Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Experimental Design
2.3. Post-Harvest Storage
2.4. Determinations
2.4.1. Biomass Production
2.4.2. Selenium, Iodine, and Nitrate Analyses
2.4.3. Leaf Photosynthetic Pigments, Flavonoids, Total Phenols, and Total Antioxidant Capacity
2.5. Contribution to Se and I Dietary Intake and Health Risk Assessment
- C = Se or I concentration (µg kg−1 FW) in the lettuce leaves
- SP = a serving size of 100 g of lettuce leaves.
- EDI = as defined above;
- UL = tolerable upper intake level of Se (300 µg day−1) and I (600 µg day−1) [34].
2.6. Statistical Analysis
3. Results
3.1. Selenium and Iodine Content
3.2. Biomass Production
3.3. Qualitative Characteristics of Leaves
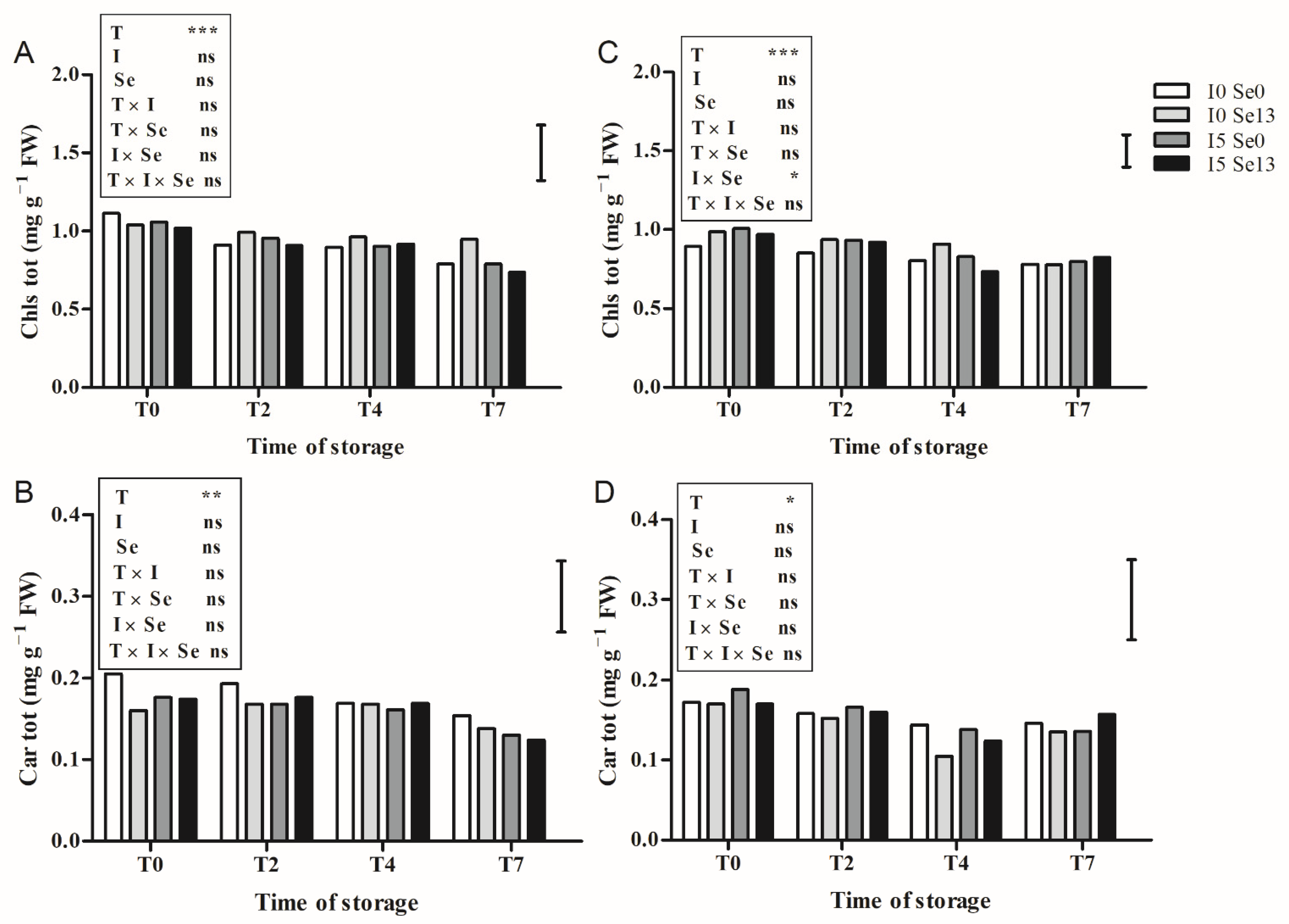
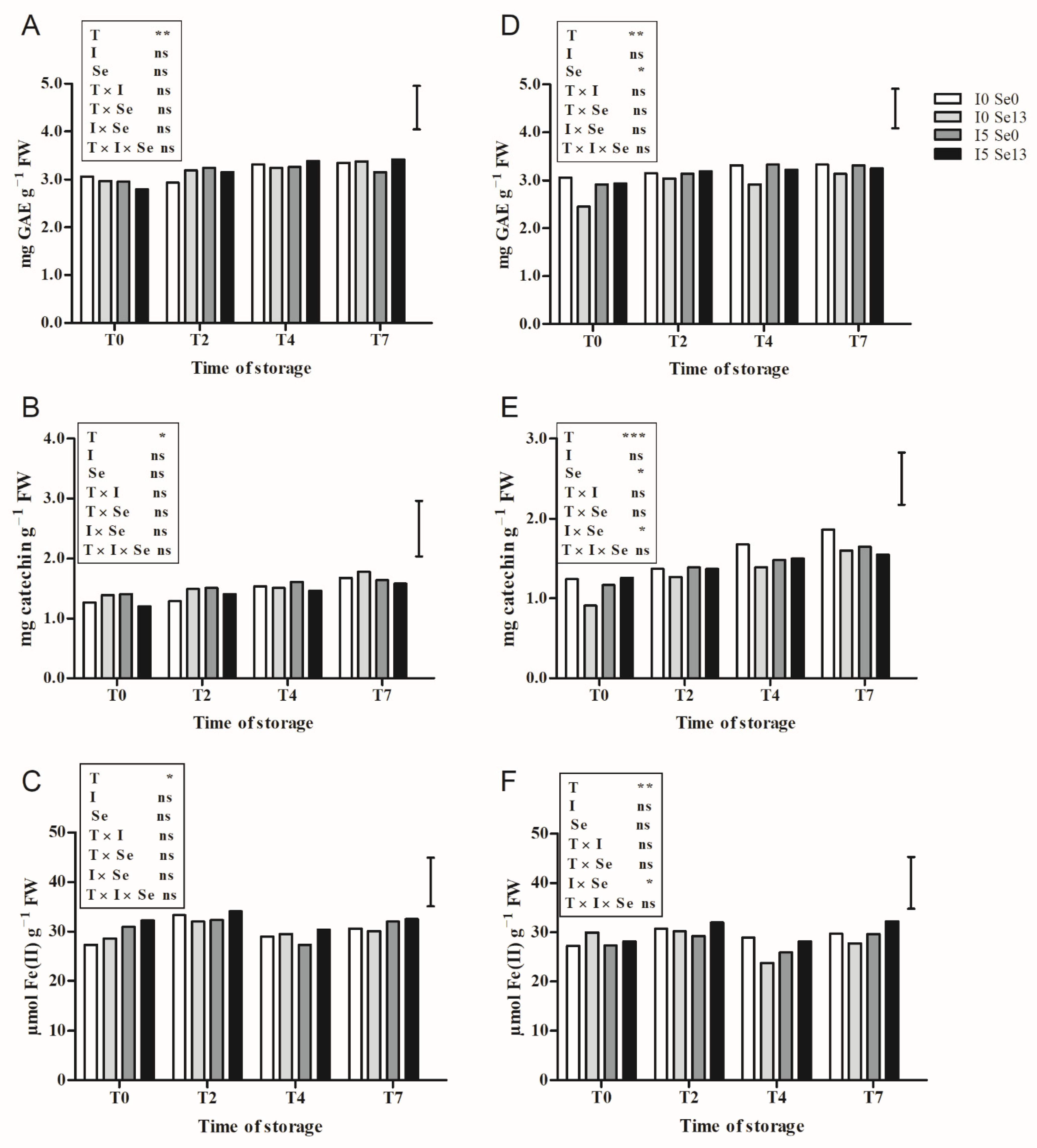
3.4. Qualitative Characteristics of Leaves during Post-Harvest Storage
3.4.1. Floating System
3.4.2. Aeroponics
4. Discussion
4.1. Selenium and Iodine Content
4.2. Effect of Biofortification on Biomass Production and Leaf Quality
4.3. Effect of Cultivation System on Biomass Production and Leaf Quality
4.4. Effects of Se and I Biofortification on Lettuce Leaf Quality during Post-Harvest Storage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets–Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Aburto, N.J.; Abudou, M.; Candeias, V.; Wu, T. Effect of Salt Iodization to Prevent Iodine Deficiency Disorders: A Systematic Review with Meta-Analyses; WHO: Geneva, Switzerland; ISBN 978 92 4 150828 5.
- EFSA Scientific Opinion on Dietary Reference Values for Iodine. EFSA J. 2014, 12, 3660.
