Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health
Abstract
1. Introduction
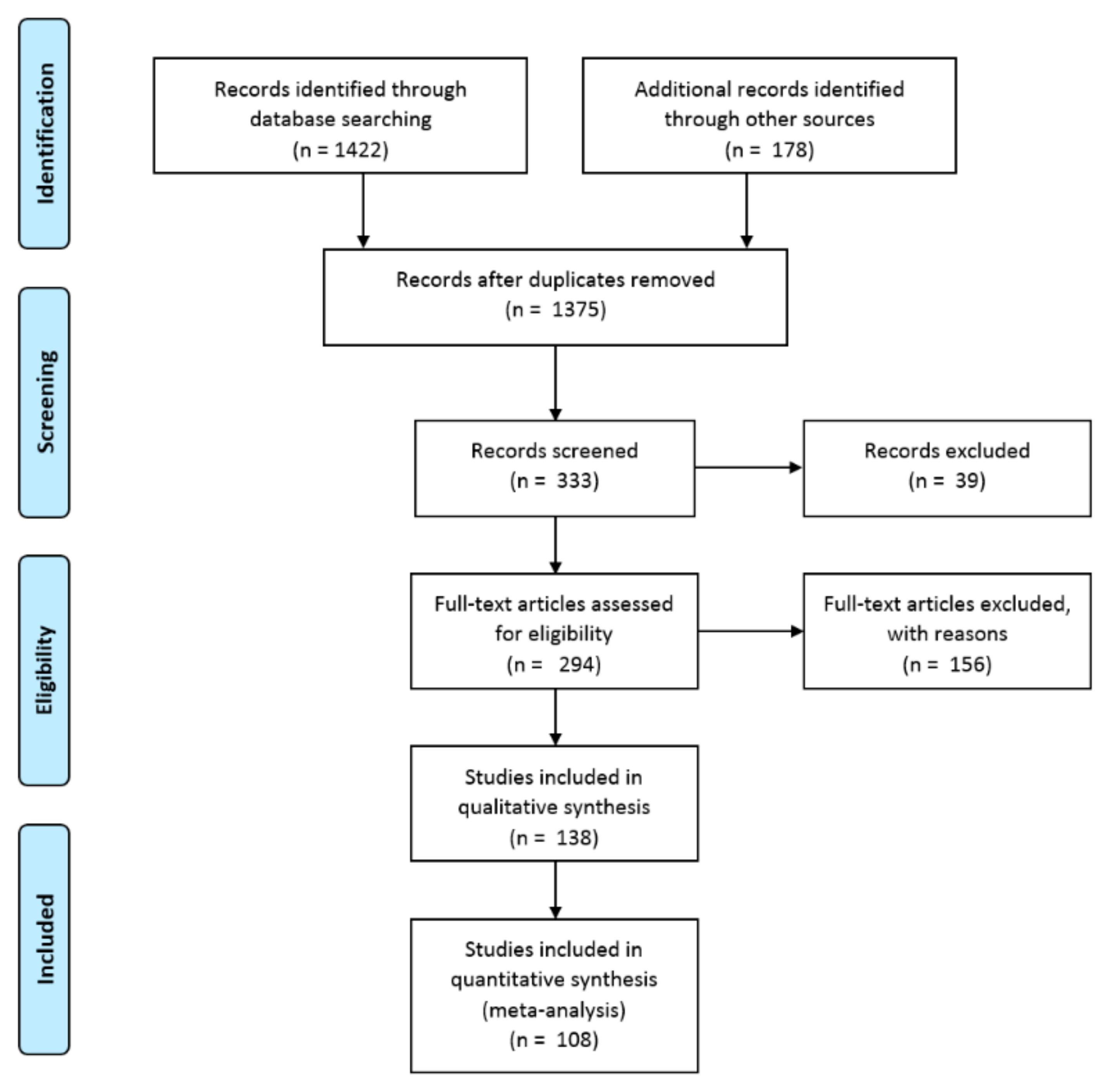
2. Methods
2.1. Data Sources
2.2. Inclusion and Exclusion of Data and Data Quality
3. Roselle Overview
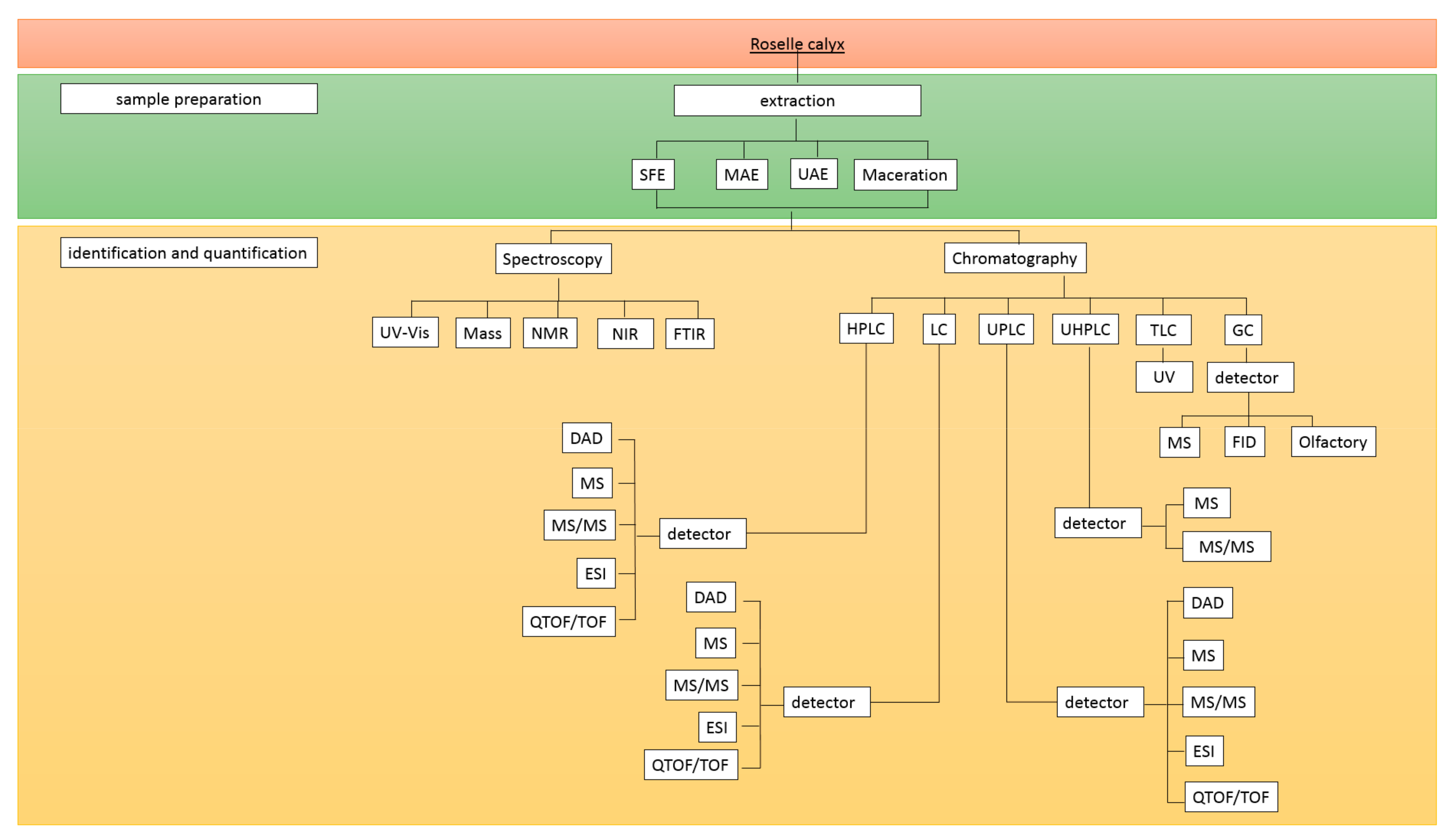
4. Analytical Methods for Phenolic Compounds in Roselle
4.1. Sample Preparation
4.1.1. Maceration
4.1.2. Ultrasound-Assisted-Extraction (UAE)
4.1.3. Microwave-Assisted Extraction (MAE)
4.1.4. Supercritical Fluid Extraction (SFE)
4.1.5. Miscellaneous Extraction Techniques
4.2. Identification and Quantification of Phenolic Compounds
4.2.1. Colorimetric Assays
4.2.2. Liquid Chromatography
4.2.3. Gas Chromatography (GC)
4.2.4. Thin-Layer Chromatography (TLC)
4.2.5. Miscellaneous Determination Techniques
5. Phenolic Compounds in Roselle
5.1. Total Phenolics
5.1.1. Total Phenolic Compounds
5.1.2. Total Flavonoid Compounds
5.1.3. Total Anthocyanin Compounds
5.2. Phenolic Acids
5.3. Flavonoids
Anthocyanins
5.4. Organic Acid and Volatile Compounds
6. Functional Properties
6.1. Antioxidant Activity
6.2. Other Health Activities
6.2.1. Anti-Diabetes
6.2.2. Anti-Hyperlipidemia and Anti-Obesity
6.2.3. Antihypertension
6.2.4. Diuretic
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Aziz, Z.; Wong, S.Y.; Chong, N.J. Effects of Hibiscus sabdariffa L. on serum lipids: A systematic review and meta-analysis. J. Ethnopharmacol. 2013, 150, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Ariyabukalakorn, V.; Panthong, S.; Itharat, A. Effects and chemical contents of hydrolysis modification of aqueous roselle extract to reflect the antioxidant and anti-inflammatory effects. Sci. Technol. Asia 2019, 24, 115–125. [Google Scholar] [CrossRef]
- Zihad, S.M.N.K.; Gupt, Y.; Uddin, S.J.; Islam, M.T.; Alam, M.R.; Aziz, S.; Hossain, M.; Shilpi, J.A.; Nahar, L.; Sarker, S.D. Nutritional value, micronutrient and antioxidant capacity of some green leafy vegetables commonly used by southern coastal people of Bangladesh. Heliyon 2019, 5, e02768. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Rui, L.; de Mejia, E.G. Hibiscus sabdariffa L.: Phytochemical Composition and Nutraceutical Properties. ACS Symp. Ser. 2012, 1109, 279–305. [Google Scholar]
- Pham, T.N.; Phu Nguyen, T.N.; Duc, L.T.; Nguyen, M.T.; Toan, T.Q.; Hong Nhan, L.T.; N-Vo, D.V.; Vo, T.S.; Bui, L.M. Response surface modeling and optimizing conditions for anthocyanins extraction from Hibiscussabdariffa L. (Roselle) grown in Lam Dong, Vietnam. IOP Conf. Ser. Mater. Sci. Eng. 2019, 544. [Google Scholar] [CrossRef]
- Villani, T.; Juliani, H.R.; Simon, J.E.; Wu, Q. Hibiscus sabdariffa: Phytochemistry, Quality Control, and Health Properties. ACS Symp. Ser. 2013, 1127, 209–230. [Google Scholar]
- Cassol, L.; Rodrigues, E.; Zapata Noreña, C.P. Extracting phenolic compounds from Hibiscus sabdariffa L. calyx using microwave assisted extraction. Ind. Crops Prod. 2019, 133, 168–177. [Google Scholar] [CrossRef]
- Paraíso, C.M.; dos Santos, S.S.; Correa, V.G.; Magon, T.; Peralta, R.M.; Visentainer, J.V.; Madrona, G.S. Ultrasound assisted extraction of hibiscus (Hibiscus sabdariffa L.) bioactive compounds for application as potential functional ingredient. J. Food Sci. Technol. 2019, 56, 4667–4677. [Google Scholar] [CrossRef]
- Pozos, G.I.P.; Ruiz-López, M.A.; Nátera, J.F.Z.; Moya, C.Á.; Ramírez, L.B.; Silva, M.R.; Macías, R.R.; García-López, P.M.; Cruz, R.G.; Pérez, E.S.; et al. Antioxidant capacity and antigenotoxic effect of Hibiscus sabdariffa L. extracts obtained with ultrasound-assisted extraction process. Appl. Sci. 2020, 10, 560. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H. Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: Kinetic modelling and process intensification. Ind. Crops Prod. 2019, 137, 528–535. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Supercritical CO2 extraction of bioactive compounds from Hibiscus sabdariffa. J. Supercrit. Fluids 2019, 147, 213–221. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. On obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef]
- Guardiola, S.; Mach, N. Therapeutic potential of Hibiscus sabdariffa: A review of the scientific evidence. Endocrinol. Nutr. 2014, 61, 274–295. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Vega, J.A.; Arteaga-Badillo, D.A.; Sánchez-Gutiérrez, M.; Morales-González, J.A.; Vargas-Mendoza, N.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Delgado-Olivares, L.; Madrigal-Bujaidar, E.; Madrigal-Santillán, E. Organic acids from Roselle (Hibiscus sabdariffa L.)-A brief review of its pharmacological effects. Biomedicines 2020, 8, 100. [Google Scholar] [CrossRef]
- Bule, M.; Albelbeisi, A.H.; Nikfar, S.; Amini, M.; Abdollahi, M. The antidiabetic and antilipidemic effects of Hibiscus sabdariffa: A systematic review and meta-analysis of randomized clinical trials. Food Res. Int. 2020, 130, 108980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yue, R.; Wang, Y.; Wang, L.; Chin, J.; Huang, X.; Jiang, Y. Effect of Hibiscus sabdariffa (Roselle) supplementation in regulating blood lipids among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis. Phyther. Res. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boushehri, S.N.; Karimbeiki, R.; Ghasempour, S.; Ghalishourani, S.S.; Pourmasoumi, M.; Hadi, A.; Mbabazi, M.; Pour, Z.K.; Assarroudi, M.; Mahmoodi, M.; et al. The efficacy of sour tea (Hibiscus sabdariffa L.) on selected cardiovascular disease risk factors: A systematic review and meta-analysis of randomized clinical trials. Phyther. Res. 2020, 34, 329–339. [Google Scholar] [CrossRef]
- Serban, C.; Sahebkar, A.; Ursoniu, S.; Andrica, F.; Banach, M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Cid-Ortega, S.; Guerrero-Beltrán, J.A. Roselle calyces (Hibiscus sabdariffa), an alternative to the food and beverages industries: A review. J. Food Sci. Technol. 2015, 52, 6859–6869. [Google Scholar] [CrossRef]
- Purbowati, I.S.M.; Maksum, A. The antioxidant activity of Roselle (Hibiscus sabdariffa Linii) phenolic compounds in different variations microwave-Assisted extraction time and power. IOP Conf. Ser. Earth Environ. Sci. 2019, 406. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Nadlene, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Yusriah, L. A Review on Roselle Fiber and Its Composites. J. Nat. Fibers 2016, 13, 10–41. [Google Scholar] [CrossRef]
- Patel, S. Hibiscus sabdariffa: An ideal yet under-exploited candidate for nutraceutical applications. Biomed. Prev. Nutr. 2014, 4, 23–27. [Google Scholar] [CrossRef]
- Qi, Y.; Chin, K.L.; Malekian, F.; Berhane, M.; Gager, J. Biological Characteristics, Nutritional and Medicinal Value of Roselle, Hibiscus sabdariffa Biological Characteristics, Nutritional and Medicinal Value of Roselle, Hibiscus sabdariffa. Agric. Res. Ext. Cent. 2005, 70813, 603–604. [Google Scholar]
- Tahir, H.E.; Arslan, M.; Mahunu, G.K.; Mariod, A.A.; Wen, Z.; Xiaobo, Z.; Xiaowei, H.; Jiyong, S.; El-Seedi, H. Authentication of the geographical origin of Roselle (Hibiscus sabdariffa L) using various spectroscopies: NIR, low-field NMR and fluorescence. Food Control. 2020, 114, 107231. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Caleja, C.; Calhelha, R.C.; Soković, M.; Catarino, L.; Barros, L.; Ferreira, I.C.F.R. Exploring the chemical and bioactive properties of: Hibiscus sabdariffa L. calyces from Guinea-Bissau (West Africa). Food Funct. 2019, 10, 2234–2243. [Google Scholar] [CrossRef]
- Hinojosa-Gómez, J.; Martin-Hernández, C.S.; Heredia, J.B.; León-Félix, J.; Osuna-Enciso, T.; Muy-Rangel, M.D. Roselle (Hibiscus sabdariffa L.) cultivars calyx produced hydroponically: Physicochemical and nutritional quality. Chil. J. Agric. Res. 2018, 78, 478–485. [Google Scholar] [CrossRef]
- Ifie, I.; Ifie, B.E.; Ibitoye, D.O.; Marshall, L.J.; Williamson, G. Seasonal variation in Hibiscus sabdariffa (Roselle) calyx phytochemical profile, soluble solids and α-glucosidase inhibition. Food Chem. 2018, 261, 164–168. [Google Scholar] [CrossRef]
- Ali, S.A.M.; Zain, C.R.C.M.; Latip, J. Influence of elevated CO2 on the growth and phenolic constituents. J. Teknol. 2019, 3, 109–118. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Obanijesu, E.O.; Alara, J.A.; Abdul Mudalip, S.K. Extract-rich in flavonoids from Hibiscus sabdariffa calyces: Optimizing microwave-assisted extraction method and characterization through LC-Q-TOF-MS analysis. J. Food Process. Eng. 2019, 43. [Google Scholar] [CrossRef]
- Zhang, Y.; Sang, J.; Chen, F.F.; Sang, J.; Li, C. qin β-Cyclodextrin-assisted extraction and green chromatographic analysis of Hibiscus sabdariffa L. anthocyanins and the effects of gallic/ferulic/caffeic acids on their stability in beverages. J. Food Meas. Charact. 2018, 12, 2475–2483. [Google Scholar] [CrossRef]
- Samadi, S.; Fard, F.R. Phytochemical properties, antioxidant activity and mineral content (Fe, Zn and Cu) in Iranian produced black tea, green tea and roselle calyces. Biocatal. Agric. Biotechnol. 2020, 23, 101472. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.I.; Esoh, R.B.; Al-U’Datt, M.H.; Alrababah, M.A.; Yang, W. Antioxidant activities, total phenolics and HPLC analyses of the phenolic compounds of extracts from common Mediterranean plants. Nat. Prod. Res. 2011, 25, 596–605. [Google Scholar] [CrossRef]
