw type, which is tropical rainy with dry summer and rainy season concentrated between June and October.
Figure 1.
Location where the experiments took place in South-East Asia (a) and within the country (b). Image of the low-tech experimental nursery used for the research (c).
2.2. Plant Material and Crop Management
The first two experiments were performed on lettuce (Lactuca sativa L.) cv. Green wave (Evergreen seeds, Sunnyvale, CA, USA), while the third one was carried out on Chinese cabbage (Brassica juncea L.) cv. Pavito (East West Seeds, Nanning, China). Both cultivars are commonly sold in the local market of the main cities of CDZ-Myanmar such as Sagaing, Magway, and Mandalay. Sowing took place on 9 January 2020 (first experiment) and 27 February 2020 (second and third experiment). Crops were sown manually in 105 cells plastic seedling trays. The sizes of the seedling tray were the following: 45 cm length; 28 cm width and 5.5 cm dept. Plant density was 833 plants m−2. Seedling trays were placed on a simple wooden/bamboo frame covered with a 70% shading net (Figure 1c) to reduce sunlight intensity while preserving air circulation from the open sides. For five days after sowing, irrigation was carried out only with clear water three times per day (at 7 a.m., 11 a.m., and 3 p.m.). When 80% of seedlings presented fully expanded cotyledons, the germination process was considered completed, and treatments started. Plant fertilization was managed to supply 1 L per tray of nutrient solution twice a day (7 a.m. and 3 p.m.) while only water was supplied once a day at 11 a.m. using a 1-L watering can.
2.3. Treatments and Experimental Design
Experiment 1: A total of four treatments were considered, obtained by the factorial combination of two substrates (soil based substrate, SBS, and Hulls Manure mix, HM-mix) and two nutrient solution concentrations (water, W0.1, and water enriched with fertilizer, NS1.2).
SBS (soil based substrate) refers to the local common substrate generally used to grow vegetables seedlings, composed by 70% of the local soil, 20% of burned rice husk, and 10% of fresh cattle manure. In this case, the rice husks are burned inside an iron tank up to turning husks into ashes. HM-mix (Hulls Manure mix) refers to a substrate formerly suggested for seedling production in the tropics [20], composed by 50% of carbonized rice husk and 50% of well sifted mature cattle manure. Physical and chemical substrates characterization was performed at the laboratory of the Yezin Agriculture University of Naypyidaw (Myanmar). Electrical conductivity (EC) and pH were determined using the conductivity meter DS-51 and pH meter F-51 (HORIBA, Kyoto, Japan). Organic matter (OM), cation exchange capacity (CEC), and water holding capacity (WHC) were determined using the Tyurin’s method, leaching method, and Keen–Razcowski measurement method, respectively [21,22,23]. Available N, P, and K were also analyzed using the Alkaline permanganate method, Olsen’s P-Malachite method, and ammonium acetate extraction [24,25].
During seedling preparation, local farmers commonly irrigate plants with harvested rainwater (W0.1), featuring EC of 0.13 dS m−1. Alternatively, a simplified nutrient solution (NS1.2, featuring EC = 1.2 dS m−1) prepared using a compound fertilizer (N: 15%, P: 15%, K: 15%, S: 2%, and CaO: 4.6%) at a concentration of 0.8 g L−1 was tested.
The experimental design was a completely randomized block design with four treatments and three replicates of each essay (Figure 2).

Figure 2.
Graphical representation of the experimental design used in the three experiments.
Experiment 2 and 3: Four treatments were applied, obtained by using different mineral fertilizer concentrations, namely: W0.1 (0 g L−1 of fertilizer, EC = 0.13 dS m−1), NS0.6 (0.4 g L−1, EC = 0.6 dS m−1), NS1.2 (0.8 g L−1, EC = 1.2 dS m−1), and NS1.8 (1.2 g L−1, EC = 1.8 dS m−1). The cost for preparing 1000 L of nutrient solution was of about 336 Kyats (0.25 USD) for NS0.6, 672 Kyats (0.50 USD) for NS1.2, and 1008 Kyats (0.75 USD) for NS1.8. The same HM-mix adopted for experiment 1 (50% of rice husk and 50% of mature cattle manure) was used as growing media.
The experimental design was a completely randomized block design with four treatments and three replicates of each essay (Figure 2).