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Ríos, J.J.; Rosales, M.A.; Blasco, B.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Biofortification of Se and Induction of the Antioxidant Capacity in Lettuce Plants. Sci. Hortic. 2008, 116, 248–255. [Google Scholar] [CrossRef]
- Malorgio, F.; Diaz, K.E.; Ferrante, A.; Mensuali-Sodi, A.; Pezzarossa, B. Effects of Selenium Addition on Minimally Processed Leafy Vegetables Grown in a Floating System. J. Sci. Food Agric. 2009, 89, 2243–2251. [Google Scholar] [CrossRef]
- Bian, Z.-H.; Lei, B.; Cheng, R.-F.; Wang, Y.; Li, T.; Yang, Q.-C. Selenium Distribution and Nitrate Metabolism in Hydroponic Lettuce (Lactuca sativa L.): Effects of Selenium Forms and Light Spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; de Pascale, S.; Rouphael, Y. Combating Micronutrient Deficiency and Enhancing Food Functional Quality Through Selenium Fortification of Select Lettuce Genotypes Grown in a Closed Soilless System. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef] [Green Version]
- Duma, M.; Alsina, I.; Dubova, L.; Stroksa, L.; Smiltina, Z. The Effect of Sodium Selenite and Selenate on the Quality of Lettuce. In Proceedings of the 6th Baltic Conference on Food Science and Technology: Innovations for Food Science and Production, FOODBALT-2011–Conference Proceedings, Jelgava, Latvia, 5–6 May 2011; pp. 39–44. [Google Scholar]
- EFSA Scientific Opinion on Dietary Reference Values for Selenium. EFSA J. 2014, 12, 3846. [CrossRef]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine Biofortification of Crops: Agronomic Biofortification, Metabolic Engineering and Iodine Bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bañuelos, G.S.; Lin, Z.Q. Use and Development of Biofortified Agricultural Products; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-6005-8. [Google Scholar]
- Díaz-Gómez, J.; Twyman, R.M.; Zhu, C.; Farré, G.; Serrano, J.C.E.; Portero-Otin, M.; Muñoz, P.; Sandmann, G.; Capell, T.; Christou, P. Biofortification of Crops with Nutrients: Factors Affecting Utilization and Storage. Curr. Opin. Biotechnol. 2016, 44, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopsell, D.A.; Kopsell, D.E. Selenium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 515–549. [Google Scholar]
- Voogt, W.; Holwerda, H.T.; Khodabaks, R. Biofortification of Lettuce (Lactuca sativa L.) with Iodine: The Effect of Iodine Form and Concentration in the Nutrient Solution on Growth, Development and Iodine Uptake of Lettuce Grown in Water Culture. J. Sci. Food Agric. 2010, 90, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Photorespiration Process and Nitrogen Metabolism in Lettuce Plants (Lactuca sativa L.): Induced Changes in Response to Iodine Biofortification. J. Plant Growth Regul. 2010, 29, 477–486. [Google Scholar] [CrossRef]
- Puccinelli, M.; Landi, M.; Maggini, R.; Pardossi, A.; Incrocci, L. Iodine Biofortification of Sweet Basil and Lettuce Grown in Two Hydroponic Systems. Sci. Hortic. 2021, 276, 109783. [Google Scholar] [CrossRef]
- Kowalska, I.; Smoleń, S.; Czernicka, M.; Halka, M.; Kęska, K.; Pitala, J. Effect of Selenium Form and Salicylic Acid on the Accumulation of Selenium Speciation Forms in Hydroponically Grown Lettuce. Agriculture 2020, 10, 584. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Huang, Y.; Hu, Y.; Liu, Y.; Christie, P. Interactions between Selenium and Iodine Uptake by Spinach (Spinacia Oleracea L.) in Solution Culture. Plant Soil 2004, 261, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of Biofortification with Iodine and Selenium of Lettuce Cultivated in the NFT Hydroponic System. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Smoleń, S.; Skoczylas, Ł.; Ledwożyw-Smoleń, I.; Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Pysz, M.; Koronowicz, A.; Kapusta-Duch, J.; et al. Iodine and Selenium Biofortification of Lettuce (Lactuca sativa L.) by Soil Fertilization with Various Compounds of These Elements. Acta Sci. Pol. Hortorum Cultus 2016, 15, 69–91. [Google Scholar]
- Smoleń, S.; Kowalska, I.; Kováčik, P.; Halka, M.; Sady, W. Biofortification of Six Varieties of Lettuce (Lactuca sativa L.) with Iodine and Selenium in Combination with the Application of Salicylic Acid. Front. Plant Sci. 2019, 10, 143. [Google Scholar] [CrossRef] [Green Version]
- Pardossi, A.; Malorgio, F.; Incrocci, L.; Tognoni, F. Hydroponic Technologies for Greenhouse Crops. In Crops: Quality, Growth and Biotechnology; WFL Publisher: Helsinki, Finland, 2006; Volume 23, pp. 360–378. [Google Scholar]
- Alshrouf, A. Hydroponics, Aeroponic and Aquaponic as Compared with Conventional Farming. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 247–255. [Google Scholar]
- Saini, R.K.; Ko, E.Y.; Keum, Y.-S. Minimally Processed Ready-to-Eat Baby-Leaf Vegetables: Production, Processing, Storage, Microbial Safety, and Nutritional Potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Zasoski, R.J.; Burau, R.G. A Rapid Nitric-perchloric Acid Digestion Method for Multi-element Tissue Analysis. Commun. Soil Sci. Plant Anal. 1977, 8, 425–436. [Google Scholar] [CrossRef]
- CEN EN 15111:2007; Foodstuffs–Determination of Trace Elements–Determination of Iodine by ICP-MS (Inductively Coupled Plasma Mass Spectrometry); European Committee for Standardization: Brussels, Belgium, 2007.