- Özdogan, F.P.; Orhan, N.; Ergun, F. Studies on the conformity of Hibiscus sabdariffa L. samples from turkish market to european pharmacopeia. Fabad J. Pharm. Sci. 2011, 36, 25–32. [Google Scholar]
- Jacob, J.P.S.; Shenbagaraman, S. Evaluation of antioxidant and antimicrobial activities of the selected green leafy vegetables. Int. J. PharmTech Res. 2011, 3, 148–152. [Google Scholar]
- Salem, M.A.; Michel, H.E.; Ezzat, M.I.; Okba, M.M.; El-desoky, A.M.; Mohamed, S.O.; Ezzat, S.M. Optimization of an Extraction Solvent for Angiotensin-Converting Enzyme Inhibitors from Hibiscus sabdariffa L. Based on Its UPLC-MS/MS Metabolic Profiling. Molecules 2020, 25, 2307. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef]
- Gulsheen; Kumar, A.; Sharma, A. Antianxiety and Antidepressant Activity Guided Isolation and Characterization of Gossypetin from Hibiscus sabdariffa Linn. Calyces. J. Biol. Act. Prod. Nat. 2019, 9, 205–214. [Google Scholar] [CrossRef]
- Portillo-Torres, L.A.; Bernardino-Nicanor, A.; Gómez-Aldapa, C.A.; González-Montiel, S.; Rangel-Vargas, E.; Villagómez-Ibarra, J.R.; González-Cruz, L.; Cortés-López, H.; Castro-Rosas, J. Hibiscus acid and chromatographic fractions from Hibiscus sabdariffa calyces: Antimicrobial activity against multidrug-resistant pathogenic bacteria. Antibiotics 2019, 8, 218. [Google Scholar] [CrossRef]
- Wilson, F.D.; Menzel, M.Y. Kenaf (Hibiscus cannabinus), Roselle (Hibiscus sabdariffa). Econ. Bot. 1964, 18, 80–91. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Lamm, M.G.; Funk, J.; Ritenbaugh, C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia 2013, 85, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.; Batista, C.M.D.; Fungwe, T.V. The Effectiveness of Hibiscus sabdariffa for Improving Metabolic Syndrome: A Systematic Review. EC Nutr. 2019, 10, 887–892. [Google Scholar]
- Kafkas, N.E.; Kosar, M.; Öz, A.T.; Mitchell, A.E. Advanced Analytical Methods for Phenolics in Fruits. J. Food Qual. 2018, 2018. [Google Scholar] [CrossRef]
- Tham, T.C.; Ng, M.X.; Gan, S.H.; Chua, L.S.; Aziz, R.; Abdullah, L.C.; Ong, S.P.; Chin, N.L.; Law, C.L. Impacts of different drying strategies on drying characteristics, the retention of bio-active ingredient and colour changes of dried Roselle. Chin. J. Chem. Eng. 2018, 26, 303–316. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642221446. [Google Scholar]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Optimization of the ultrasound-assisted extraction of tryptophan and its derivatives from rice (Oryza sativa) grains through a response surface methodology. J. Cereal Sci. 2017, 75, 192–197. [Google Scholar] [CrossRef]
- Alam, G.; Sartini; Alfath, A. Comparison of microwave assisted extraction (MAE) with variations of power and infusion extraction method on antibacterial activity of rosella calyx extract (Hibiscus sabdariffa). J. Phys. Conf. Ser. 2019, 1341. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Ruiz-López, I.I. Mass transfer modeling of the antioxidant extraction of roselle flower (Hibiscus sabdariffa). J. Food Sci. Technol. 2019, 56, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodrigues, M.M.; Balaban, M.O.; Marshall, M.R.; Rouseff, R.L. Hot and Cold Water Infusion Aroma Profilesof Hibiscus sabdariffa: Fresh Compared with Dried. J. Food Sci. 2011, 76, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Aryanti, N.; Nafiunisa, A.; Wardhani, D.H. Conventional and ultrasound-assisted extraction of anthocyanin from red and purple roselle (Hibiscus sabdariffa L.) calyces and characterisation of its anthocyanin powder. Int. Food Res. J. 2019, 26, 529–535. [Google Scholar]
- Amaya-Cruz, D.M.; Perez-Ramirez, I.F.; Ortega-Diaz, D.; Rodriguez-Garcia, M.E.; Reynoso-Camacho, R. Roselle (Hibiscus sabdariffa) by-product as functional ingredient: Effect of thermal processing and particle size reduction on bioactive constituents and functional, morphological, and structural properties. J. Food Meas. Charact. 2018, 12, 135–144. [Google Scholar] [CrossRef]
- Aryanti, N.; Nafiunisa, A.; Wardhani, D.H.; Kumoro, A.C. Extraction characteristic and microencapsulation of anthocyanin as natural food colouring from roselle calyces by ultrasound-assisted extraction. J. Bahan Alam Terbarukan 2017, 6, 87–96. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Castaño-Tostado, E.; Ramírez-De León, J.A.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015, 172, 885–892. [Google Scholar] [CrossRef]
- Sindi, H.A.; Marshall, L.J.; Morgan, M.R.A. Comparative chemical and biochemical analysis of extracts of Hibiscus sabdariffa. Food Chem. 2014, 164, 23–29. [Google Scholar] [CrossRef]
- Salazar-González, C.; Vergara-Balderas, F.T.; Ortega-Regules, A.E.; -Beltrán, J.Á. Antioxidant properties and color of Hibiscus sabdariffa extracts. Cienc. Investig. Agrar. 2012, 39, 79–90. [Google Scholar] [CrossRef]
- Cissé, M.; Bohuon, P.; Sambe, F.; Kane, C.; Sakho, M.; Dornier, M. Aqueous extraction of anthocyanins from Hibiscus sabdariffa: Experimental kinetics and modeling. J. Food Eng. 2012, 109, 16–21. [Google Scholar] [CrossRef]
- Cisse, M.; Vaillant, F.; Soro, D.; Reynes, M.; Dornier, M. Crossflow microfiltration for the cold stabilization of roselle (Hibiscus sabdariffa L.) extract. J. Food Eng. 2011, 106, 20–27. [Google Scholar] [CrossRef]
- Pham, T.N.; Nguyen, T.N.P.; Lam, T.D.; Tran, T.H.; Nguyen, D.C.; Vo, D.V.N.; Le, X.T.; Do, S.T.; Bach, L.G. Effects of various solvent concentration, liquid-solid ratio, temperatures and time values on the extraction yield of anthocyanin from Vietnam Hibiscus sabdariffa L. (Roselle). IOP Conf. Ser. Mater. Sci. Eng. 2019, 542. [Google Scholar] [CrossRef]
- Bergmeier, D.; Berres, P.H.D.; Filippi, D.; Bilibio, D.; Bettiol, V.R.; Priamo, W.L. Extraction of total polyphenols from hibiscus (Hibiscus sabdariffa L.) and waxweed/‘sete-sangrias’ (Cuphea carthagenensis) and evaluation of their antioxidant potential. Acta Sci. Technol. 2014, 36, 545–551. [Google Scholar] [CrossRef]
- Duy, N.Q.; Thoai, H.; Lam, T. Effects of Different Extraction Solvent Systems on Total Phenolic, Total Flavonoid, Total Anthocyanin Contents and Antioxidant Activities of Roselle (Hibiscus sabdariffa L.) Extracts. ASIAN J. Chem. 2019, 31, 2517–2521. [Google Scholar] [CrossRef]
- Anokwuru, C.P.; Esiaba, J.; Ajibaye, O.; Adesuyi, A.O. Polyphenolic content and antioxidant activity of Hibiscus sabdariffa calyx. Res. J. Med. Plant. 2011, 5, 557–566. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Salawu, N.A.; Yakubu, M.T.; Oladiji, A.T.; Akanji, M.A.; Okogun, J.I. Antioxidant and drug detoxification potentials of Hibiscus sabdariffa anthocyanin extract. Drug Chem. Toxicol. 2011, 34, 109–115. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Pekel, A.G.; Bilgin, M.; Makris, D.P.; Şahin, S. Citric acid-based deep eutectic solvent for the anthocyanin recovery from Hibiscus sabdariffa through microwave-assisted extraction. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Vargas-León, E.A.; Díaz-Batalla, L.; González-Cruz, L.; Bernardino-Nicanor, A.; Castro-Rosas, J.; Reynoso-Camacho, R.; Gómez-Aldapa, C.A. Effects of acid hydrolysis on the free radical scavenging capacity and inhibitory activity of the angiotensin converting enzyme of phenolic compounds of two varieties of jamaica (Hibiscus sabdariffa). Ind. Crops Prod. 2018, 116, 201–208. [Google Scholar] [CrossRef]
- Aryanti, N.; Nafiunisa, A.; Bella, N.; Sanjaya, R.; Wardhani, D.H.; Kumoro, A.C. Kinetics of ultrasound-assisted extraction of anthocyanin from purple roselle calyces under different PH conditions. Chem. Chem. Technol. 2018, 12, 523–528. [Google Scholar] [CrossRef]
- Sipahli, S.; Mohanlall, V.; Mellem, J.J. Stability and degradation kinetics of crude anthocyanin extracts from H. sabdariffa. Food Sci. Technol. 2017, 37, 209–215. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Barouh, N.; Baréa, B.; Fernandes, A.; De Freitas, V.; Salas, E. Isolation and Characterization of Anthocyanins from Hibiscus sabdariffa Flowers. J. Nat. Prod. 2016, 79, 1709–1718. [Google Scholar] [CrossRef]
- Deli, M.; Ndjantou, E.B.; Ngatchic Metsagang, J.T.; Petit, J.; Njintang Yanou, N.; Scher, J. Successive grinding and sieving as a new tool to fractionate polyphenols and antioxidants of plants powders: Application to Boscia senegalensis seeds, Dichrostachys glomerata fruits, and Hibiscus sabdariffa calyx powders. Food Sci. Nutr. 2019, 7, 1795–1806. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Vanegas-Espinoza, P.E.; Escudero-Gilete, M.L.; Heredia, F.J.; Paredes-López, O.; Del Villar-Martínez, A.A. Colorimetric Analysis of Hibiscus Beverages and their Potential Antioxidant Properties. Plant. Foods Hum. Nutr. 2018, 73, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Duy, N.Q.; Pham, T.N.; Binh, M.L.T.; Thuan, M.; Van, N.T.T.; Lam, T.D.; Nhan, P.N.T. Effects of extraction conditions on antioxidant activities of roselle (Hibiscus sabdariffa L.) extracts. Mater. Sci. Forum 2020, 977, 201–206. [Google Scholar] [CrossRef]