Rice Husk Carbonization Process
The HM-mix is composed by cattle manure enriched with carbonized rice husk. The carbonization of the rice husk is performed by using a metal chimney (height of 1.5 m and diameter of 0.15 m, Figure 3a,b) that features a square perforated metal burner (28 cm height) at its base, where combustion takes place. The carbonization process starts with lighting a wood fire, which is quickly covered with the chimney. At this point, the rice husk is poured directly over the burner, creating a cone in contact with the chimney itself (Figure 3b) and undergoing a pyrolysis process. The carbonization process continued for around 3 h, during which rice husk was turned several times to ensure homogeneous carbonization. When full carbonization was reached, the chimney was removed and water was poured over the carbonized rice husk to stop the combustion (Figure 3c). The obtained carbonized rice husk (biochar) was then ready for use (Figure 3d).

Figure 3.
Phases of rice husk carbonization process: fire lighting and arrangement of the chimney (a,b) used for carbonization of rice husk (b). After cooling the rice husk with water (c), the carbonized rice husk (d) is ready for use.
2.4. Measurements
At 21 days after sowing, morphological (experiment 1: width and length of all plant leaves; experiment 2 and 3: seedling fresh weight, leaf number, width, and length of all plant leaves) and physiological (experiment 1: leaf chlorophyll content and temperature; experiment 2 and 3: stomatal conductance, leaf chlorophyll content, and leaf temperature) parameters were taken. Leaf temperature was assessed using an infrared thermometer model FLUKE 61 (Fluke Corporation, Everett, WA, USA). Stomatal conductance was measured using a handheld photosynthesis measurement system model CI-340 (Camas, WA, USA). Leaf chlorophyll content was estimated using SPAD 502 (Minolta, Osaka, Japan). Morphological measurements were obtained from 6 plants per treatment per each of the 3 replicates (n = 18). Physiological measurements were made on the upper surface of the canopy on three leaves per each sampled plant, on 6 plants per treatment in each of the three replications (n = 18).
2.5. Statistical Analysis
Data from experiment 1 were analyzed using two-way ANOVA (substrate × nutrient solution), while data from experiment 2 and 3 were analyzed using one-way ANOVA. Means were separated using the Tukey HSD (honestly significant difference) test [26] at p ≤ 0.05. Before the analysis, all data were checked for normality and homogeneity of the variance. Averages and standard errors (SE) were calculated. Statistical analysis was carried out using R statistical software (version 3.3.2).
3. Results
3.1. Climate during the Experiments
The first experiment was carried out from 9 January to 29 January 2020. During cultivation, maximum air temperatures ranged between 28.5 and 34.0 °C, with an average of 32.3 °C. Minimum temperatures ranged between 14.4 and 19.4 °C, with an average of 16.4 °C. The average maximum relative humidity (RH) was 73%, and the minimum average RH was 50%. No rainfall occurred during the experiment.
The second and third experiments were carried out from 27 February to 19 March 2020. Maximum air temperatures ranged between 30.7 and 38.6 °C, with an average of 35.8 °C. Minimum temperatures ranged between 16.0 and 23.0 °C, with an average of 21.0 °C. The average maximum relative humidity (RH) was 59%, and the minimum average RH was 30%. No rainfall occurred during the experiment.
3.2. Experiment 1
3.2.1. Substrate
Results of physical and chemical substrates characterization are reported in Table 1.

Table 1.
Physical and chemical substrates characterization.
Both substrates have a subacid pH and a quite high EC, comparable to a moderately saline soil. Among the macronutrient analyzed, the available nitrogen content was higher in SBS than in the HM-mix. Contrarily, the HM-mix had higher phosphorous and potassium, organic matter content, and water holding capacity. The observed higher cation exchange capacity (CEC) of the HM-mixed substrate is also an indicator of a larger nutrient reserve available for the plant. Moreover, during irrigation/fertilization, water stagnation, and runoff on the surface trays with the SBS substrate occurred, highlighting its low permeability, low infiltration capacity, and poor drainage compared to the HM-mixed substrate.
3.2.2. Seedling Growth
For all parameters analyzed, no-significant interactions (p = 0.26 for leaf length; p = 0.20 for leaf width; p = 0.45 for leaf temperature; and p = 0.77 for leaf chlorophyll content) between the substrate and nutrient solution were observed, therefore the effect of the two factors was discussed separately. The adopted substrate only affected leaf length and width (Table 2), whereas the nutrient solution affected all parameters analyzed (Table 3). As compared with SBS, HM-mix use resulted in increased leaf length and width by 14% and 17%, respectively (Table 2). Leaf length and width and leaf chlorophyll content were the highest in seedlings grown with nutrient solution NS1.2 (18.8 SPAD value), with a 152%, 17%, and 39% increase from seedling watered with clear water (W0.1), respectively (Table 3). On the other hand, the highest leaf temperature was observed in seedling watered with W0.1 (26.2 °C).

Table 2.