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Welburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Carotenoids and Chlorophyll a and b of Leaf Extracts in Different Solvents. In Advances in Photosyntesis Research; Springer: Dordrecht, The Netherlands, 1984; Volume II. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Tolerable Upper Intake Level for Vitamins and Minerals; EFSA: Parma, Italy, 2006. [Google Scholar]
- Li, L.; Chen, S.; Yang, C.; Meng, F.; Sigrimis, N. Prediction of Plant Transpiration from Environmental Parameters and Relative Leaf Area Index Using the Random Forest Regression Algorithm. J. Clean. Prod. 2020, 261, 121136. [Google Scholar] [CrossRef]
- Shi, K.; Ding, X.T.; Dong, D.K.; Zhou, Y.H.; Yu, J.Q. Root Restriction-Induced Limitation to Photosynthesis in Tomato (Lycopersicon Esculentum Mill.) Leaves. Sci. Hortic. 2008, 117, 197–202. [Google Scholar] [CrossRef]
- Blok, C.; Jackson, B.E.; Guo, X.; de Visser, P.H.B.; Marcelis, L.F.M. Maximum Plant Uptakes for Water, Nutrients, and Oxygen Are Not Always Met by Irrigation Rate and Distribution in Water-Based Cultivation Systems. Front. Plant Sci. 2017, 8, 562. [Google Scholar] [CrossRef] [Green Version]
- Ferrarese, M.; Mahmoodi Sourestani, M.; Quattrini, E.; Schiavi, M.; Ferrante, A. Biofortification of Spinach Plants Applying Selenium in the Nutrient Solution of Floating System. Veg. Crop. Res. Bull. 2012, 76, 127–136. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Gonnella, M.; Renna, M.; D’Imperio, M.; Santamaria, P.; Serio, F.; Gonnella, M.; Renna, M.; D’Imperio, M.; Santamaria, P.; Serio, F. Iodine Biofortification of Four Brassica Genotypes Is Effective Already at Low Rates of Potassium Iodate. Nutrients 2019, 11, 451. [Google Scholar] [CrossRef] [Green Version]
- Kiferle, C.; Ascrizzi, R.; Martinelli, M.; Gonzali, S.; Mariotti, L.; Pistelli, L.; Flamini, G.; Perata, P. Effect of Iodine Treatments on Ocimum basilicum L.: Biofortification, Phenolics Production and Essential Oil Composition. PLoS ONE 2019, 14, 1–23. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The Dual Effects of Two Inorganic Selenium Forms on the Growth, Selected Physiological Parameters and Macronutrients Accumulation in Cucumber Plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef] [Green Version]
- Smoleń, S.; Baranski, R.; Ledwożyw-Smoleń, I.; Skoczylas, Ł.; Sady, W. Combined Biofortification of Carrot with Iodine and Selenium. Food Chem. 2019, 300, 125202. [Google Scholar] [CrossRef]
- Smoleń, S.; Wierzbińska, J.; Sady, W.; Kołton, A.; Wiszniewska, A.; Liszka-Skoczylas, M. Iodine Biofortification with Additional Application of Salicylic Acid Affects Yield and Selected Parameters of Chemical Composition of Tomato Fruits (Solanum lycopersicum L.). Sci. Hortic. 2015, 188, 89–96. [Google Scholar] [CrossRef]
- Mezeyova, I.; Hegedusova, A.; Andrejiová, A.; Mezeyová, I.; Hegedűsová, A.; Hegedűs, O.; Golian, M. Phytomass and Content of Essential Oils in Ocimum Basilicum after Foliar Treatment with Selenium. J. Int. Sci. Publ. 2016, 4, 19–27. [Google Scholar]
- EFSA Statement on Possible Public Health Risks for Infants and Young Children from the Presence of Nitrates in Leafy Vegetables. EFSA J. 2010, 8, 1935. [CrossRef]