- Duy, N.Q.; Binh, M.L.T.; Thuan, M.; Van, N.T.T.; Lam, T.D.; Tran, T.H.; Nhan, P.N.T. Effects of extraction conditions on total phenolic content and total flavonoid content of roselle (Hibiscus sabdariffa L.) extracts. Key Eng. Mater. 2019, 814, 469–474. [Google Scholar] [CrossRef]
- Maciel, L.G.; do Carmo, M.A.V.; Azevedo, L.; Daguer, H.; Molognoni, L.; de Almeida, M.M.; Granato, D.; Rosso, N.D. Hibiscus sabdariffa anthocyanins-rich extract: Chemical stability, in vitro antioxidant and antiproliferative activities. Food Chem. Toxicol. 2018, 113, 187–197. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef]
- Miranda-Medina, A.; Hayward-Jones, P.M.; Carvajal-Zarrabal, O.; de Guevara-Vela, L.d.A.L.; Ramírez-Villagómez, Y.D.; Barradas-Dermitz, D.M.; Luna-Carrillo, G.; Aguilar-Uscanga, M.G. Optimization of Hibiscus sabdariffa L. (Roselle) anthocyanin aqueous-ethanol extraction parameters using response surface methodology. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 53–62. [Google Scholar]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef]
- Lukmanto, S.; Roesdiyono, N.; Ju, Y.H.; Indraswati, N.; Soetaredjo, F.E.; Ismadji, S. Supercritical Co2 Extraction of Phenolic Compounds in Roselle(Hibiscus sabdariffa L.). Chem. Eng. Commun. 2013, 200, 1187–1196. [Google Scholar] [CrossRef][Green Version]
- Lourith, N.; Kanlayavattanakul, M. Antioxidant activity and stability of natural colour recovered from Roselle juice production. Agro Food Ind. Hi Tech. 2013, 24, 40–43. [Google Scholar]
- Jung, E.; Kim, Y.; Joo, N. Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.). J. Sci. Food Agric. 2013, 93, 3769–3776. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodrigues, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J. Food Sci. 2011, 76, C428–C435. [Google Scholar] [CrossRef]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical fluid extraction of plant flavors and fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef]
- Idham, Z.; Nasir, H.M.; Yunus, M.A.C.; Yian, L.N.; Peng, W.L.; Hassan, H.; Setapar, S.H.M. Optimisation of supercritical CO2 extraction of red colour from roselle (Hibiscus sabdariffa Linn.) calyces. Chem. Eng. Trans. 2017, 56, 871–876. [Google Scholar] [CrossRef]
- Opletal, L.; Chocholousova-Havlikova, L.; Siatka, T.; Cahliková, L.; Locarek, M.; Ali, B.H.; Manoj, P.; Ramkumar, A.; Al Suleimani, Y.M.; Al Za’Abi, M.; et al. Preparation and validated analysis of anthocyanin concentrate from the calyces of Hibiscus sabdariffa. Nat. Prod. Commun. 2017, 12, 44–45. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, D.; Bao, T.; Xie, J.; Chen, W. A simple and rapid method for the preparation of pure delphinidin-3-O-sambubioside from Roselle and its antioxidant and hypoglycemic activity. J. Funct. Foods 2017, 39, 9–17. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Figueroa-Espinoza, M.C. Lipophilization and MS characterization of the main anthocyanins purified from hibiscus flowers. Food Chem. 2017, 230, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Cisse, M.; Vaillant, F.; Kane, A.; Ndiaye, O.; Dornier, M. Impact of the extraction procedure on the kinetics of anthocyanin and colour degradation of roselle extracts during storage. J. Sci. Food Agric. 2012, 92, 1214–1221. [Google Scholar] [CrossRef]
- Trinh, L.T.P.; Choi, Y.S.; Bae, H.J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Ind. Crops Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Amlashi, H.A.; Madani, H.; Sonboli, A.; Khaghani, S.; Ramezani, M. Volatile composition of the leaves and calyces essential oil of roselle (Hibiscus sabdariffa L.) from Iran. J. Essent. Oil Bear. Plants 2020, 23, 743–755. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Kaewdoo, K.; Leeprechanon, W.; Sivamaruthi, B.S. Influence of extraction methods on total phenolic content and antioxidant properties of some of the commonly used plants in Thailand. Pakistan J. Biol. Sci. 2019, 22, 117–126. [Google Scholar] [CrossRef][Green Version]
- Ramírez-Cortés, B.; de Caro-Velarde, F.J.; Valdivia-Reynoso, M.G.; Ramírez-Lozano, M.H.; Machuca-Sánchez, L. Changes in size and chemical characteristics of roselle (Hibiscus sabdariffa L.) calyces during maturation. Rev. Chapingo Ser. Hortic. 2011, XVII, 19–31. [Google Scholar]
- Pragalyaashree, M.M.; Tiroutchelvame, D.; Sashikumar, S. Degradation kinetics of anthocyanin extracted from roselle calyces (Hibiscus sabdariffa). J. Appl. Pharm. Sci. 2018, 8, 57–63. [Google Scholar] [CrossRef]
- Inggrid, H.M.; Jaka; Santoso, H. Natural red dyes extraction on roselle petals. IOP Conf. Ser. Mater. Sci. Eng. 2016, 162. [Google Scholar] [CrossRef]
- Tavakolifar, F.; Givianrad, M.H.; Saber-Tehrani, M. Extraction of anthocyanins from Hibiscus sabdariffa and assessment of its antioxidant properties in extra virgin olive oil. Fresenius Environ. Bull. 2016, 25, 3709–3713. [Google Scholar]
- Oboh, G.; Adewuni, T.M.; Ademiluyi, A.O.; Olasehinde, T.A.; Ademosun, A.O. Phenolic Constituents and Inhibitory Effects of Hibiscus sabdariffa L. (Sorrel) Calyx on Cholinergic, Monoaminergic, and Purinergic Enzyme Activities. J. Diet. Suppl. 2018, 15, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Méndez, G.A.; Jara-Palacios, M.J.; Escudero-Gilete, M.L.; Gordillo, B.; Hernanz, D.; Paredes-López, O.; Vanegas-Espinoza, P.E.; Del Villar-Martínez, A.A.; Heredia, F.J. Comparative Study of Phenolic Profile, Antioxidant Capacity, and Color-composition Relation of Roselle Cultivars with Contrasting Pigmentation. Plant. Foods Hum. Nutr. 2016, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Sinela, A.M.; Mertz, C.; Achir, N.; Rawat, N.; Vidot, K.; Fulcrand, H.; Dornier, M. Exploration of reaction mechanisms of anthocyanin degradation in a roselle extract through kinetic studies on formulated model media. Food Chem. 2017, 235, 67–75. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef]
- Piovesana, A.; Rodrigues, E.; Noreña, C.P.Z. Composition analysis of carotenoids and phenolic compounds and antioxidant activity from hibiscus calyces (Hibiscus sabdariffa L.) by HPLC-DAD-MS/MS. Phytochem. Anal. 2019, 30, 208–217. [Google Scholar] [CrossRef]
- Juhari, N.H.; Bredie, W.L.P.; Toldam-Andersen, T.B.; Petersen, M.A. Characterization of Roselle calyx from different geographical origins. Food Res. Int. 2018, 112, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Ningrum, A.; Schreiner, M.; Luna, P.; Khoerunnisa, F.; Tienkink, E. Free volatile compounds in red and purple roselle (Hibiscus sabdariffa) pomace from Indonesia. Food Res. 2019, 3, 749–754. [Google Scholar] [CrossRef]
- Avalos-Martínez, E.; Pino, J.A.; Sáyago-Ayerdi, S.; Sosa-Moguel, O.; Cuevas-Glory, L. Assessment of volatile compounds and sensory characteristics of Mexican hibiscus (Hibiscus sabdariffa L.) calyces hot beverages. J. Food Sci. Technol. 2019, 56, 360–366. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Ragazzo-Sánchez, J.A.; Jiménez-Aparicio, A.R.; Vanegas-Espinoza, P.E.; Paredes-López, O.; Del Villar-Martínez, A.A. Comparative Study of Anthocyanin and Volatile Compounds Content of Four Varieties of Mexican Roselle (Hibiscus sabdariffa L.) by Multivariable Analysis. Plant. Foods Hum. Nutr. 2013, 68, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Rasheed, D.M.; Kamal, I.M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle) cultivars via headspace SPME-GC-MS and chemometrics. Food Res. Int. 2015, 78, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Xiaobo, Z.; Mariod, A.A.; Mahunu, G.K.; Abdualrahman, M.A.Y.; Tchabo, W. Assessment of antioxidant properties, instrumental and sensory aroma profile of red and white Karkade/Roselle (Hibiscus sabdariffa L.). J. Food Meas. Charact. 2017, 11, 1559–1568. [Google Scholar] [CrossRef]
- Ifie, I.; Abrankó, L.; Villa-Rodriguez, J.A.; Papp, N.; Ho, P.; Williamson, G.; Marshall, L.J. The effect of ageing temperature on the physicochemical properties, phytochemical profile and α-glucosidase inhibition of Hibiscus sabdariffa (roselle) wine. Food Chem. 2018, 267, 263–270. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Fitrotunnisa, Q.; Arsianti, A.; Tejaputri, N.A.; Qorina, F. Antioxidative activity and phytochemistry profile of Hibiscus sabdariffa herb extracts. Int. J. Appl. Pharm. 2019, 11, 29–32. [Google Scholar] [CrossRef]
- Builders, P.; Kabele-Toge, B.; Builders, M.; Chindo, B.; Anwunobi, P.; Isimi, Y. Wound healing potential of formulated extract from Hibiscus sabdariffa calyx. Indian J. Pharm. Sci. 2013, 75, 45–52. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Teralı, K.; Olofinsan, K.A.; Surgun, S.; Ogbaga, C.C.; Ajiboye, T.O. Antidiabetic activity-guided isolation of gallic and protocatechuic acids from Hibiscus sabdariffa calyxes. J. Food Biochem. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Wongsa, P.; Chaiwarit, J.; Zamaludien, A. In vitro screening of phenolic compounds, potential inhibition against α-amylase and α-glucosidase of culinary herbs in Thailand. Food Chem. 2012, 131, 964–971. [Google Scholar] [CrossRef]