Experiment 1. Effect of substrate (SBS (soil based substrate) = substrate composed of 70% soil, 20% burned rice husk, and 10% fresh cattle manure; HM-mix (Hulls Manure mix) = substrate composed of 50% mature cattle manure and 50% carbonized rice husk) on lettuce seedlings leaf width and length. Significance codes: **, significant differences at p ≤ 0.01.

Table 3.
Experiment 1. Effect of nutrient solution (W0.1: 0 g L−1 of fertilizer, EC = 0.13 dS m−1; NS1.2: 0.8 g L−1 of fertilizer, EC = 1.2 dS m−1) on morphological and physiological parameters of lettuce seedlings. Significance codes: *, significant differences at p ≤ 0.05; ***, significant differences at p ≤ 0.001.
3.3. Experiment 2
Seedling Growth
Productive and morphological parameters: Seedling weight was affected by nutrient solution, reaching the highest value in seedlings grown in NS1.2 (Figure 4a). The nutrient solution also influenced the number of leaves (Table 4), although significant differences were detected only between W0.1 and the other nutrient solution treatments. Leaf length was the highest in NS1.2 and NS1.8 treatments and decreased when the nutrient solution’s mineral fertilizer concentration was reduced (Table 4). Finally, leaf width was not affected by nutrient solution treatments (Table 4).
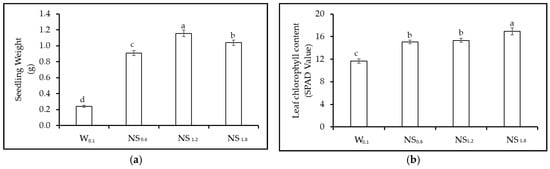
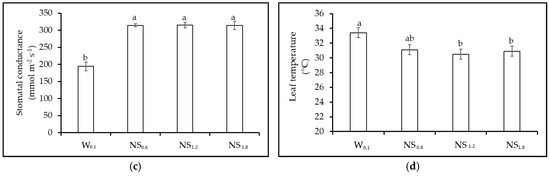
Figure 4.
Experiment 2. Effect of different nutrient solution concentration (W0.1 = 0 g L−1 of fertilizer, EC = 0.13 dS m−1; NS0.6 = 0.4 g L−1 of fertilizer, EC = 0.60 dS m−1; NS1.2 = 0.8 g L−1 of fertilizer, EC = 1.2 dS m−1, and NS1.8 = 1.2 g L−1 of fertilizer, EC = 1.8 dS m−1) on morphological and physiological parameters of lettuce. (a) Seedling fresh weight (g plant−1); (b) leaf chlorophyll content; (c) leaf stomatal conductance (mmol m−2 s−1); and (d) leaf temperature (°C). NS = Nutrient Solution. Vertical bars indicate SE; different letters indicate significant differences with the Tukey HSD test at p ≤ 0.05.

Table 4.
Experiment 2. Effect of different nutrient solution concentration (W0.1 = 0 g L−1 of fertilizer, EC = 0.13 dS m−1; NS0.6 = 0.4 g L−1 of fertilizer, EC = 0.60 dS m−1; NS1.2 = 0.8 g L−1 of fertilizer, EC = 1.2 dS m−1, and NS1.8 = 1.2 g L−1 of fertilizer, EC = 1.8 dS m−1) on morphological parameters of lettuce. Significance codes: ***, significant differences at p ≤ 0.001, “ns” = not significant. Different letters indicate significant differences with the Tukey HSD test at p ≤ 0.05.
Physiological parameter: Leaf chlorophyll content was the highest in NS1.8 and diminished with decreasing nutrient solution concentrations (Figure 4b). No significant difference was observed between NS1.2 and NS0.6, while the lowest value was obtained in W0.1 treatment (Figure 4b). Nutrient solution treatments affected stomatal conductance, which resulted to be the lowest in seedlings grown in W0.1, while no significant differences were detected among the other nutrient solution treatments (average value of 315 mmol m−2 s−1) (Figure 4c). Leaf temperature was also affected by the nutrient solution concentration, with the highest values in W0.1 (33.4 °C) and NS0.6 (31.1 °C), although, in this second case, without statistically significant differences as compared to NS1.2 and NS1.8 (Figure 4d).
3.4. Experiment 3
Seedling Growth
Productive and morphological parameters: When NS1.8 was supplied, Chinese cabbage seedlings weight was the highest, and progressively decreased with the reduction of nutrient solution concentration (Figure 5a). Additionally, leaf morphological parameters (leaf number, width, and length) were affected by nutrient solution concentration, showing higher values in plants grown with NS1.8 and NS1.2 (Table 5).