- Tomasi, N.; Pinton, R.; Gottardi, S.; Mimmo, T.; Scampicchio, M.; Cesco, S. Selenium Fortification of Hydroponically Grown Corn Salad (Valerianella locusta). Crop. Pasture Sci. 2015, 66, 1128–1136. [Google Scholar] [CrossRef]
- Hernández-Castro, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Rodríguez-Mendoza, M.N.; Sánchez-García, P.; Robledo-Paz, A. Bioaccumulation of Iron, Selenium, Nitrate, and Proteins in Chard Shoots. J. Soil Sci. Plant Nutr. 2015, 15, 694–710. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Maggini, R.; Rosellini, I.; Pezzarossa, B. Biofortification of Ocimum basilicum L. Plants with Selenium. Acta Hortic. 2019, 1242, 663–670. [Google Scholar] [CrossRef]
- Strzetelski, P.; Smoleń, S.; Rożek, S.; Sady, W. The Effect of Diverse Iodine Fertillization on Nitrate Accumulation and Content of Selected Compounds in Radish Plants (Raphanus sativus L.). Hortorum Cultus 2010, 9, 65–73. [Google Scholar]
- Sarrou, E.; Siomos, A.S.; Riccadona, S.; Aktsoglou, D.C.; Tsouvaltzis, P.; Angeli, A.; Franceschi, P.; Chatzopoulou, P.; Vrhovsek, U.; Martens, S. Improvement of Sea Fennel (Crithmum maritimum L.) Nutritional Value through Iodine Biofortification in a Hydroponic Floating System. Food Chem. 2019, 296, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Sady, W. Influence of Iodine Form and Application Method on the Effectiveness of Iodine Biofortification, Nitrogen Metabolism as Well as the Content of Mineral Nutrients and Heavy Metals in Spinach Plants (Spinacia oleracea L.). Sci. Hortic. 2012, 143, 176–183. [Google Scholar] [CrossRef]
- Eveleens, B.; Blok, C. Cultivation of Chrysanthemum without Substrate. Acta Hortic. 2014, 1034, 185–192. [Google Scholar] [CrossRef]
- Godfrey, M. The Quick Reference Guide for Hydroponic Farmers–Upstart University. Available online: https://university.upstartfarmers.com/blog/the-quick-reference-guide-for-hydroponic-farmers (accessed on 1 September 2020).
- Ercan, N.; Bayyurt, R. The Effects of Applications Which Increase the O2 of the Water on Yield and Quality of Lettuce Grown in a Floating System. Acta Hortic. 2014, 1034, 77–84. [Google Scholar] [CrossRef]
- Devienne-Barret, F.; Justes, E.; Machet, J.M.; Mary, B. Integrated Control of Nitrate Uptake by Crop Growth Rate and Soil Nitrate Availability under Field Conditions. Ann. Bot. 2000, 86, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Manzocco, L.; Foschia, M.; Tomasi, N.; Maifreni, M.; Dalla Costa, L.; Marino, M.; Cortella, G.; Cesco, S. Influence of Hydroponic and Soil Cultivation on Quality and Shelf Life of Ready-to-Eat Lamb’s Lettuce (Valerianella locusta L. Laterr). J. Sci. Food Agric. 2011, 91, 1373–1380. [Google Scholar] [CrossRef]
- Nam, S.Y. Color Changes of Leaf Lettuce during Postharvest Storage. HortScience 2000, 35, 410. [Google Scholar] [CrossRef] [Green Version]
- Agüero, M.V.; Barg, M.V.; Yommi, A.; Camelo, A.; Roura, S.I. Postharvest Changes in Water Status and Chlorophyll Content of Lettuce (Lactuca sativa L.) and Their Relationship with Overall Visual Quality. J. Food Sci. 2007, 73, S47–S55. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Antioxidant Activity and Total Phenolics in Post-Harvest Iceberg Lettuce (Lactuca sativa). In Proceedings of the ISHS Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2003; Volume 628, pp. 687–691. [Google Scholar]