- Sarkar, B.; Vyas, P.; Haque, I.; Mukhopadhyay, K. A rapid UPLC method for simultaneous separation and detection of anthocyanidins from Ocimum, Hibiscus and Syzygium species and estimation of their antioxidant activity. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 658–667. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Pasini, F.; Joven, J.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011, 44, 1490–1495. [Google Scholar] [CrossRef]
- Daniel, D.L.; Huerta, B.E.B.; Sosa, I.A.; Mendoza, M.G.V. Effect of fixed bed drying on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.). Ind. Crops Prod. 2012, 40, 268–276. [Google Scholar] [CrossRef]
- Reyes-Luengas, A.; Salinas-Moreno, Y.; Ovando-Cruz, M.E.; Arteaga-Garibay, R.I.; Martínez-Peña, M.D. Análisis de ácidos fenólicos y actividad antioxidante de extractos acuosos de variedades de Jamaica (Hibiscus sabdariffa L.) con cálices de colores diversos analysis of phenolic acids and antioxidant activity of aqueous. Agrociencia 2015, 49, 277–290. [Google Scholar]
- Chaiyasut, C.; Sivamaruthi, B.S.; Pengkumsri, N.; Sirilun, S.; Peerajan, S.; Chaiyasut, K.; Kesika, P. Anthocyanin profile and its antioxidant activity of widely used fruits, vegetables, and flowers in Thailand. Asian J. Pharm. Clin. Res. 2016, 9, 218–224. [Google Scholar] [CrossRef]
- Achir, N.; Sinela, A.; Mertz, C.; Fulcrand, H.; Dornier, M. Monitoring anthocyanin degradation in Hibiscus sabdariffa extracts with multi-curve resolution on spectral measurement during storage. Food Chem. 2019, 271, 536–542. [Google Scholar] [CrossRef]
- Obouayeba, A.P.; Djyh, N.B.; Sekou, D.; Djaman, A.J.; N’guessan, J.D.; Kone, M.; Kouakou, T.H. Phytochemical and Antioxidant Activity of Roselle (Hibiscus sabdariffa L.) Petal Extracts. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1694–1720. [Google Scholar]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J.F.; Segura-Carretero, A. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crops Prod. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Ali, S.A.M.; Zainalabidin, S.; Latip, J. Quantitative analysis of phenolics content in two roselle varieties (Hibiscus sabdariffa) by high performance liquid chromatography. Malaysian J. Anal. Sci. 2019, 23, 715–724. [Google Scholar] [CrossRef]
- Ali, S.A.M.; Mohd, C.R.C.; Latip, J. Comparison of phenolic constituent in Hibiscus sabdariffa cv. UKMR-2 calyx at different harvesting times. Sains Malaysiana 2019, 48, 1417–1424. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Blancas-Benitez, F.J.; Velderrain-Rodríguez, G.R.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols released and associated to dietary fibre in calyces and decoction residues of Roselle (Hibiscus sabdariffa L.). J. Funct. Foods 2015, 18, 171–181. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Švajdlenka, E. Biological evaluation and molecular docking of protocatechuic acid from Hibiscus sabdariffa L. as a potent urease inhibitor by an ESI-MS based method. Molecules 2017, 22, 1696. [Google Scholar] [CrossRef]
- Chin, K.L.; Zhen, J.; Qi, Y.; Chin, S.L.; Breithaupt, M.; Wu, Q.L.; Simon, J.; Henson, J.; Ferchaud, V. A comparative evaluation: Phytochemical composition and antioxidant capacity of three roselle (Hibiscus sabdariffa L.) accessions. Acta Hortic. 2016, 1125, 99–107. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.; Peréz-Ramírez, I.F.; Pérez-Jiménez, J.; Nava, G.M.; Reynoso-Camacho, R. Comparison of the bioactive potential of Roselle (Hibiscus sabdariffa L.) calyx and its by-product: Phenolic characterization by UPLC-QTOF MSE and their anti-obesity effect in vivo. Food Res. Int. 2019, 126. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Rani, A.P.; Amir Hamzah, R.; Arumwardana, S.; Afifah, E.; Kusuma, H.S.W.; Rihibiha, D.D.; Nufus, H.; Amalia, A. Antioxidant and antiaging assays of Hibiscus sabdariffa extract and its compounds. Nat. Prod. Sci. 2017, 23, 192–200. [Google Scholar] [CrossRef]
- Zannou, O.; Kelebek, H.; Selli, S. Elucidation of key odorants in Beninese Roselle (Hibiscus sabdariffa L.) infusions prepared by hot and cold brewing. Food Res. Int. 2020, 133, 109133. [Google Scholar] [CrossRef]
- Juhari, N.H.; Petersen, M.A. Physicochemical properties and oxidative storage stability of milled roselle (Hibiscus sabdariffa L.) seeds. Molecules 2018, 23, 385. [Google Scholar] [CrossRef]
- Abdel-shafi, S.; Al-Mohammadi, A.-R.; Sitohy, M.; Mosa, B.; Ismaiel, A.; Enan, G.; Osman, A. Antimicrobial Activity and Chemical Constitution of the Crude, Phenolic-Rich Extracts of Hibiscus sabdariffa, Brassica oleracea and Beta vulgaris. Molecules 2019, 24, 4280. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.; Achir, N.; Cisse, M.; Pallet, D.; Sakho, M.; Dornier, M. Identification of roselle varieties through simple discriminating physicochemical characteristics using multivariate analysis. Food Sci. Technol. 2019, 39, 321–327. [Google Scholar] [CrossRef]
- Sukkhaeng, S.; Promdang, S.; Doung-ngern, U. Fruit characters and physico-chemical properties of roselle (Hibiscus sabdariffa L.) in Thailand—A screening of 13 new genotypes. J. Appl. Res. Med. Aromat. Plants 2018, 11, 47–53. [Google Scholar] [CrossRef]
- Soradech, S.; Kusolkumbot, P.; Reungpatthanaphong, P.; Thubthimthed, S. Investigation of DPPH radical scavenging, antioxidant and melanogenesis stimulating activities of various pigment extracts from Thai herbal plants. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 392–399. [Google Scholar]
- Bi, W.; He, C.; Ma, Y.; Shen, J.; Zhang, L.H.; Peng, Y.; Xiao, P. Investigation of free amino acid, total phenolics, antioxidant activity and purine alkaloids to assess the health properties of non-Camellia tea. Acta Pharm. Sin. B 2016, 6, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Sagdic, O.; Ekici, L. Natural food colorants and bioactive extracts from some edible flowers. J. Appl. Bot. Food Qual. 2015, 88, 170–176. [Google Scholar] [CrossRef]
- Aishah, B.; Nursabrina, M.; Noriham, A.; Norizzah, A.R.; Mohamad Shahrimi, H. Anthocyanins from Hibiscus sabdariffa, melastoma Malabathricum and Ipomoea batatas and its color properties. Int. Food Res. J. 2013, 20, 827–834. [Google Scholar]
- Chunthanom, P.; Chaikham, P.; Intaket, R. Biochemical and antibacterial properties of Thai medicine herbal infusions. Int. Food Res. J. 2013, 20, 1901–1907. [Google Scholar]
- Singh, S.; Singh, D.R.; Salim, K.M.; Srivastava, A.; Singh, L.B.; Srivastava, R.C. Estimation of proximate composition, micronutrients and phytochemical compounds in traditional vegetables from Andaman and Nicobar Islands. Int. J. Food Sci. Nutr. 2011, 62, 765–773. [Google Scholar] [CrossRef]
- Rezende, F.A.G.G.; Sande, D.; Coelho, A.C.; Oliveira, G.P.; Boaventura, M.A.D.; Takahashi, J.A. Edible flowers as innovative ingredients for future food development: Anti-Alzheimer, antimicrobial and antioxidant potential. Chem. Eng. Trans. 2019, 75, 337–342. [Google Scholar] [CrossRef]
- Sánchez-Feria, C.; González-Hernández, V.A.; Salinas-Moreno, Y.; Cruz-Huerta, N. Genotype and Environmental Effects on Physical and Chemical Qualities of Mexican Varieties of Hibiscus sabdariffa L. Flowers 2017, 51, 525–541. [Google Scholar]
- Kashyap, D.; Sarmah, P.; Sarma, A. Nutritional evaluation of calyces and epi-calyces from two variants of Hibiscus sabdariffa l.: A comparison. Res. J. Med. Plant. 2015, 9, 241–247. [Google Scholar] [CrossRef][Green Version]
- Sáyago-Ayerdi, S.G.; Velázquez-López, C.; Montalvo-González, E.; Goñi, I. By-product from decoction process of Hibiscus sabdariffa L. calyces as a source of polyphenols and dietary fiber. J. Sci. Food Agric. 2014, 94, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.; Ramos, D.; Vieira, M.; Ramalho, S.; Silva, M.; Zárate, N.; Foglio, M.; Carvalho, J. Phenolic compounds of Hibiscus sabdariffa and influence of organic residues on its antioxidant and antitumoral properties. Brazilian J. Biol. 2015, 75, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Loyola Arenas, K.S.; Cruz Y Victoria, M.T.; Vizcarra Mendoza, M.G.; Martínez Vera, C.; Anaya Sosa, I. Effect of agitated bed drying on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.). Int. J. Food Sci. Technol. 2016, 51, 1457–1464. [Google Scholar] [CrossRef]
- Kalla, M.L.M.; Jong, E.N.; Kayem, J.G.; Sreekumar, M.M.; Nisha, P. Effect of re-extraction parameters and drying temperature on the antioxidant properties and dietary fiber of Red sorrel (Hibiscus sabdariffa L.) calyces residues. Ind. Crops Prod. 2015, 74, 680–688. [Google Scholar] [CrossRef]
- Leyva Daniel, D.E.; Barragán Huerta, B.E.; Vizcarra Mendoza, M.G.; Anaya Sosa, I. Effect of drying conditions on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.) added to yogurt. Int. J. Food Sci. Technol. 2013, 48, 2283–2291. [Google Scholar] [CrossRef]