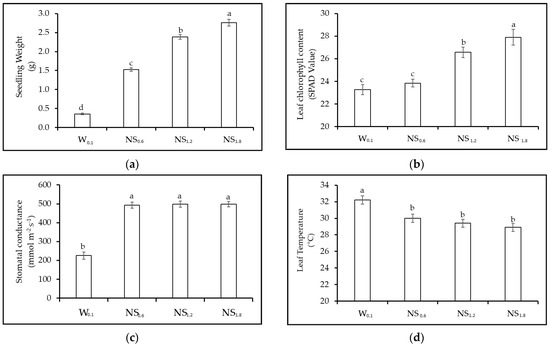
Figure 5.
Experiment 3. Effect of different nutrient solution concentration (W0.1 = 0 g L−1 of fertilizer, EC = 0.13 dS m−1; NS0.6 = 0.4 g L−1 of fertilizer, EC = 0.6 dS m−1; NS1.2 = 0.8 g L−1 of fertilizer, EC = 1.2 dS m−1, and NS1.8 = 1.2 g L−1 of fertilizer, EC = 1.8 dS m−1) on Chinese cabbage seedling. (a) Seedling fresh weight (g plant−1); (b) leaf chlorophyll content; (c) leaf stomatal conductance (mmol m−2 s−1); and (d) leaf temperature (°C). NS = Nutrient solution; Vertical bars indicate SE; different letters indicate significant differences with the Tukey HSD test at p ≤ 0.05.

Table 5.
Experiment 3. Effect of different nutrient solution concentration (W0.1 = 0 g L−1 of fertilizer, EC = 0.13 dS m−1; NS0.6 = 0.4 g L−1 of fertilizer, EC = 0.60 dS m−1; NS1.2 = 0.8 g L−1 of fertilizer, EC = 1.2 dS m−1, and NS1.8 = 1.2 g L−1 of fertilizer, EC = 1.8 dS m−1) on morphological parameters of Chinese cabbage. NS = Nutrient Solution. Significance codes: ***, significant at p ≤ 0.001. Different letters indicate significant differences with Tukey HSD test at p ≤ 0.05.
Physiological parameters: Concerning leaf chlorophyll content, the highest values were found in NS1.8, while the lowest values were observed in W0.1 and NS0.6 (Figure 5b). The response in both stomatal conductance and leaf temperature was consistent with lettuce’s observations, with lowest values in seedlings grown in W0.1 and no statistically significant differences among other nutrient solution treatments (average value of 481 mmol m−2 s−1) (Figure 5c). Leaf temperature was the greatest in W0.1, while no statistically significant differences were detected among the other treatments (Figure 5d).
4. Discussion
Adopting different substrates and nutrient solutions significantly affected growth (fresh weight and leaf morphology) and physiological parameters (stomatal conductance, leaf temperature, and chlorophyll content values) of lettuce and Chinese cabbage seedlings grown in CDZ-Myanmar. Seedlings’ quality affects their growth and yield after transplanting, given that good-quality seedlings exhibit morphological characteristics such as thick stems, thick leaves, dark green leaves, and large white roots [27]. In the first experiment, lettuce seedlings with the longest leaf length and width were associated with the HM-mix substrate and NS1.2 nutrient solution treatments. Physiological parameters (leaf temperature and chlorophyll content values) were affected only by the nutrient solution (Table 3). The combination of HM-mix and NS1.2 allowed obtaining the most appropriate seedlings for transplanting. Although both substrates have similar EC and pH, the former quite high while the latter adequate for lettuce growth, the obtained results differ probably due to other HM-mix substrate’s intrinsic physical and chemical features. According to the substrate analysis, SBS substrate showed larger nitrogen content as compared to the HM-mix substrate, probably resulted by the inclusion of fresh cattle manure [28] that negatively affected seedling growth. In a previous study by Sapkota et al. [29], elevated nitrogen concentration in the substrate depressed root metabolism leading to shorter roots. The high content of carbonized rice husks (biochar) in the HM-mixed substrate may be responsible of its good agronomic performances, given that rice husk features elevate the content of silicon and potassium [30]. The carbonization process is needed to avoid that rice husk turns to ash, keeping the original shape and ensuring several benefits associated with its capacity for decreasing bulk density, enhancing water holding capacity, adding organic carbon, increasing available nutrients, and ultimately increasing crop yields [31,32]. Accordingly, carbonized rice husk use as a growing substrate should be promoted among farmers, especially in those areas where it is readily available as in Asian countries, where rice residues are estimated at 560 million tons for rice straw and 112 million tons for rice husks [30]. Therefore, the promotion of this biochar production should also be stimulated to increase vegetable local quality seedling production.