- Ferrante, A.; Incrocci, L.; Maggini, R.; Serra, G.; Tognoni, F. Colour Changes of Fresh-Cut Leafy Vegetables during Storage. J. Food Agric. Environ. 2004, 22, 40–44. [Google Scholar]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Selenium—An Antioxidative Protectant in Soybean during Senescence. Plant Soil 2005, 272, 77–86. [Google Scholar] [CrossRef]
| Hydroponic System | I Added (µM) | Se Added (µM) | I (mg kg−1 DW) | Se (mg kg−1 DW) | FW (g m−2) | DW (g m−2) | DW/FW (%) | NO3 (mg kg−1 FW) |
|---|---|---|---|---|---|---|---|---|
| FS | 0 | 0 | 4.70 | 0.09 | 174.2 c | 6.3 | 3.63 | 924.7 |
| 13 | 2.46 | 3.33 | 198.3 c | 7.9 | 3.99 | 1135.8 | ||
| 5 | 0 | 86.30 | 0.03 | 165.8 c | 6.6 | 3.99 | 1066.0 | |
| 13 | 72.55 | 3.44 | 193.8 c | 5.7 | 2.92 | 755.6 | ||
| A | 0 | 0 | 4.02 | 0.10 | 478.1 b | 16.5 | 3.49 | 1012.5 |
| 13 | 2.45 | 6.54 | 735.0 a | 20.9 | 2.84 | 499.4 | ||
| 5 | 0 | 65.43 | 0.15 | 598.3 b | 19.6 | 3.31 | 1248.1 | |
| 13 | 71.90 | 6.46 | 538.4 b | 17.2 | 3.24 | 601.1 | ||
| Mean effect | ||||||||
| FS | 41.50 | 1.72 b | 183.0 b | 6.6 b | 3.63 | 970.5 a | ||
| AE | 35.95 | 3.31 a | 587.5 a | 18.6 a | 3.22 | 840.3 b | ||
| 0 | 3.37 b | 2.67 | 438.4 | 14.1 | 3.42 | 865.7 | ||
| 5 | 72.97 a | 2.68 | 412.9 | 13.5 | 3.35 | 919.1 | ||
| 0 | 39.03 | 0.10 b | 390.9 b | 13.4 | 3.56 | 1076.3 a | ||
| 13 | 37.3 | 5.25 a | 460.4 a | 14.1 | 3.21 | 708.5 b | ||
| FS | 0 | 3.58 | 1.71 | 186.3 | 7.1 | 3.81 | 1030.2 a | |
| 5 | 79.43 | 1.73 | 179.8 | 6.1 | 3.46 | 910.8 ab | ||
| AE | 0 | 3.23 | 3.32 | 606.5 | 18.7 | 3.17 | 756.0 b | |
| 5 | 68.67 | 3.31 | 568.4 | 18.4 | 3.27 | 924.6 ab | ||
| FS | 0 | 45.5 | 0.06 c | 170.0 c | 6.5 | 3.81 | 995.4 a | |
| 13 | 37.51 | 3.38 b | 196.0 c | 6.8 | 3.46 | 945.8 a | ||
| AE | 0 | 24.37 | 0.13 c | 538.2 b | 18.1 | 3.40 | 1130.3 a | |
| 13 | 37.18 | 6.50 a | 636.7 a | 19.0 | 3.04 | 550.4 b | ||
| 0 | 0 | 4.29 | 0.09 | 356.5 b | 12.4 b | 3.55 | 977.4 ab | |
| 13 | 2.45 | 5.25 | 520.3 a | 15.7 a | 3.30 | 753.9 bc | ||
| 5 | 0 | 73.78 | 0.10 | 425.3 ab | 14.4 ab | 3.58 | 1175.3 a | |
| 13 | 72.16 | 5.25 | 400.6 ab | 12.6 b | 3.11 | 663.0 b | ||
| Significance | ||||||||
| HS | ns | *** | *** | *** | ns | * | ||
| I | *** | ns | ns | ns | ns | ns | ||
| Se | ns | *** | * | ns | ns | *** | ||
| HS × I | ns | ns | ns | ns | ns | * | ||
| HS × Se | ns | *** | * | ns | ns | *** | ||
| I × Se | ns | ns | ** | ** | ns | * | ||
| HS × I × Se | ns | ns | * | ns | ns | ns | ||
| Hydroponic System | I Added (µM) | Se Added (µM) | I (mg kg−1 DW) | Se (mg kg−1 DW) | FW (g m−2) | DW (g m−2) | DW/FW (%) | NO3 (mg kg−1 FW) |
|---|---|---|---|---|---|---|---|---|
| FS | 0 | 0 | 6.18 | 0.00 | 183.3 | 7.3 | 4.0 | 915.8 |
| 13 | 4.02 | 4.84 | 179.5 | 7.1 | 3.9 | 950.9 | ||
| 5 | 0 | 140.5 | 0.00 | 187.2 | 7.4 | 4.0 | 779.2 | |
| 13 | 138.0 | 4.24 | 185.9 | 6.9 | 3.7 | 836.3 | ||
| A | 0 | 0 | 4.10 | 0.00 | 326.7 | 13.6 | 4.2 | 846.7 |
| 13 | 3.75 | 5.07 | 407.9 | 15.4 | 3.8 | 572.9 | ||