- Paraíso, C.M.; dos Santos, S.S.; Ogawa, C.Y.L.; Sato, F.; dos Santos, O.A.A.; Madrona, G.S. Hibiscus sabdariffa L. extract: Characterization (FTIR-ATR), storage stability and food application. Emirates J. Food Agric. 2020, 32, 55–61. [Google Scholar] [CrossRef]
- Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Arámbula-Villa, G.; Flores-Casamayor, V.; Álvarez-Fitz, P.; Ramírez-Ruano, M.; Salazar, R. Effect of water activity on extractable polyphenols and some physical properties of Hibiscus sabdariffa L. calyces. J. Food Meas. Charact. 2019, 13, 687–696. [Google Scholar] [CrossRef]
- Mollah, M.A.F.; Tareq, M.Z.; Bashar, K.K.; Zahidul Hoque, A.B.M.; Karim, M.M.; Al Rafiq, M.Z. Antioxidant properties of BJRI vegetable mesta-1 (Hibiscus sabdariffa L.). Plant. Sci. Today 2020, 7, 154–156. [Google Scholar] [CrossRef]
- Apáez Barrios, P.; Granados, M.D.C.R.; Santos, M.E.P.; Montaño, Y.A.R. Effect of foliar copper application on yield and anthocyanin concentration in Hibiscus sabdariffa calyces. Rev. Fac. Ciencias Agrar. 2018, 50, 65–75. [Google Scholar]
- Ibrahim, R.; Mazuki, N.A.F. The quality of roselle (Hibiscus sabdariffa L.) juices made from roselle calyces stored at different cold temperatures. Malaysian Appl. Biol. 2013, 42, 67–71. [Google Scholar]
- Bechoff, A.; Cissé, M.; Fliedel, G.; Declemy, A.L.; Ayessou, N.; Akissoe, N.; Touré, C.; Bennett, B.; Pintado, M.; Pallet, D.; et al. Relationships between anthocyanins and other compounds and sensory acceptability of Hibiscus drinks. Food Chem. 2014, 148, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Yang, K.M.; Chiang, P.Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Alonso, J.; Zamilpa, A.; Aguilar, F.A.; Herrera-Ruiz, M.; Tortoriello, J.; Jimenez-Ferrer, E. Pharmacological characterization of the diuretic effect of Hibiscus sabdariffa Linn (Malvaceae) extract. J. Ethnopharmacol. 2012, 139, 751–756. [Google Scholar] [CrossRef]
- Nuryanti, S.; Matsjeh, S.; Anwar, C.; Raharjo, T.J. Isolation anthocyanin from roselle petals (Hibiscus sabdariffa L) and the effect of light on the stability. Indones. J. Chem. 2012, 12, 167–171. [Google Scholar] [CrossRef]
- Pulgarín, J.A.M.; Bermejo, L.F.G.; Durán, A.C. A fast and simple FIA-chemiluminescence method for the evaluation of Roselle flowers as scavenger of the free radicals generated by UV irradiated antibiotics. J. Pharm. Biomed. Anal. 2019, 164, 630–635. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mariod, A.A.; Wiliam, T. Rapid Determination of Antioxidant Compounds and Antioxidant Activity of Sudanese Karkade (Hibiscus sabdariffa L.) Using Near Infrared Spectroscopy. Food Anal. Methods 2016, 9, 1228–1236. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oboh, G. Aqueous extracts of roselle (Hibiscus sabdariffa Linn.) varieties inhibit α-amylase and α-glucosidase activities in vitro. J. Med. Food 2013, 16, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, K.L.; Burris, K.P.; Zivanovic, S.; Davidson, P.M.; Stewart, C.N. Aqueous extracts of Hibiscus sabdariffa calyces as an antimicrobial rinse on hot dogs against Listeria monocytogenes and methicillin-resistant Staphylococcus aureus. Food Control. 2014, 40, 274–277. [Google Scholar] [CrossRef]
| Country of Origin | Extraction Method | Solvent | Extraction Condition | Phenolics and Antioxidant Activity | Ref |
|---|---|---|---|---|---|
| Vietnam | maceration | 50% ethanol | sample:solvent ratio 1:7.7 extraction temperature 56.9 °C extraction time 33.29 min | TAC (186.01 mg/L) | [6] |
| Mexico | maceration | purified water | sample:solvent ratio 1:16.7 extraction temperature 95 °C extraction time 20 min particle size 250–177 μm | TPC (14.10 mg GAE/g), TFC (10.18 mg CE/g), and TAC (5.68 mg Cy-3-GE/g) | [55] |
| Saudi Arabia | maceration | water | sample:solvent ratio 1:10 extraction temperature 100 °C extraction time 10 min | TPC, TAC, FRAP, ABTS, delphinidin 3-sambubioside, delphinidin 3-glucoside, cyanidin 3-sambubioside, and cyanidin 3-glucoside | [58] |
| Mexico | maceration | 50% ethanol | sample:solvent ratio 1:10 extraction time 2 h light protection with aluminum foil | TPC, TAC, DPPH, delphinidin-3-O-sambubioside, delphinidin-3-O-glucoside (myrtillin), and cyanidin-3-O-sambubioside) | [59] |
| Vietnam | maceration | 50% ethanol | sample:solvent ratio 1:8 extraction temperature 60 °C extraction time 30 min | TAC (180,82 mg Cy-3-GE/L) | [62] |
| Brazil | maceration | 50% ethanol | extraction temperature 56 °C pH 10.2 extraction time 110 min, stirring | TPC (5.01 mg GAE/g) | [63] |
| Nigeria | maceration | methanol | sample:solvent ratio 1:12.5 extraction time 72 h | Yield (27.3%), TPC (29.2 mg GAE/g) TFC (36.7 mg QE/g), DPPH (78%), and TBARS (21%) | [65] |
| Mexico | maceration | 96% ethanol | sample:solvent ratio 1:50 extraction temperature 65 °C | TAC (1.50 mg Cy-3-GE/g) | [78] |
| Thailand | maceration | 95% ethanol | sample:solvent ratio 1:4 extraction time 24 h shaking at 150 rpm | Yield (4.91%), TPC (0.05 mg GAE/g), DPPH, gallic acid (0.75 g/kg), caffeic acid (0.39 g/kg), ferulic acid (0.06 g/kg), chlorogenic acid (0.74 g/kg) and quercetin (0.45 g/kg) | [81] |
| Germany | maceration | 70% ethanol | sample:solvent ratio 1:10 extraction temperature 50 °C extraction time 48 h shaking at 150 rpm | TPC (0.04 μg/mL), TAC (3.16 mg Cy-3-GE/L), and DPPH (60.38%) | [82] |
| Senegal | maceration | water | sample:solvent ratio 1:15 extraction temperature 30 °C extraction time 240 min | TAC (220 mg delphinidin 3-xylosylglucoside/L), ORAC (165 μmol Trolox/g) | [89] |
| Iran | maceration | 80% ethanol and water 80% methanol | sample:solvent ratio 1:10 extraction time 24 h sample:solvent ratio 1:10 extraction time 24 h | TPC (80% ethanol: 9.34 mg/g; water: 9.59 mg/g), TFC (80% ethanol: 4.76 mg/g; water: 3.53 mg/g), TAC (80% ethanol: 0.042 mg/g; water: 0.044 mg/g) Caffeic acid (5.08 mg/g), chlorogenic acid (1.09 mg/g), p-coumaric acid (0.07 mg/g), catechin (0.92 mg/g), quercetin (0.16 mg/g), hesperidin (0.14 mg/g), hesperetin (0.51 mg/g) | [34] |
| Egypt | maceration | 50% ethanol | sample:solvent ratio 1:50 extraction temperature 180 °C extraction time 30 min | TPC (43.1 mg GAE/g), gallic acid (0.91 mg/g), protocatechuic acid (163.20 mg/g) | [90] |
| Mexico | maceration | distilled water | sample:solvent ratio 1:50 extraction temperature 95 °C extraction time 60 min | TPC, phenolic acids, flavonoids, anthocyanins | [57] |
| China | maceration UAE | 0.1% HCl aqueous solution HPβ-CD aqueous solution | sample:solvent ratio 1:15 extraction temperature 25 °C extraction time 30 min sample:solvent ratio 1:27 extraction temperature 54 °C extraction time 53 min 300 W; 25 kHz | TAC (4.80 mg/g) | [33] |
| Malaysia | UAE | 80% methanol | sample:solvent ratio 1:20 extraction temperature 60 °C extraction time 30 min | TPC, ACEI activity (0.01 μg/mL), 36 identified metabolites, including flavonoids, anthocyanins, and organic acids | [38] |
| Brazil | UAE | 25% ethanol | extraction temperature 65 °C extraction time 45 min | TPC (2.24 mg GAE/g), TAC (3.58 mg Cy-3-GE/g) | [9] |
| Mexico | UAE | 80% ethanol | sample:solvent ratio 1:20 extraction temperature 60 °C extraction time 32 min 180 W; 40 KHz | Yield (red calyx: 20.84%; white calyx: 13.74%), TPC (red calyx: 13.02 mg GAE/g; white calyx: 12.74 mg GAE/g), TFC (red calyx: 4.42 mg CE/g; white calyx: 4.53 mg CE/g), TAC (red calyx: 1.80 mg Cy-3-GE/g; white calyx: 0.01 mg Cy-3-GE/g), DPPH activity (red calyx: 74.58%; white calyx: 36.95%) | [10] |
| Indonesia | UAE | water | sample:solvent ratio 1:15 extraction at room temperature extraction time 30 min frequency 40 kHz | TAC | [54] |
| Portugal | UAE | 39.1% ethanol | extraction time 26.1 min power 296.6 W | TAC (51.76 mg/g), delphinidin-3-O-sambubioside, cyanidin-3-O-sambubioside | [79] |
| Malaysia | MAE | 52% ethanol | sample:solvent ratio 1:15 extraction time 4 min microwave power 450 W | TFC (94.32 mg QE/g), 95 flavonoid compounds | [32] |
| NA | MAE | water | sample:solvent ratio 1:10 extraction time 15 min microwave power 10 W | yield (24.6%), TPC (2.72%) | [51] |
| Turkey | MAE | DES in 50% water | microwave power 550 W | TPC (31.90 mg GAE/g), TAC (3.00 mg Cy-3-GE/g), DPPH 95.89% | [67] |
| Malaysia | MAE | distilled water | sample:solvent ratio 1:14 extraction temperature 60 °C extraction time 3 minmicrowave power 500 W | TPC (70.53 mg GAE/g) 77 phenolic compounds | [11] |
| Spain | SFE | 16.7% ethanol | extraction temperature 64 °C pressure 391 bar | TPC (8.63 mg GAE/g) 22 identified and quantified phenolic compounds | [12] |
| Malaysia | SFE | CO2, co-solvent 75% ethanol | extraction temperature 70 °C extraction pressure 8.90 MPa flow rate 9.49%particle size 350 μm | yield 26.73% | [85] |
| Indonesia | SFE | CO2, co-solvent acetone (5% V/V) | extraction time 2.5 h extraction temperature 343.15 K pressure 24 Mpa | TPC (8.63 mg GAE/g) | [80] |
| Pre-Analysis Step | Determination Method | Analysis Condition | Target Compound | Ref |
|---|---|---|---|---|
| Solvent evaporation at 35 °C and the obtained residue redissolved in water | LC-DAD-ESI/MS | Column: Waters C18 (4.6 mm × 150 mm, 3 μm) Mobile phase: (A) 0.1% trifluoroacetic acid in water (B) acetonitrile Elution: gradient | Delphinidin-3-O-sambubioside and cyanidin-3-O-sambubioside | [79] |