The low permeability and poor drainage showed by SBS substrate during the irrigation/fertilization time, probably determined continuous waterlogged conditions reducing oxygen in the substrate and seedling growth [33,34]. Main constraints generated by water stagnation in agriculture include detrimental effects on both soil/substrate chemical and physical properties when anaerobic conditions persist repetitively [34]. Furthermore, the application of adequate nutrient solution assures higher and prompt nutrient availability to the seedlings, and the root growing media may affect plants’ response to salinity [35,36]. According to the results obtained in the first experiment, the combination of HM-mixed substrate and NS1.2 nutrient solution proved to be a valid option to produce quality lettuce seedling in CDZ-Myanmar.
Within second and third experiments, the definition of the optimal nutrient solution concentration was targeted. Albeit limited literature has addressed to date the definition of optimal nutrient solution strength on lettuce and Chinese cabbage nursery production in tropical areas, comparison can be made against existing literature on commercial hydroponic production. Accordingly, it was observed that whenever nutrient solution salinity levels are elevated (above 2.0 dS m−1), lettuce fresh yield and plant growth might be reduced [35]. Although the adopted substrate featured high electrical conductivity (EC) (Table 1), the present study points out that the optimal nutrient solution EC for lettuce seedlings production was 1.2 dS m−1 (NS1.2), while for the cultivation of Chinese cabbage seedlings ranged between 1.2 and 1.8 dS m−1 (NS1.2; NS1.8) (Table 4 and Table 5). The effect of the initial high EC of the substrate on production was probably reduced by washing practices of substrates performed in the first five days by watering trays with only clear water. Bustamante et al. [37], in an experiment on the effect of washing treatments on different composts used as nursery growing media for seedling pepper production, showed that EC clearly decreased (up to 40.7%) in all growing substrates subjected to washing treatments. From an economic point of view, maximum recommendable fertilizer addition would be in the range of 0.8 g of fertilizer L−1. Considering the cost for 1000 L nutrient solution preparation, it is possible to estimate a 33% economic saving that the farmer could obtain in preparing NS1.2 as compared to NS1.8 without compromising crop growth in lettuce. It is also clear that the use of clear water or nutrient solutions with low electrical conductivity for seedling production, such as in the case of W0.1 and NS0.6 treatments, does not guarantee the achievement of satisfactory seedlings size, resulting in lower leaf dimensions probably due to limited nutrients availability (Table 4 and Table 5). For instance, in lettuce, leaf size was formerly affected by nutrient solution composition and water availability [29,38,39]. Moreover, seedlings grown under W0.1 and NS0.6 showed lower values of leaf chlorophyll content as compared to NS1.2 and NS1.8. According to Trani et al. [19], lettuce seedlings, at the time of transplanting, should have intense green leaves of approximately 5 cm in length, a condition that was not met, in the hereby presented study, in W0.1. It emerges how obtaining lettuce seedlings of elevated quality is highly dependent on whether their nutritional requirements are met [40]. Besides, W0.1 treatment, both in lettuce and Chinese cabbage, showed the lowest values for stomatal conductance, the highest foliar temperature, and the lowest chlorophyll content values compared to the other treatments (Figure 4). It is evident that seedlings under W0.1 treatment had to deal with abiotic stressors, most probably nutritional deficits. Plants’ stomatal conductance is closely related to plants’ nutritional status, with a confirmed linear relationship with leaf nitrogen concentration for broad categories of vegetables [41]. The application of clear water (W0.1) clearly did not meet the nutritional requirements of the seedlings, and fertigation yet at the nursery stage would be recommended to obtain seedlings suitable for transplant and marketing. On the other hand, it resulted in being also important not to provide too elevate fertilizer concentration, which would ultimately result in limited improvements of plant growth. Indeed, significant decreases in total, marketable yields and leaf transpiration in different lettuce cultivars were observed by Orsini et al., when salinity stress associated with excessive minerals was experienced [42]. Accordingly, lettuce has been considered a moderately salt-sensitive crop with a threshold electrical conductivity (EC) of 1.3 dS m−1 and a negative slope of 13.0 for each unit added salinity above this threshold value [43,44]. Finally, the three experiments highlighted the importance of using the natural resource available accurately, considering the impact of some technical choices also on climate change. The HM-mix substrate used in the three experiments has proven to be a suitable local substrate for seedling production. Considering the growth potential of the horticultural sector in Myanmar to contribute to food security [8], the adoption of such a substrate will have a lower impact on climate change by limiting the use of substrates commonly used for seedling production, such as peat. Peat comes from peatland ecosystems, which are very important for carbon sequestration. Hence, when peat is used as a substrate, the stored carbon is released, negatively affecting the environment and CO2 balance [45]. The same is also true for the use of mineral fertilizers. Indeed, N2O is considered to be highly damaging to the ozone layer, and about 80% of anthropogenic N2O emissions are attributed to agriculture [46,47,48,49]. The second and third experiments provide indications on how to obtain good quality seedlings without exceeding in the use of mineral fertilizer, which, besides determining an economic loss for the growers, negatively impacts the environment.