| 5 | 0 | 91.85 | 0.00 | 332.5 | 15.0 | 4.5 | 947.4 | |
| 13 | 120.00 | 5.15 | 342.3 | 13.6 | 4.0 | 711.5 | ||
| Mean effect | ||||||||
| FS | 72.17 a | 2.27 b | 184.0 b | 7.2 b | 3.9 | 870.5 a | ||
| AE | 54.92 b | 2.55 a | 352.4 a | 14.4 a | 4.1 | 769.6 b | ||
| 0 | 4.51 b | 2.49 | 292.9 | 11.6 | 4.0 | 799.2 | ||
| 5 | 122.59 a | 2.39 | 277.1 | 11.5 | 4.1 | 820.8 | ||
| 0 | 60.66 | 0.00 b | 271.8 b | 11.5 | 4.2 a | 877.2 a | ||
| 13 | 66.44 | 4.88 a | 298.2 a | 11.5 | 3.9 b | 742.8 b | ||
| FS | 0 | 5.10 c | 2.42 | 181.4 | 7.2 | 4.0 | 933.3 a | |
| 5 | 139.25 c | 2.12 | 186.5 | 7.2 | 3.9 | 807.8 ab | ||
| AE | 0 | 3.92 c | 2.53 | 367.3 | 14.5 | 4.0 | 709.8 b | |
| 5 | 105.93 b | 2.58 | 337.4 | 14.3 | 4.2 | 829.4 ab | ||
| FS | 0 | 73.34 | 0.00 c | 185.3 c | 14.3 | 4.3 | 847.5 a | |
| 13 | 71.01 | 4.54 b | 182.7 c | 14.5 | 3.9 | 893.6 a | ||
| AE | 0 | 47.97 | 0.00 c | 329.6 b | 7.4 | 4.0 | 897.0 a | |
| 13 | 61.88 | 5.11 a | 375.1 a | 7.0 | 3.8 | 642.2 b | ||
| 0 | 0 | 5.14 | 0.00 | 269.3 | 11.1 | 4.1 | 874.3 | |
| 13 | 3.89 | 4.98 | 316.6 | 12.1 | 3.8 | 724.1 | ||
| 5 | 0 | 116.18 | 0.00 | 274.4 | 12.0 | 4.3 | 880.1 | |
| 13 | 129.00 | 4.78 | 279.8 | 11.0 | 3.9 | 761.5 | ||
| Significance | ||||||||
| HS | *** | * | *** | *** | ns | * | ||
| I | *** | ns | ns | ns | ns | ns | ||
| Se | ns | *** | * | ns | ** | ** | ||
| HS × I | ** | ns | ns | ns | ns | ** | ||
| HS × Se | ns | * | * | ns | ns | *** | ||
| I × Se | ns | ns | ns | ns | ns | ns | ||
| HS × I × Se | ns | ns | ns | ns | ns | ns | ||
| Hydroponic System | I Added (µM) | Se Added (µM) | Total Phenols (mg GAE g−1 FW) | FRAP (µmol Fe (II) g−1 FW) | chl a/chl b | Chls Tot (mg g−1 FW) | Car (mg g−1 FW) |
|---|---|---|---|---|---|---|---|
| FS | 0 | 0 | 4.52 | 21.21 | 2.35 | 0.645 b | 0.108 e |
| 13 | 4.45 | 20.50 | 3.07 | 0.661 b | 0.136 cd | ||
| 5 | 0 | 4.35 | 21.36 | 2.78 | 0.654 b | 0.121 de | |
| 13 | 4.84 | 19.83 | 2.80 | 0.665 b | 0.122 de | ||
| A | 0 | 0 | 4.28 | 21.36 | 3.53 | 0.711 b | 0.160 ab |
| 13 | 4.06 | 22.44 | 3.41 | 0.639 b | 0.141 bcd | ||
| 5 | 0 | 4.42 | 25.39 | 3.36 | 0.645 b | 0.148 bc | |
| 13 | 4.26 | 20.22 | 3.40 | 0.831 a | 0.178 a | ||
| Mean effect | |||||||
| FS | 4.54 | 20.73 | 2.75 b | 0.656 b | 0.122 b | ||
| AE | 4.25 | 22.35 | 3.43 a | 0.707 a | 0.157 a | ||
| 0 | 4.29 | 21.48 | 3.17 | 0.666 | 0.139 | ||
| 5 | 4.43 | 21.92 | 3.14 | 0.707 | 0.146 | ||
| 0 | 4.38 | 22.54 | 3.09 | 0.666 | 0.138 | ||
| 13 | 4.35 | 20.87 | 3.22 | 0.706 | 0.147 | ||
| FS | 0 | 4.49 | 20.86 | 2.71 | 0.653 | 0.122 | |
| 5 | 4.59 | 20.59 | 2.79 | 0.659 | 0.121 | ||
| AE | 0 | 4.17 | 21.90 | 3.47 | 0.675 | 0.150 | |
| 5 | 4.34 | 22.81 | 3.38 | 0.738 | 0.163 | ||
| FS | 0 | 4.43 | 21.28 | 2.56 | 0.649 | 0.115 | |
| 13 | 4.64 | 20.17 | 2.94 | 0.663 | 0.129 | ||
| AE | 0 | 4.35 | 23.38 | 3.45 | 0.678 | 0.154 | |
| 13 | 4.16 | 21.33 | 3.41 | 0.735 | 0.159 | ||
| 0 | 0 | 4.37 | 21.30 | 3.06 | 0.685 | 0.139 | |
| 13 | 4.22 | 21.67 | 3.27 | 0.648 | 0.139 | ||
| 5 | 0 | 4.39 | 23.78 | 3.13 | 0.648 | 0.137 | |
| 13 | 4.49 | 20.07 | 3.16 | 0.765 | 0.155 | ||
| Significance | |||||||
| HS | ns | ns | *** | * | *** | ||
| I | ns | ns | ns | ns | ns | ||