| N/A | LC-DAD-MS HPLC-PDA | Column: Dionex C18 (250 mm × 4.6 mm, 5 μm) Mobile phase: (A) water (B) 60% methanol in water Elution: gradient Column: Dionex C18 (250 mm × 4.6 mm, 5 μm) Mobile phase: (A) water (B) 60% methanol in water Elution: gradient | Chlorogenic acid, gallic acid, protocatechuic acid, quercetin, delphinidin-3-glucoside, and cyanidin-3-glucoside | [83] |
| N/A | LC-ESI-MS/MS | Column: C18 (100 mm × 2.1 mm × 1.8 μm, 5 μm) Elution: gradient | 77 phenolic compounds | [11] |
| N/A | LC-MS-TOFHPLC-DAD | Column: Phenomenex Gemini C18 (250 mm × 4.6 mm, 5 μm). Mobile phase: (A) 0.1% (v/v) trifluoroacetic acid (B) trifluoroacetic acid/acetonitrile/water (50:49.9:0.1) Flow rate: 1 mL/min Elution: gradient | Gallic acid, protocatechuic acid, 3-O-caffeoylquinic acid caffeic acid myricetin 3-O-arabinogalactoside quercetin 3-O-sambubioside delphinidin 3-O-sambubioside delphinidin 3-O-glucoside cyanidin 3-O-sambubioside | [30] |
| N/A | HPLC-PDA HPLC-Q/TOF/MS/ESI | Column: Gemini C18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) 0.1% (v/v) trifluoroacetic acid (B) trifluoroacetic acid/acetonitrile/water (50:49.9:0.1) Elution: gradient Column: Kinetex C18 (4.6 mm × 150 mm, 2.6 µm) Mobile phase: (A) 0.5% (v/v) formic acid in water (B) 0.5% (v/v) formic acid in acetonitrile Elution: gradient | Quantification: gallic acid, caffeic acid, delphinidin 3-O-sambubioside, cyanidin 3-O-sambubioside, myricetin-3-arabinogalactoside, quercetin-3-sambubioside Identification: 11 derivatives anthocyanins and pyranoanthocyanins | [109] |
| N/A | HPLC | Column: C18 column (250 mm × 4.6 μm, 5 µm) Mobile phase: (A) 100% methanol (B) 0.1% trifluoroacetic acid Elution: gradient | Gallic acid, protocatechuic acid, p-hydroxybenzoic, chlorogenic acid, caffeic acid, syringic acid, p-coumaric acid, and ferulic acid | [92] |
| N/A | HPLC-UV | Column: RP (150 mm × 4.6 mm, 3 μm) Mobile phase: (A) 1% acetic acid in acetonitrile (B) 1% acetic acid in water Elution: gradient | Gallic acid and protocatechuic acid | [90] |
| N/A | HPLC-UV-Vis | Column: Supelco Discovery HS C18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) 10% acetonitrile (B) 90% formic acid (2%) Elution: gradient | Delphinidin-3-sambubioside, cyanidin-3-sambubioside, rutin, chlorogenic acid | [86] |
| The extract was diluted in methanol | HPLC-UV-Vis | Column: RP-Phenomenex C18 (250 mm × 4.6 mm, 5 μm) Mobile phase: (A) formic acid–water 1:99 v/v (B) acetonitrile Elution: gradient | Phenolic acids, flavonoids, anthocyanins | [57] |
| N/A | HPLC-UV ESI-TOF-MS | Column: Zorbax Eclipse Plus C18 (150 mm × 4.6 mm, 1.8 μm) Flow rate: 0.5 mL/min Injection volume: 10 μL Mobile phase for non-anthocyanin compounds: (A) acidified (1% acetic acid) water:acetonitrile 90:10 (B) acetonitrile Elution: gradient Mobile phase for anthocyanin compounds: (A) acidified water (10% acetic acid) and (B) acetonitrile Elution: gradient Instrument: microTOF, ESI-TOF mass spectrometer | Chlorogenic acid, quercetin 3-rutinoside, quercetin 3-glucoside, kaempferol 3-O-rutinoside and kaempferol 3-(p-coumarylglucoside), quercetin, 4-hydroxycoumarin and delphinidin-3-sambubioside | [116] |
| Dried extract was redissolved in 4% acetic acid in water | HPLC-DAD | Column: C18 column (300 mm × 3.9 mm, 125 A°) Mobile phase: (A) 4% acetic acid in water (B) 100% acetonitrile Elution: gradient | Cyanidin 3-sambubioside | [117] |
| N/A | HPLC -DAD | Column: RP C18 (25 cm × 0.4 cm, 5 mm) Mobile phase: acetonitrile:formic acid (4.5%) Elution: isocratic | Anthocyanins (delphinidin-3-O-sambubioside, delphinidin-3-O-glucoside (myrtillin), and cyanidin-3-O-sambubioside) | [59] |
| N/A | HPLC -DAD | Column: C18 (250 mm × 4.6 mm) Mobile phase: (A) water:acetonitrile:trifluoroacetic acid (50:50:0.1) and (B) acetonitrile:water:trifluoroacetic acid (10:90:0.1) Elution: gradient | Gallic acid, protocatechuic acid, caffeic acid, gentisic, chlorogenic acid, vanillic acid, benzoic acid, syringic acid, catechin, and epicatechin | [35] |
| 80% methanol extract | HPLC-DAD | Column: zorbax eclipse XDB-C18 column (4.6 mm × 150 mm, 5 μm) Mobile phase: (A) methanol 100% (B) formic acid 1%; Elution: gradient | Caffeic acid, chlorogenic acid, p-coumaric acid, catechin, quercetin, hesperidin, hesperetin | [34] |
| Extract was re-dissolved in 2.5 mL methanol | HPLC-DAD | Column: C18 reversed-phase Xbridge (250 mm × 4.6 mm, 5 μm) Mobile phase: (A) 0.1% acetic acid in distilled water (B) acetonitrile Injection volume: 10 μL Flow rate: 0.8 mL/min | Protocatechuic acid, catechin | [46] |
| N/A | HPLC-DAD | Column: C18 (150 mm × 4.6 mm, 5 µm) Mobile phase: (A) 2% acetic acid (B) 100% methanol Elution: gradient | Gallic acid, chlorogenic acid, caffeic acid, ferulic acid, catechin, epicatechin, rutin, quercetin, quercitrin, and kaempferol | [97] |
| N/A | HPLC-DAD | Column: Zorbax C18 (250 mm × 4.6 mm, 5 μm) Mobile phase: (A) 0.1% trifluoroacetic acid (B) acetonitrile Elution: gradient | Cyanidin 3-sambubioside (Cy-3-Sa), delphinidin-3-sambubioside | [73] |
| N/A | HPLC-DAD | Column: C18 (250 mm × 4 mm, 5 μm) Mobile phase: (A) water/acetonitrile/formic acid (87I:3I:10% v/v/v) (B) water/acetonitrile/formic acid (40I:50I:10% v/v/v) Elution: gradient | delphinidin chloride, malvidin chloride, cyanidin chloride and pelargonidin chloride | [70] |
| N/A | HPLC-DAD | Non-anthocyanins column: C18 Poroshell (50 mm × 4 mm × 6 mm, 2.7 μm) Mobile phase: (A) 0.1% formic acid in water (B) acetonitrile Anthocyanins column: Zorbax C18 column (250 mm × 4.6 mm, 5 μm); Mobile phase: (A) trifluoroacetic acid, 0.1%) (B) acetonitrile Elution: gradient | Flavanols, flavonols, benzoic, hibiscus and phenolic acids as well as two main anthocyanins (cyanidin 3sambubioside and delphinidin 3-sambubioside) | [98] |
| N/A | HPLC-DAD | Column: hypersil ODS.2 analytical (250 mm × 46 mm, 5 µm) Mobile phase: methanol:acetic acid:water (25:1:75 v/v/v) Elution: isocratic | Caffeic acid, chlorogenic acid, ferulic acid, gallic acid, protocatechuic acid, p-coumaric acid, p-hydroxybenzoic acid and syringic acid | [118] |
| N/A | HPLC-DAD | Column: RP Alltech Prevail C18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) AcCN (B) 3% aq. AcOH Elution: gradient | Gallic acid, caffeic acid, ferulic acid, chlorogenic acid and quercetin | [81] |
| N/A | HPLC-DAD | Column: Kromasil C18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) 10 mM phosphoric acid (pH 2.5) (B) 100% methanol Elution: gradient | Caffeic acid and p-coumaric acid | [114] |
| N/A | HPLC-DAD | Column: ACE® C18 (250 mm × 4.6 mm,5 μm) Mobile phase: (A) acetonitrile (B) 0.1% trifluoroacetic acid (TFA) Elution: gradient | Anthocyanins (cyanidin 3-glucoside, delphinidin 3-glucoside, malvidin 3-glucoside, and peonidin 3-glucoside) and anthocyanidins (Cyanidin chloride, delphinidin chloride, malvidin chloride, and peonidin chloride) | [119] |
| N/A | HPLC-DAD | Column: RP- ACEC18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) water/formic acid/acetonitrile (97.2/2.0/0.8 v/v/v) (B) acetonitrile Elution: gradient | Anthocyanins (Del 3-O-Sb and Cya 3-O-Sb), gallic and protocatechuic acids | [120] |
| N/A Extract in methanol | HPLC-DAD HPLC-ESI-MS | Column: promosil C18 (250 mm × 4.6 mm, 5 µm); Mobile phase: (A) 1.5% formic acid aqueous solution (B) acetonitrile Column: promosil C18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) 0.1% formic acid aqueous solution (B) methanol containing 0.1% formic acid Elution: gradient | Delphinidin-3-Osambubioside (D3S) | [87] |
| N/A | HPLC-DADLC-MS | Column: Gemini C18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) 0.2% (v/v) formic acid in water, (B) acetonitrile Elution: gradient | Delphinidin 3-sambubioside, delphinidin 3-glucoside, cyanidin 3-sambubioside and cyanidin 3-glucoside | [58] |
| Purification using TLC | HPLC-DAD HPLC-ESI-MS | Column: Kinetex C18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) formic acid (5%, v/v) (B) acetonitrile (5%, v/v formic acid) Elution: gradient Column: A DIONEX Acclaim 120 C18 (150 mm × 2.1 mm, 3 µm) Mobile phase: (A) formic acid (5%, v/v) (B) acetonitrile (5% formic acid, v/v) Elution: gradient | Anthocyanins | [88] |
| Diluted with purified distilled water | HPLC-DAD NMR | Column: prontosil C-18 (250 mm × 4 mm, 5 μm) Mobile phase: (A) 0.1% trifluoroacetic acid in water (B) 0.1% trifluoroacetic acid in acetonitrile Elution: gradient | Protocatechuic acid and anthocyanins | [121] |
| Diluted with ultrapure water | HPLC-DAD-ESI-TOF | Column: Zorbax C18 (250 mm × 4.6 mm, 5 μm) Mobile phase: 50:49.99:0.01 (v/v) methanol:water:trifluoroacetic acid Elution: isocratic | Anthocyanins | [122] |
| Diluted with solvent A | HPLC-DAD-ESI-MS/MS | Column: C18 (150 mm × 4.6 mm, 4.0 μm) Mobile phase: (A) water/formic acid (99.5:0.5, v/v) (B) acetonitrile/formic acid (99.5:0.5, v/v) Elution: gradient | 3-caffeoylquinic acid, delphinidin 3-sambubioside, 3-p-coumaroylquinic acid, cyanidin 3-sambubioside, 5-caffeoylquinic acid, 4-caffeoylquinic acid, myricetin 3-sambubioside, quercetin 3-sambubioside, 5-O-caffeoylshikimic acid, 5-p-coumaroylquinic acid, quercetin 3-rutinoside, quercetin 3-glucoside, kaempferol 3-O-rutinoside | [8] |