5. Conclusions
The three experiments highlight the importance of adopting accurate substrates and nutrient solution concentration to improve vegetable seedlings production in semi-arid areas such as the Central Dry Zone of Myanmar. The study confirmed that proper substrate selection associated with a proper nutrient solution management might increase seedling quality and improve physiological parameters of lettuce and Chinese cabbage seedling production. Under limited resources, the substrate named HM-mixed and nutrient solution NS1.2 allowed for obtaining high quality seedlings. An interdisciplinary approach and appropriate dissemination and knowledge transfer will be essential to guarantee that the seedling management methodology and technology for nursery purposes will be put in place adequately by local institutions and farmers.
Author Contributions
Conceptualization, N.M. and G.G.; methodology, N.M.; validation, N.M., G.G., G.P., F.O.; formal analysis, N.M., G.P., G.G.; investigation, N.M., N.O.M.; resources, F.O., G.P.; data curation, N.M., G.P.; writing—original draft preparation, N.M.; writing—review and editing, G.P., G.G., F.O.; visualization, N.M., G.P., G.G.; supervision, F.O., G.G.; project administration, N.M.; funding acquisition, F.O., G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Nang Hseng Hom, Rector of Yezin agriculture University of Myanmar for providing the area, infrastructure and equipment to carry out the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bezerra, F.C. Produção de Mudas de Hortaliças em Ambiente Protegido. Embrapa Agroindústria Tropical-Documentos; Infoteca-e; Embrapa: Brasília, Brazil, 2003; Volume 21. [Google Scholar]
- Laborde, D.; Martin, W.; Swinnen, J.; Vos, R. COVID-19 risks to global food security. Science 2020, 369, 500–502. [Google Scholar] [CrossRef]
- Vittuari, M.; Bazzocchi, G.; Blasioli, S.; Cirone, F.; Maggio, A.; Orsini, F.; Penca, J.; Petruzzelli, M.; Specht, K.; Amghar, S.; et al. Envisioning the future of European food systems: Approaches and research priorities after COVID-19. Front. Sustain. Food Syst. 2021, 5, 58, in press. [Google Scholar]
- Burton, P.; Lyons, K.; Richards, C.; Amati, M.; Rose, N.; Des Fours, L.; Pires, V.; Barclay, R. Urban Food Security, Urban Resilience and Climate Chang; National Climate Change Adaptation Research Facility: Southport, Australia, 2013; p. 160. [Google Scholar] [CrossRef]
- Soilless Horticulture and Other Water-Saving Innovative Technologies for Landless and Marginal Farmers, Baseline Survey; Report; Terre des Hommes Italy: Milan, Italy, 2015.
- Gianquinto, G.; Orsini, F. Feasibility Study for the Improvement of Food Security in Central Dry Zone, Myanmar; Report; Terre des Hommes Italy: Milan, Italy, 2007. [Google Scholar]
- McCartney, M.P.; Pavelic, P.; Lacombe, G.; Latt, K.; Zan, A.K.; Thein, K.; Cho, C. Water Resources Assessment of the Dry Zone of Myanmar: Final Report for Component; Project Report of the Livelihoods and Food Security Trust Fund (LIFT), Dry Zone Program. 2013. No. 615-2016-40947. Available online: https://www.researchgate.net/publication/266261203_Water_resource_assessment_of_the_dry_zone_of_Myanmar (accessed on 15 October 2020).
- Ministry of Agriculture, Livestock and Irrigation the Republic of the Union of Myanmar. Data Collection Survey for Food Value Chain Development Assistance in the Republic of the Union of Myanmar. Final Report. 2018. Available online: https://openjicareport.jica.go.jp/pdf/12321998.pdf (accessed on 15 October 2020).
- Livelihood and Food Security Trust Fund (LIFT). Available online: http://www.iwmi.cgiar.org/Publications/Other/Reports/PDF/improving-water-management-in-myanmars-dry-zone-for-food-security-livelihoods-and-health.pdf (accessed on 15 October 2020).