| Se | ns | ns | ns | ns | ns | ||
| HS × I | ns | ns | ns | ns | ns | ||
| HS × Se | ns | ns | ns | ns | ns | ||
| I × Se | ns | ns | ns | ** | ns | ||
| HS × I × Se | ns | ns | ns | ** | ** | ||
| Hydroponic System | I Added (µM) | Se Added (µM) | Total Phenols (mg GAE g−1 FW) | Flavonoids (mg Catechin g−1 FW) | FRAP (µmol Fe (II) g−1 FW) | chl a/chl b | Chls Tot (mg g−1 FW) | Car (mg g−1 FW) |
|---|---|---|---|---|---|---|---|---|
| FS | 0 | 0 | 3.06 | 1.27 | 27.2 | 2.83 | 1.112 | 0.205 |
| 13 | 2.95 | 1.41 | 30.0 | 2.66 | 0.908 | 0.160 | ||
| 5 | 0 | 2.97 | 1.39 | 27.3 | 2.58 | 1.065 | 0.176 | |
| 13 | 2.80 | 1.20 | 28.2 | 2.85 | 0.952 | 0.174 | ||
| A | 0 | 0 | 3.06 | 1.24 | 28.9 | 2.87 | 0.892 | 0.172 |
| 13 | 2.45 | 0.91 | 23.8 | 2.89 | 0.918 | 0.170 | ||
| 5 | 0 | 2.92 | 1.17 | 25.9 | 2.87 | 1.009 | 0.188 | |
| 13 | 2.93 | 1.26 | 28.1 | 3.22 | 0.838 | 0.170 | ||
| Mean effect | ||||||||
| FS | 2.94 | 1.32 | 28.2 | 2.73 b | 1.009 | 0.179 | ||
| AE | 2.84 | 1.14 | 26.7 | 2.96 a | 0.914 | 0.175 | ||
| 0 | 2.88 | 1.21 | 27.5 | 2.81 | 0.957 | 0.177 | ||
| 5 | 2.90 | 1.25 | 27.4 | 2.88 | 0.966 | 0.177 | ||
| 0 | 3.00 | 1.27 | 27.3 | 2.79 | 1.019 | 0.185 | ||
| 13 | 2.78 | 1.19 | 27.5 | 2.90 | 0.904 | 0.168 | ||
| FS | 0 | 3.01 a | 1.34 | 28.6 | 2.75 | 1.010 | 0.182 | |
| 5 | 2.88 ab | 1.29 | 27.8 | 2.72 | 1.008 | 0.175 | ||
| AE | 0 | 2.76 b | 1.07 | 26.4 | 2.88 | 0.905 | 0.171 | |
| 5 | 2.93 ab | 1.22 | 27.0 | 3.04 | 0.923 | 0.179 | ||
| FS | 0 | 3.01 | 1.33 | 27.3 | 2.71 | 1.088 | 0.191 | |
| 13 | 2.87 | 1.30 | 29.1 | 2.76 | 0.930 | 0.167 | ||
| AE | 0 | 2.99 | 1.21 | 27.4 | 2.87 | 0.950 | 0.180 | |
| 13 | 2.69 | 1.08 | 26.0 | 3.05 | 0.878 | 0.170 | ||
| 0 | 0 | 3.06 | 1.25 | 28.1 | 2.85 | 1.002 | 0.189 | |
| 13 | 2.70 | 1.16 | 26.9 | 2.78 | 0.913 | 0.165 | ||
| 5 | 0 | 2.94 | 1.28 | 26.6 | 2.72 | 1.037 | 0.182 | |
| 13 | 2.86 | 1.23 | 28.2 | 3.03 | 0.895 | 0.172 | ||
| Significance | ||||||||
| HS | ns | ns | ns | *** | ns | ns | ||
| I | ns | ns | ns | ns | ns | ns | ||
| Se | ns | ns | ns | ns | ns | ns | ||
| HS × I | * | ns | ns | ns | ns | ns | ||
| HS × Se | ns | ns | ns | ns | ns | ns | ||
| I × Se | ns | ns | ns | ns | ns | ns | ||
| HS × I × Se | ns | ns | ns | ns | ns | ns | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccinelli, M.; Malorgio, F.; Incrocci, L.; Rosellini, I.; Pezzarossa, B. Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems. Horticulturae 2021, 7, 590. https://doi.org/10.3390/horticulturae7120590
Puccinelli M, Malorgio F, Incrocci L, Rosellini I, Pezzarossa B. Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems. Horticulturae. 2021; 7(12):590. https://doi.org/10.3390/horticulturae7120590
Chicago/Turabian StylePuccinelli, Martina, Fernando Malorgio, Luca Incrocci, Irene Rosellini, and Beatrice Pezzarossa. 2021. "Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems" Horticulturae 7, no. 12: 590. https://doi.org/10.3390/horticulturae7120590
APA StylePuccinelli, M., Malorgio, F., Incrocci, L., Rosellini, I., & Pezzarossa, B. (2021). Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems. Horticulturae, 7(12), 590. https://doi.org/10.3390/horticulturae7120590