| Concentrated and resuspended in the mobile phase | HPLC-DAD-MS | Column: phenomenex C18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) water: acetonitrile: formic acid 90:10:1 (v/v) (B) acetonitrile (v/v) Elution: gradient | Anthocyanins | [55] |
| Dissolved in 20 mL of a methanol/water mixture (50/50 v/v) | HPLC–DAD-MS | Identification column: ACEC18 (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) water/formic acid/acetonitrile (99.1/0.1/0.8 v/v/v) (B) acetonitrile Injection volume: 20 µL Flow rate: 0.7 mL min Elution: gradient. Quantification: the same operating conditions as for identification with Mobile phase: (A) 97.2/2/0.8 (v/v/v) of water/formic acid/acetonitrile. | Anthocyanins, flavonols, chlorogenic acid, gallic acid, and protocatechuic acid | [100] |
| N/A | HPLC-DAD-MS/MS | Column: Synergi Hydro-RP C18 (250 mm × 4.6 mm, 4 μm) Mobile phase: (A) water–formic acid (99.5:0.5, v/v) (B) acetonitrile–formic acid (99.5:0.5, v/v) Elution: gradient | 20 identified and quantified phenolic compounds. Major found as delphinidin-3-O-sambubioside and 3-caffeoylquinic acid | [102] |
| N/A | HPLC-PDA | Column: C18 Phenomenax Luna (150 mm × 4.6 mm, 10 µm) Mobile phase: (A) 0.1% phosphoric acid (B)100% acetonitrile Elution: gradient | Chlorogenic acid, coumaric acid, ferulic acid, quercetin and cyanidine-3-sambubioside | [3] |
| Dissolved in 1 mL of 0.1% formic acid in water, sonicated for 5 min | HPLC-PDA | Column: Purospher STAR RP-18e LichroCART (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) 0.1% formic acid in water (B) 0.1% formic acid in acetonitrile Elution: gradient | Ascorbic acid, chlorogenic acid, caffeic acid, delphinidin-3-O-sambubioside and cyanidin-3-O-sambubioside | [123] |
| Dissolved in 1 mL of 0.1% formic acid in water. Samples were sonicated for 5 min | HPLC-PDA | Column: Purospher STAR RP-18e LichroCART column (250 mm × 4.6 mm, 5 µm) Mobile phase: (A) 0.1% formic acid in water (B) 0.1% formic acid in acetonitrile Elution: gradient | Gallic acid, ascorbic acid, chlorogenic acid, caffeic acid, delphinidin-3-O-sambubioside, and cyanidin-3-O-sambubioside | [124] |
| N/A | HPLC-PDA | Column: RP C18 (3.0 mm, 5 µm) Mobile phase: (A) 0.1% trifluoroacetic acid (B) acetonitrile (85, v/v, 0.085%)Elution: gradient | (+)catechin, gallic, chlorogenic, caffeic, syringic and ferulic acids | [125] |
| Purification and fractionation | HPLC-ESI-MSNMR | Column: RP-18 Lichrocart (150 mm × 4.6 mm, 5 μm) Mobile phase: (A) [formic acid (1%, v/v) (B) (MeCN(formic acid 1%, v/v)) Elution: gradient | delphinidin-3-O-sambubioside (Dp-samb) and cyanidin-3-O-sambubioside (cy-samb) | [71] |
| N/A | HPLC-ESI-TOF-MS | Column: Zorbax Eclipse Plus C18 (150 mm × 4.6 mm, 1.8 μm) Mobile phase: (A) water plus 0.1% of formic acid (B) acetonitrile Elution: gradient | 22 identified and quantified phenolic compounds, including: organic acid, phenolic acid, and flavonoids | [12] |
| N/A | HPLC-ESI-TOF-MS | Column: Zorbax Eclipse Plus C18 (150 mm × 4.6 mm, 1.8 μm) Mobile phase: (A) water plus 0.1% of formic acid (B) acetonitrile Flow rate: of 0.5 mL/min Injection volume: 10 µL Elution: gradient | organic acids, anthocyanins, flavonoids, phenolic acid | [77] |
| N/A | HPLC-MS | N/A | protocatechuic acid | [126] |
| Sonicated for 30 min | HPLC-MS | Column: Polaris C18 Amide (250 mm × 4.6 mm, 5 μm) Mobile phase: (A) 0.4% formic acid/water and (B) 0.4% formic acid/acetonitrile Elution: gradient | Delphinidin (De) and cyanidin (Cy), delphinidin-3-sambubioside (De-Sam) and cyanidin-3sambubioside (Cy-Sam) | [127] |
| N/A | HPLC-DAD HPLC-MS | Column: Agilent ZORBAX Eclipse Plus C18 (100 mm × 4.5 mm, 3.5 µm) Mobile phase: ethanol and tartaric acid aqueous solution (0.25 mol/L) Elution: gradient Column: Agilent ZORBAX Eclipse Plus C18 (100 mm × 4.5 mm, 3.5 µm) Mobile phase: ethanol and formic acid aqueous solution (0.1%, v/v) Elution: gradient | Delphinidin-3-O-sambubioside and cyanidin-3-O-sambubioside | [33] |
| Dissolved in methanol | UPLC | Column: UPLC BEH RP C18 (50 mm × 2.1 mm, 1.7 µm) Mobile phase: (A) 0.3% phosphoric acid in water (B) acetonitrile Elution: gradient | Anthocyanidins: delphinidin, cyanidin, petunidin, peonidin, pelargonidin, malvidin | [115] |
| N/A | UPLC-DAD-ESI/MS | Column: Waters Spherisorb S3 ODS-2 C18 (150 mm × 4.6 mm, 3 μm) Mobile phase for anthocyanin separation: (A) 0.1% trifluoroacetic acid in water (B) acetonitrile Mobile phase for non-anthocyanin separation: (A) 0.1% formic acid in water (B) acetonitrile Elution: gradient | 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, caffeic acid, myricetin-O-sambubioside, quercetin-O-sambubioside, quercetin-3-O-rutinoside, quercetin-3-O-glucoside, kaempferol-3-O-rutinoside | [28] |
| N/A | UPLC-DAD-ESI/MS | Column: Waters Spherisorb S3 ODS-2 C18 (150 mm × 4.6 mm, 3 μm) Mobile phase for anthocyanin separation: (A) 0.1% trifluoroacetic acid in water (B) acetonitrile Mobile phase for non-anthocyanin separation: (A) 0.1% formic acid in water (B) acetonitrile Elution: gradient | Anthocyanin and non-anthocyanin | [99] |
| N/A | UPLC-MS/MS | Column: RP HSS T3 C18 (100 mm × 2.1 mm, 1.7 µm) Mobile phase: (A) formic acid 0.1% (B) acetonitrile containing 0.1% formic acid Elution: gradient | 36 identified metabolites, including flavonoids, anthocyanins, phenolic and organic acids | [38] |
| N/A | UPLC-MS/MS | Column: BEH C18 (50 mm × 2.1 mm, 1.7 µm) Mobile phase: (A) ultrapure water acidified with 0.1% formic acid (B) methanol Elution: gradient | Hydroxycitric acid, caffeoylquinic acid, quercetin 3-O-glycoside, cyanidin 3-O-rutinoside, myricetin, rutin, pelargodine, cyanidin, delphinidin, 3-O-sambubioside delphinidin and cyanidin 3-O-sambubioside, quercetin hexoside, quercetin, myricitin, and 4-caffeoylquinic acid | [9] |
| The dried extract was reconstituted in the mobile phase (water with 0.1% formic acid) | UPLC-QTOF-MS-ESI | Column: BEH C18 (100 mm × 2.1 mm, 1.7μm) Mobile phase: (A)water with 0.1% formic acid (B) acetonitrile with 0.1% formic acid Elution: gradient | 34 identified extractable polyphenols (EPP), hydrolysable non-extractable polyphenols (NEPP), and 2 organic acids | [128] |
| The dried extract was dissolved in 1 mL methanol | UHPLC-MS/MS | Column: N/A Mobile phase: Formic acid 0.1% Electrospray Ionization (ESI) (voltage 3 kV; evaporation temperature 250 °C; capillary temperature 300 °C; nitrogen 40 psi, and Aux 10 psi with argon gas) | Myricetin, β-carotene, ascorbic acid | [129] |
| The dried extract was reconstituted in 300 µL of methanol | UHPLC-MS NMR | Column: Waters Acquity HSS T3 RP (150 mm × 1 mm, 1.8 µm) Mobile phase: (A) water contain 0.1% (v/v) formic acid (B) acetonitrile contain 0.1% (v/v) formic acid Elution: gradient | 33 metabolites: sugars, flavonoids, anthocyanins, and phenolic and aliphatic organic acids | [39] |
| Solid-phase microextraction (SPME) | GC GC-MS | N/A | Volatile compounds | [82] |
| The extract was dried over anhydrous sodium sulfate and concentrated to 0.6 mL on a Kuderna–Danish evaporator with a 12-cm Vigreux column and further evaporated to 0.2 mL with a gentle nitrogen stream | GC–FID GC-MS | Column: AT-5 ms (30 m × 0.25 mm, 0.5 µm) or DB-Wax column (30 m × 0.25 mm, 0.25 µm) Split mode (1:50) at 250 °C Carrier gas: Helium Flow rate: 1 mL/min Same condition with GC-FID | Volatile compounds | [105] |
| Solid-phase microextraction (SPME) | GC-MS | Column: Agilent J&W DB-WAX 122–7062 (60 m × 0.25 mm, 25 μm) Split ratio of 1:10 Flow rate: 1 mL/min Carrier gas: Helium | Volatile compounds | [108] |
| SPME | GC-MS | Column: DB5-MS (30 m × 0.25 mm, 0.25 μm) Splitless mode for 30 s Carrier gas: Helium Flow rate: 1 mL/min | Volatile compounds | [107] |
| SPME | GC-MS | Column: CP-Wax 52 CB (30 m × 0.25 mm, 0.25 μm) Split mode (1:10) Initial temperature: 60 °C (2 min) Carrier gas: Nitrogen Flow rate of 1 mL/min | Volatile compounds | [106] |
| SPME | GC-MS | Column: HP5 (30 m × 0.25 mm, 0.25 µm and BPX70 (70% Cyanopropyl-Polysylphenylene-Siloxane) capillary column (50 m × 0.22 mm × 0.25 µm) Carrier gas: Helium Flow rate of 1.7 mL/min Initial temperature 50 °C | Volatile compounds | [104] |
| LLE | GC-MS-O | Column: DB-Wax (30 m × 0.25 mm, 0.5 µm) and a Gerstel ODP-2 (Linthicum, MD) sniffing port using deactivated capillary column (30 cm–0.25 mm) Flow rate of 1.5 mL/min Carrier gas: Helium | Volatile compounds | [130] |
| Dynamic headspace sampling using Tenax TA cold trap | GC–MS GC-O | Column: DBWax (30 m × 0.25 mm, 0.50 μm Flow rate of 1.4 mL min Carrier gas: Hydrogen Column: DB-Wax column (30 m × 0.25 mm i.d., 0.5 μm film thickness) Carrier gas: Helium Flow rate: 1 mL min; split ratio, 1:20 | Volatile compounds | [131] |