- Prasad, K.N.; Shivamurthy, G.R.; Aradhya, S.M. Ipomoea aquatica, an underutilized green leafy vegetable: A review. Int. J. Bot. 2008, 4, 123–129. [Google Scholar]
- Nicolle, C.; Cardinault, N.; Gueux, E.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amouroux, P.; Rémésy, C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004, 23, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.M.; Khachik, F. Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. J. Agric. Food Chem. 2003, 51, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Cashin, J. Undernutrition in Myanmar; Part 1: A Critical Review of Literature. LIFT. Leveraging Essential Nutrition Actions to Reduce Malnutrition (LEARN) Programme. 2016. Available online: https://www.lift-fund.org/sites/lift-fund.org/files/uploads/LEARN%20Report%20Part%201.compressed.pdf (accessed on 15 October 2020).
- Fillion, L.; Henry, C.J.K. Nutrient losses and gains during frying: A review. Int. J. Food Sci. Nutr. 1998, 49, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Ceglie, F.G.; Bustamante, M.A.; Amara, M.B.; Tittarelli, F. The challenge of peat substitution in organic seedling production: Optimization of growing media formulation through mixture design and response surface analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef]
- Everaarts, A.P.; de Putter, H. Opportunities and constraints for improved vegetable production technology in tropical Asia. Acta Hortic. 2008, 809, 55–68. [Google Scholar] [CrossRef]
- Oliveira, R.P.; Scivittaro, W.B.; Vasconcellos, L.A.B.C. Avaliação de mudas de maracujazeiro em função do substrato e do tipo de bandejas. Sci. Agric. 1993, 50, 261–266. [Google Scholar] [CrossRef][Green Version]
- Cañizares, K.A.; Costa, P.C.; Goto, R.; Vieira, A.R.M. Desenvolvimento de mudas de pepino em diferentes substratos com e sem uso de solução nutritiva. Hortic. Bras. 2002, 20, 227–229. [Google Scholar] [CrossRef]
- Trani, P.E.; Novo, M.D.C.S.; Cavallaro Júnior, M.L.; Telles, L.M. Production of lettuce seedlings in different trays and commercial substrates. Hortic. Bras. 2004, 22, 290–294. [Google Scholar] [CrossRef]
- Fecondini, M.; Casati, M.; Dimech, M.; Michelon, N.; Orsini, F.; Gianquinto, G. Improved cultivation of lettuce with a low cost soilless system in indigent areas of northeast Brazil. Acta Hortic. 2008, 807, 501–508. [Google Scholar] [CrossRef]
- Seo, M.C.; So, K.H.; Ko, B.G.; Son, Y.K. Comparison of Tyurin Method and Dry Combustion Method for Carbon Analysis in Soils of Low Iorganic Carbon content. Korean J. Soil Sci. Fert. 2004, 37, 315–321. [Google Scholar]
- Gillman, G.P.; Bruce, R.C.; Davey, B.G.; Kimble, J.M.; Searle, P.L.; Skjemstad, J.O. A comparison of methods used for determination of cation exchange capacity. Commun. Soil Sci. Plant Anal. 1983, 14, 1005–1014. [Google Scholar] [CrossRef]
- Gupta, R.D.; Arora, S.; Gupta, G.D.; Sumberia, N.M. Soil physical variability in relation to soil erodibility under different land uses in foothills of Siwaliks in NW India. Trop. Ecol. 2010, 51, 183. [Google Scholar]
- Moe, K.; Mg, K.W.; Win, K.K.; Yamakawa, T. Combined effect of organic manures and inorganic fertilizers on the growth and yield of hybrid rice (Palethwe-1). Am. J. Plant Sci. 2017, 8, 1022–1042. [Google Scholar] [CrossRef]
- Beegle, D.B.; Oravec, T.C. Comparison of field calibrations for Mehlich 3 P and K with Bray-Kurtz P1 and ammonium acetate K for corn. Commun. Soil Sci. Plant Anal. 1990, 21, 1025–1036. [Google Scholar] [CrossRef]
- Acutis, M.; Scaglia, B.; Confalonieri, R. Perfunctory analysis of variance in agronomy, and its consequences in experimental results interpretation. Eur. J. Agron. 2012, 43, 129–135. [Google Scholar] [CrossRef]
- Oda, M. Raising of vigorous and valuable seedlings. Regul. Plant Grow. Dev. 2007, 42, 176–182. [Google Scholar] [CrossRef]
- Muhereza, I.; Pritchard, D.; Murray-Prior, R.; Collins, D. Nitrogen value of stockpiled cattle manure for crop production. Afr. J. Agric. Res. 2020, 16, 574–584. [Google Scholar] [CrossRef]
- Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Varela Milla, O.; Rivera, E.B.; Huang, W.J.; Chien, C.; Wang, Y.M. Agronomic properties and characterization of rice husk and wood biochars and their effect on the growth of water spinach in a field test. J. Soil Sci. Plant Nutr. 2013, 13, 251–266. [Google Scholar] [CrossRef]
- Williams, N.A.; Morse, N.D.; Buckman, J.F. Burning vs. incorporation of rice crop residues. Agron. J. 1972, 64, 467–468. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. J. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Parent, C.; Capelli, N.; Berger, A.; Crèvecoeur, M.; Dat, J.F. An overview of plant responses to soil waterlogging. Plant Stress 2008, 2, 20–27. [Google Scholar]
- Patel, P.K.; Singh, A.K.; Tripathi, N.; Yadav, D.; Hemantaranjan, A. Flooding: Abiotic constraint limiting vegetable productivity. APAR 2014, 1, 96–103. [Google Scholar]
- Andriolo, J.L.; Luz, G.L.D.; Witter, M.H.; Godoi, R.D.S.; Barros, G.T.; Bortolotto, O.C. Growth and yield of lettuce plants under salinity. Hortic. Bras. 2005, 23, 931–934. [Google Scholar] [CrossRef]
- Samarakoon, U.C.; Weerasinghe, P.A.; Weerakkody, W.A.P. Effect of electrical conductivity (EC) of the nutrient solution on nutrient uptake, growth and yield of leaf lettuce (Lactuca sativa L.) in stationary culture. Trop. Agric. Res. 2006, 18, 21. [Google Scholar]
- Bustamante, M.A.; Gomis, M.P.; Pérez-Murcia, M.D.; Gangi, D.; Ceglie, F.G.; Paredes, C.; Moral, R. Use of livestock waste composts as nursery growing media: Effect of a washing pre-treatment. Sci. Hortic. 2021, 281. [Google Scholar] [CrossRef]
- Fraile-Robayo, R.D.; Álvarez-Herrera, J.G.; Reyes, M.; Johana, A.; Álvarez-Herrera, O.F.; Fraile-Robayo, A.L. Evaluation of the growth and quality of lettuce (Lactuca sativa L.) in a closed recirculating hydroponic system. Agron. Colomb. 2017, 35, 216–222. [Google Scholar] [CrossRef]
- Michelon, N.; Pennisi, G.; Myint, N.O.; Dall’Olio, G.; Batista, L.P.; Salviano, A.A.C.; Gianquinto, G. Strategies for Improved Yield and Water Use Efficiency of Lettuce (Lactuca sativa L.) through Simplified Soilless Cultivation under Semi-Arid Climate. Agronomy 2020, 10, 1379. [Google Scholar] [CrossRef]
- Soundy, P.; Cantliffe, D.J.; Hochmuth, G.J.; Stoffella, P.J. Management of nitrogen and irrigation in lettuce transplant production affects transplant root and shoot development and subsequent crop yields. HortScience 2005, 40, 607–610. [Google Scholar] [CrossRef]
- Schulze, E.D.; Kelliher, F.M.; Körner, C.; Lloyd, J.; Leuning, R. Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: A global ecology scaling exercise. Annu. Rev. Ecol. Evol. Syst. 1994, 25, 629–662. [Google Scholar] [CrossRef]
- Orsini, F.; Pennisi, G.; Mancarella, S.; Al Nayef, M.; Sanoubar, R.; Nicola, S.; Gianquinto, G. Hydroponic lettuce yields are improved under salt stress by utilizing white plastic film and exogenous applications of proline. Sci. Hortic. 2018, 233, 283–293. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production: A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Antón, A.; Torrellas, M.; Montero, J.I.; Ruijs, M.; Vermeulen, P.; Stanghellini, C. Environmental impact assessment of Dutch tomato crop production in a Venlo glasshouse. Acta Hortic. 2012, 927, 781–791. [Google Scholar] [CrossRef]
- Rashti, M.R.; Wang, W.; Moody, P.; Chen, C.; Ghadiri, H. Fertiliser-induced nitrous oxide emissions from vegetable production in the world and the regulating factors: A review. Atmos. Environ. 2015, 112, 225–233. [Google Scholar] [CrossRef]
- Hénault, C.; Grossel, A.; Mary, B.; Roussel, M.; Léonard, J. Nitrous oxide emission by agricultural soils: A review of spatial and temporal variability for mitigation. Pedosphere 2021, 22, 426–433. [Google Scholar] [CrossRef]
- Liu, S.; Lin, F.; Wu, S.; Ji, C.; Sun, Y.; Jin, Y.; Zou, J. A meta-analysis of fertilizer-induced soil NO and combined NO + N2O emissions. Glob. Chang. Biol. 2017, 23, 2520–2532. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).