| N/A | GC-MS | Column: direct capillary column TG ram negative 5MS (30 mm × 0.25 mm, 0.25 µm) Carrier gas: Helium Elution: gradient | Hydrocarbons (alkan)-saturated compounds, alcoholic compounds, triazine derivatives, unsat. alcoholic compounds, unsat. ester, merceoto compound, alkenes, primary alcohols, natural product (cholesterol) | [132] |
| The extract was diluted in methanol | TLC | mixture of chloroform (CHCl3) and methanol (CH3OH) in ratio 4:1 | glycosides, alkaloids, steroids, triterpenoids, tannins, and flavonoid | [111] |
| N/A | TLC | Column: (silica gel G60 F254 TLC plates of E. Merck, layer thickness 0.2 mm) Mobile phased: butanol:acetic:water (4:1:5) and methanol:water (95:5) Wavelength: 365–254 nm | flavonoid | [112] |
| Country | TPC | TFC | TAC | Sample | Ref |
|---|---|---|---|---|---|
| Sudan | N/A | N/A | L: 0.02 mg Cy-3-G/g (Al-Ubayyid) H: 107.7 mg Cy-3-G/g (Al-Rahad) | 8 cultivars Al-Ubayyid (white), Al-Gezira, Nyala, Al-Rahad, Al-Ubayyid, Kaduqli, El Geneina, Al-Fashir, China | [27] |
| Senegal | L: 19.30 mg GAE/g (Thai) H: 28.20 mg GAE/g (Vimto) | N/A | L: 8.20 mg D-3-D-X/g (Koor) H: 17.30 mg D-3-D-X/g (CLT92) | 4 cultivars (Vimto, Koor, Thaï, CLT92) | [133] |
| Thailand | N/A | N/A | L: 0.02 mg Cy-3-G/g (White) H: 19.48 mg Cy-3-G/g (HAC, Dark-purple closed calyx) | 15 genotypes Purple-Jumbo; Red-Jumbo; Pink-Jumbo; White calyx; Purple-Jumbo-Opened; Red-Jumbo-Opened; Pink-Jumbo-Opened; Purple-Jumbo-Closed; Red-Jumbo-Closed; Pink-Jumbo-Closed; Dark-purple-Closed; Dark-purple-Opened; Purple calyx-5-lobed leaf; Dark-red; Orange-Red | [134] |
| Mexico | L: 0.14 mg GAE/g (UAN16-2) H: 291.78 mg GAE/g (Cruza Negra) | N/A | L: 10.00 Cy-3-G/g (UAN25-1) H: 180.00 Cy-3-G/g (Cruza Negra) | 6 cultivars Cruza Negra, Criolla Huajicori, UAN25-1, UAN16-2, 4Q4, UAN6-Puga | [29] |
| Nigeria | L: 36.04 mg GAE/g (dark-red wet season) H: 38.00 mg GAE/g (dark red-dry season) | N/A | L: 19.57 mg/g (dark-red wet season) H: 27.10 mg/g (dark-red dry season) | dark red cultivar in wet and dry season | [30] |
| Mexico | L: 11.64 mg GAE/g (Alma Blanca) H: 17.57 mg GAE/g (Criolla) | L: 0.58 mg QE/g, 2.86 mg CE/g (Organic Criolla) H: 1.43 mg QE/g, 7.84 mg CE/g (Criolla) | L: 0.12 mg Cy-3-G/g (Alma Blanca) H: 3.98 mg Cy-3-G/g (Criolla) | 2 cultivars Criolla, Alma Blanca, Criolla variety, Organic Criolla | [68] |
| Sudan | L: 0.80 mg GAE/g (White) H: 1.48 mg GAE/g (Al-Rahad) | L: 0.18 mg QE/g (White) H: 0.27 mg QE/g (Al-Rahad) | L: 0.00 mg Cy-3-G/g (White) H: 32.96 mg Cy-3-G/g (Al-Rahad) | 4 cultivars White, Al-Gezira, Al-Fashir, Al-Rahad | [108] |
| Mexico | L: 6.20 mg GAE/g (DMS) H: 36.40 mg GAE/g (UAN21) | N/A | L: 1.48 mg Cy-3-G/g (DMS) H: 7.57 mg Cy-3-G/g (UAN18) | 53 genotypes Tempranilla Negra, Tempranilla Flor, Colima, Jersey Acriollada, Criolla Roja Violeta, Criolla Huajicor, Negra UAN, Criolla Morada, UAN5, Criolla Súper Precoz, Criolla Puebla Precoz, Criolla Precoz, Negra Quiviquinta, China, UAN 6 Puga, UAN 31, UAN 6-1, UAN 16-2, UAN 6 Novillero, MoradaXRoja, UAN 25, UAN 7, Tempranilla Roja, UAN 23, UAN 11, UAN 24, UAN 21, UAN 8, UAN 13, UAN 17, UAN 26, UAN 27, UAN 12-1, UAN 15, UAN 24-1, UAN 12, UAN 20, UAN 22, UAN 10-1, UAN 29, UAN 19, UAN 30, UAN 16, UAN 18, UAN 21-1, UAN 10-2, 2MQ2, 3Q3, 6Q6, 7Q7, 10, CONEJA, Q12, DMS | [142] |
| USA | L: 15.92 mg GAE/g (Jamaica) H: 19.25 mg GAE/g (Senegal) | N/A | N/A | 3 accessions from Jamaica, Senegal, Malaysia | [127] |
| Mexico | N/A | N/A | L: 1.71 mg Cy-3-S/g, mg Dep-sam/g (Rosa) H: 6.56 mg Cy-3-S/g, 23.74 mg Dep-sam/g (Sudan) nd: Blanca | 4 cultivars Negra, Sudan, Rosa, Blanca | [106] |
| Mexico | L: 24.00 mg GAE/g (Blanca) H: 100.00 mg GAE/g (Real) | L: 4.19 mg QE/g (Talpa) H: 22.6 mg QE/g (Reyna) | L: 0.00 mg Cy-cl/g, 0.00 mgDep-cl/g (JB 00001 SM) H: 8.73 mg Cy-cl/g, 35.35 mgDep-cl/g (Tempranilla) nd: Reyna | 25 cultivars Americana, Tepalcatepec, Diamante, Colima, Tempranilla, Talpa, Violenta, Quesería, CriollaTala, Tecoman, El Bordo, Tempranilla, Sudan, Media Luna, Pisila/Colima, JB 00001 SM, JCP 0001 T, Mutante Blanca, JJ 00001 SM, JR 00001 C, Americana, Puerta de Anzar, Variedad Blanca, Real, Reyna | [122] |
| India | L: 134.32 mg GAE/g (Red) H: 154.60 mg GAE/g (White) | N/A | N/A | 2 cultivars red and white | [143] |
| Mexico | L: 13.50 mg GAE/g (Alma Blanca, White) H: 36.50 mg GAE/g (Sudan, Dark-red) | N/A | L: 0.20 mg Cy-3-G/g Alma (Blanca, white) H: 10.99 mg Cy-3-G/g (Sudan, dark-red) | 3 cultivars Alma Blanca (white), Criolla Nayarit (light red), Sudan (dark red) | [118] |
| Mexico | L: 2.80 mg GAE/g (Criolla) H: 6.80 mg GAE/g (China) | N/A | N/A | 4 cultivars Criolla, Rosalis, Tecoanapa, China | [144] |
| Mexico | N/A | N/A | L: 1.14 mg Cy-3-G/g (China) vH: 7.24 mg Cy-3-G/g (Reyna) | 3 cultivars in different maturing stages Criolla/Creole, Reina/Queen, China | [93] |
| Turkey | L: 16.40 mg GAE/g (Turkey/Kızılay) H: 49.1 mg GAE/g (Turkey/Söğütözü) | N/A | N/A | 8 regions Kızılay, Söğütözü, Tunalı Hilmi, Kızılay, Ulus, Kızılay, Ulus, Söğütözü | [36] |
| Country | Cultivar | Phenolic Acids (mg/g) | Ref | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GlA | PhA | SyA | VaA | GeA | 4-HyA | ChA | CfA | FeA | CoA | p-Ca | 3-p-CA | 5-p-CA | 3-O-CfA | 4-O-CfA | 5-CfA | |||
| Iran | N/A | 1.09 | 5.08 | 0.07 | [34] | |||||||||||||
| Thailand | N/A | Nd–5.76 | 0.09–13.84 | 2.23–10.84 | [3] | |||||||||||||
| Malaysia | UKMR-2 | 5.11 | 0.76 | [123] | ||||||||||||||
| Malaysia | UMKL-1 | 4.06 | 1.58 | [123] | ||||||||||||||
| Malaysia | UKMR-2 | 0.63 | 0.2 | [31] | ||||||||||||||
| Brazil | N/A | 0.07 | 0.04 | 0.79 | 0.01 | 0.68 | [102] | |||||||||||
| Thailand | N/A | 2.44 | 0.72 | 0.74 | [92] | |||||||||||||
| Egypt | N/A | 0.91 | 1.63 | [90] | ||||||||||||||
| Nigeria | N/A | 0.23–0.35 | 0.14–0.18 | 0.30–0.35 | 3.19–4.90 | [30] | ||||||||||||
| Nigeria | N/A | 67.12 | 15.38 | [97] | ||||||||||||||
| Portugal | N/A | 2.60 | 1.44 | 1.53 | [99] | |||||||||||||
| Brazil | Alma Blanca | nd | 0.26 | nd | 0.30 | 1.88 | 0.49 | 0.01 | nd | nd | [118] | |||||||
| Thailand | N/A | 0.75 | 0.74 | 0.39 | 0.06 | [81] | ||||||||||||
| Thailand | N/A | 0.34 | 0.13 | [114] | ||||||||||||||
| Jordan | N/A | 0.38 | 0.42 | 0.01 | 0.20 | 3.00 × 10−3 | 1.40 × 10−3 | 0.01 | 0.11 | [35] | ||||||||
| Country | Flavonoids | Ref | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cth | Epc | Epg | EpgG | Kmf | K3-O-R | Myr | Myr-3-S | Myr-3-O-AS | Qrc | Qrc3-O-G | Qrc3-O-S | Qrc3-O-R | Qrc-pe | Rtn | Qrin | Hptn | Hpdn | ||
| Iran | 0.92 | 0.16 | 0.51 | 0.14 | [34] | ||||||||||||||
| Thailand | 0.57–2.03 | [3] | |||||||||||||||||
| Brazil | 0.02 | 0.04 | 0.01 | 0.09 | 0.08 | [102] | |||||||||||||
| Nigeria | 0.28–0.35 | 0.21–0.24 | [30] | ||||||||||||||||
| Nigeria | 53.21 | 12.75 | 3.51 | 15.03 | 12.29 | 16.25 | 3.86 | [97] | |||||||||||
| Portugal | 1.03 | tr | 0.96 | tr | 1.07 | [99] | |||||||||||||
| Thailand | 0.45 | [81] | |||||||||||||||||
| Country | Cultivar | Anthocyanin | Ref | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cy-3-O-S | Cy-3-O-G | Cy-3,5-O-Dg | Cy-3-O-6Sg | Cy-3-O-R | Cy-3-O-Dmg | Cy-3-O-Dxg | Dep-3-O-S | Dep-3-O-G | Dep-3-O-A | Dep-3-O-Fg | Dep-3,5-O-Dg | Mal-3-G | Pet-3-5-O-Dg | Pet-3-O-G | Pet-3-O-Gs | Pel-3-O-G | Cy-cl | Dep-cl | |||
| Thailand | N/A | nd-0.56 | [3] | ||||||||||||||||||
| Malaysia | UKMR-2 | 8.80 | 18.98 | [123] | |||||||||||||||||
| Malaysia | UMKL-1 | 2.91 | 7.10 | [123] | |||||||||||||||||
| Malaysia | UKMR-2 | 1.39 | 3.18 | [31] | |||||||||||||||||
| Portugal | N/A | 32.39 | [79] | ||||||||||||||||||
| Brazil | N/A | 0.70 | 2.18 | [102] | |||||||||||||||||
| Nigeria | 3.06–5.17 | 16.10–21.20 | 0.42–0.76 | [30] | |||||||||||||||||
| Mexico | N/A | 1.10 × 10−3 | 6.10 × 10−3 | 1.81 × 10−3 | 1.00 | 5.90 × 10−3 | 3.40 × 10−3 | 0.04 | 3.40 × 10−3 | 2.70 × 10−3 | 3.10 × 10−3 | 1.00 × 10−3 | 7 × 10−4 | 1.40 × 10−3 | 2.17 × 10−3 | [55] | |||||
| Mexico | Negra | 96.58 | 202.09 | [73] | |||||||||||||||||
| Mexico | Sudan | 88.71 | 224.96 | [73] | |||||||||||||||||
| Mexico | Rosa | 14.26 | 27.46 | [73] | |||||||||||||||||
| Portugal | N/A | 4.08 | 7.00 | 1.30 | [99] | ||||||||||||||||
| South Africa | N/A | 0.77 | 0.69 | [70] | |||||||||||||||||
| Saudi Arabia | N/A | 3.81 | 0.46 | 4.11 | 0.15 | [58] | |||||||||||||||
| Mexico | N/A | 2.77 | [117] | ||||||||||||||||||
| Mexico | N/A | 0.75 | 1.33 | 0.08 | [59] | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hapsari, B.W.; Manikharda; Setyaningsih, W. Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health. Horticulturae 2021, 7, 35. https://doi.org/10.3390/horticulturae7020035
Hapsari BW, Manikharda, Setyaningsih W. Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health. Horticulturae. 2021; 7(2):35. https://doi.org/10.3390/horticulturae7020035
Chicago/Turabian StyleHapsari, Bety W., Manikharda, and Widiastuti Setyaningsih. 2021. "Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health" Horticulturae 7, no. 2: 35. https://doi.org/10.3390/horticulturae7020035
APA StyleHapsari, B. W., Manikharda, & Setyaningsih, W. (2021). Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health. Horticulturae, 7(2), 35. https://doi.org/10.3390/horticulturae7020035







