Fruit Quality and Yield of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars Grown in Two Planting Systems under Different Protected Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Environmental Conditions
2.3. Plant Volume and Fruit Harvest
2.4. Fruit Firmness and Color
2.5. Individual Sugar and Organic Acid Extraction and Determination
2.6. Individual Phenolics Extraction and Determination
2.7. Statistical Evaluation
3. Results
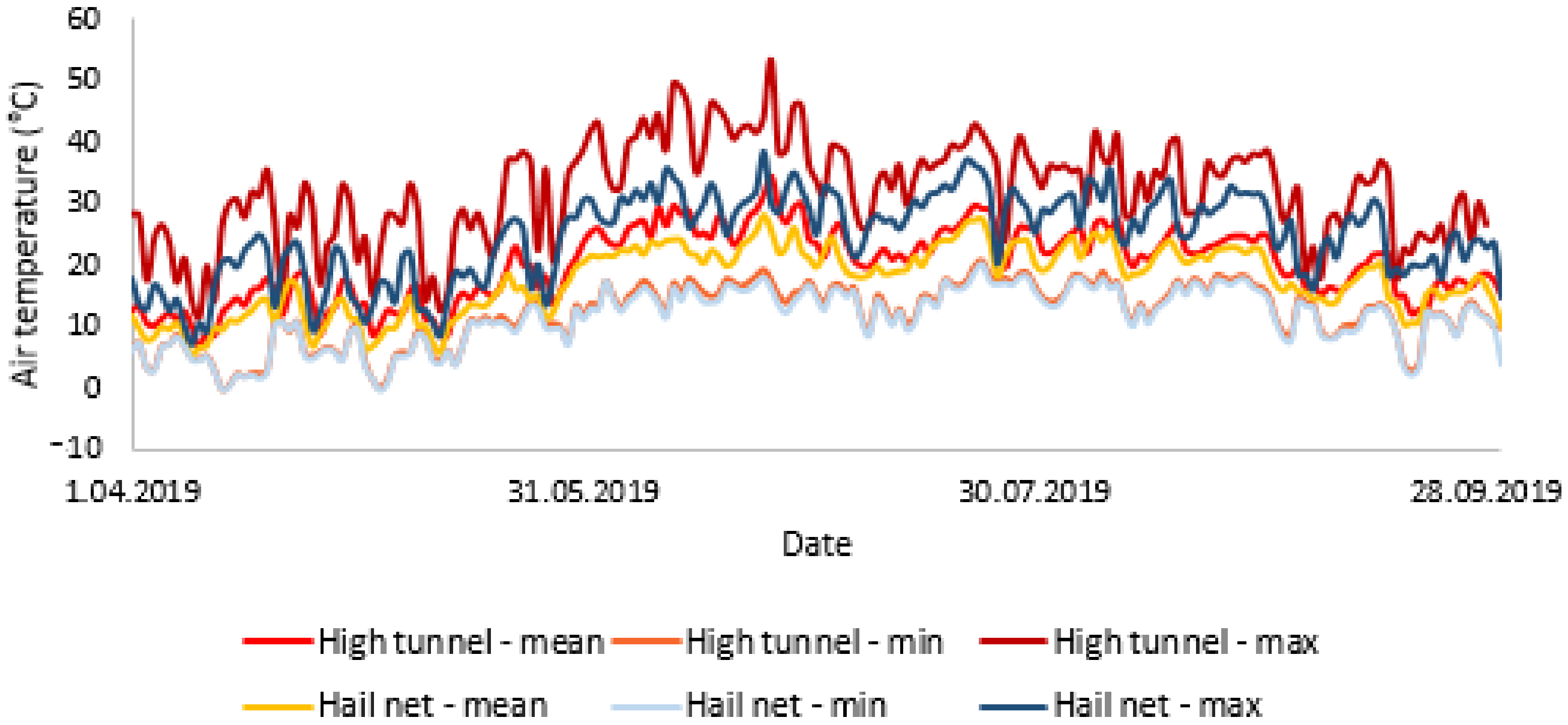
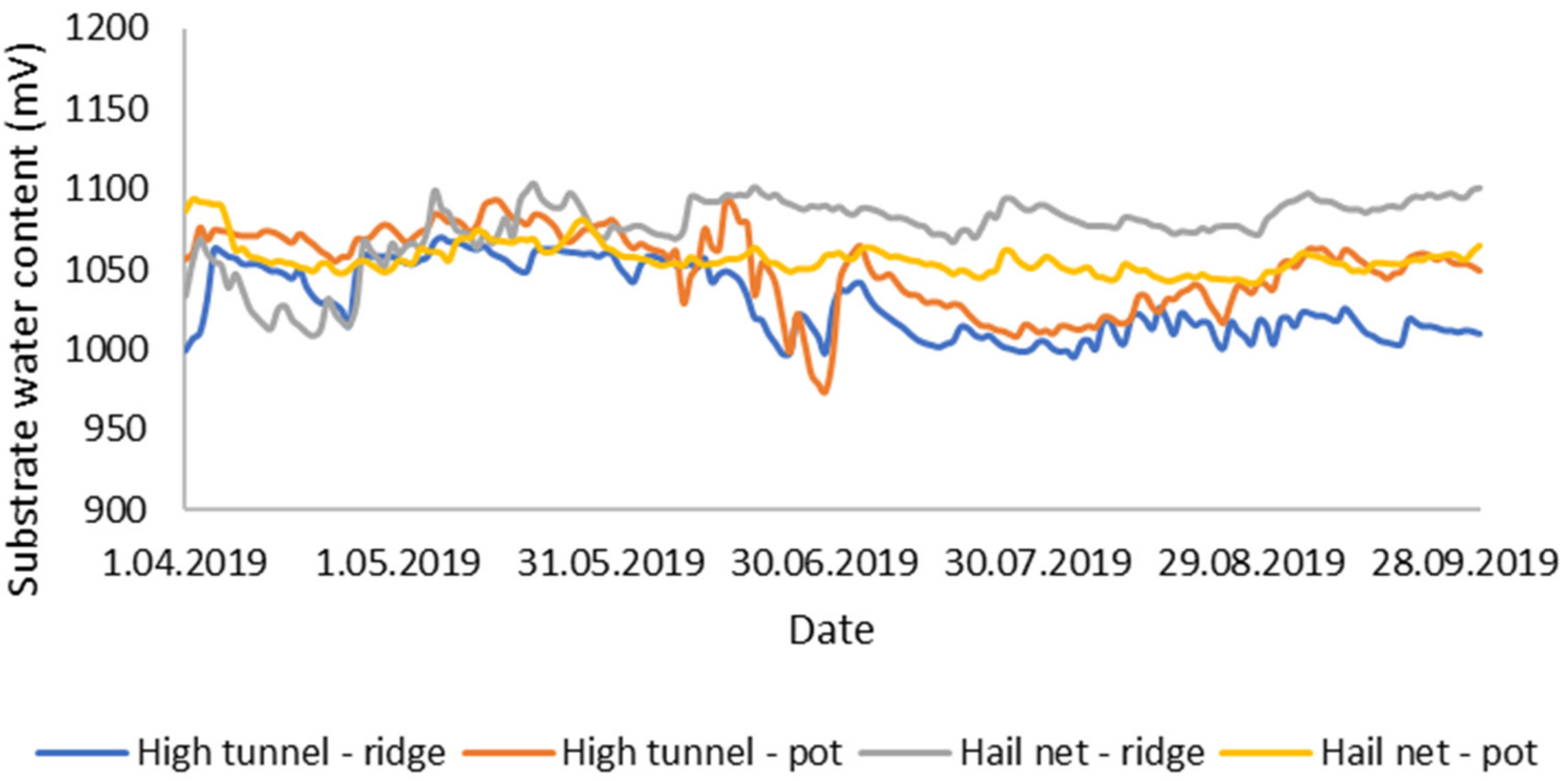
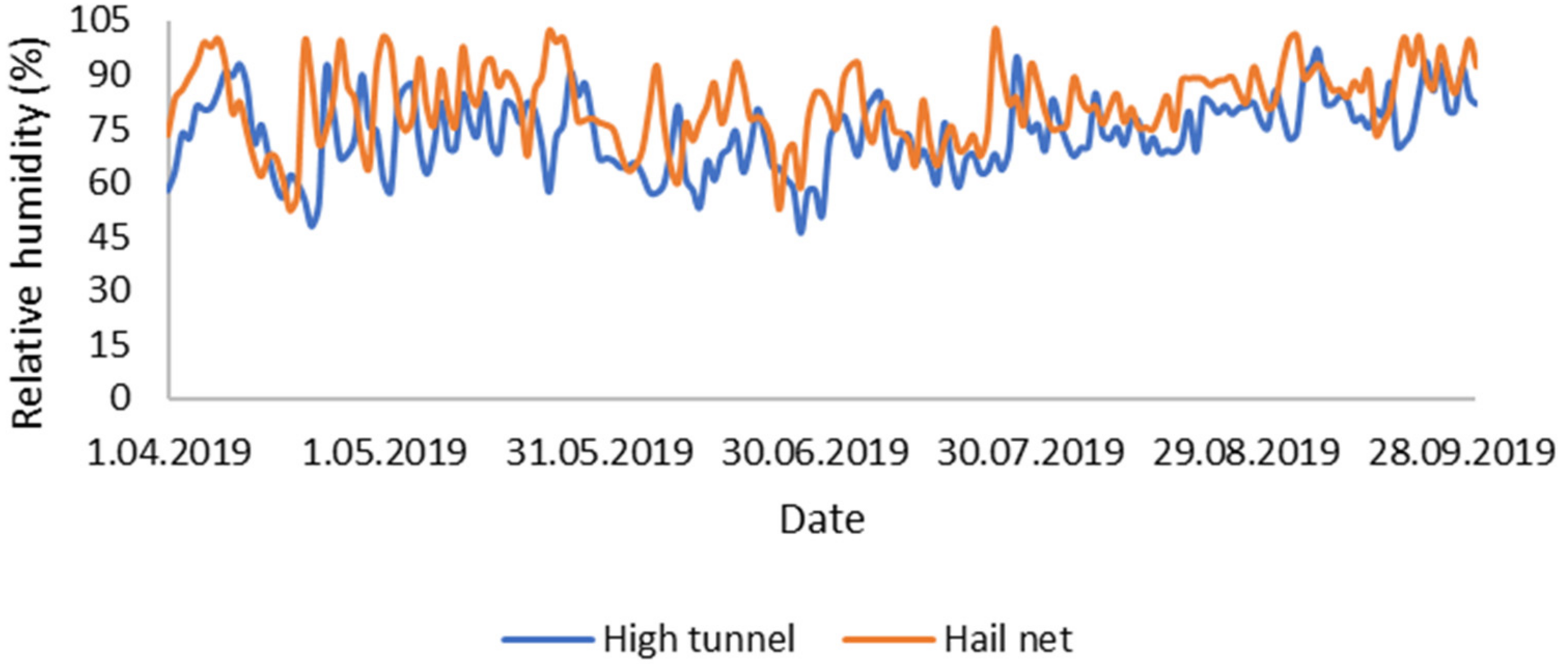
3.1. Environmental Conditions
3.2. Plant Volume and Yield
3.3. Fruit Firmness and Color
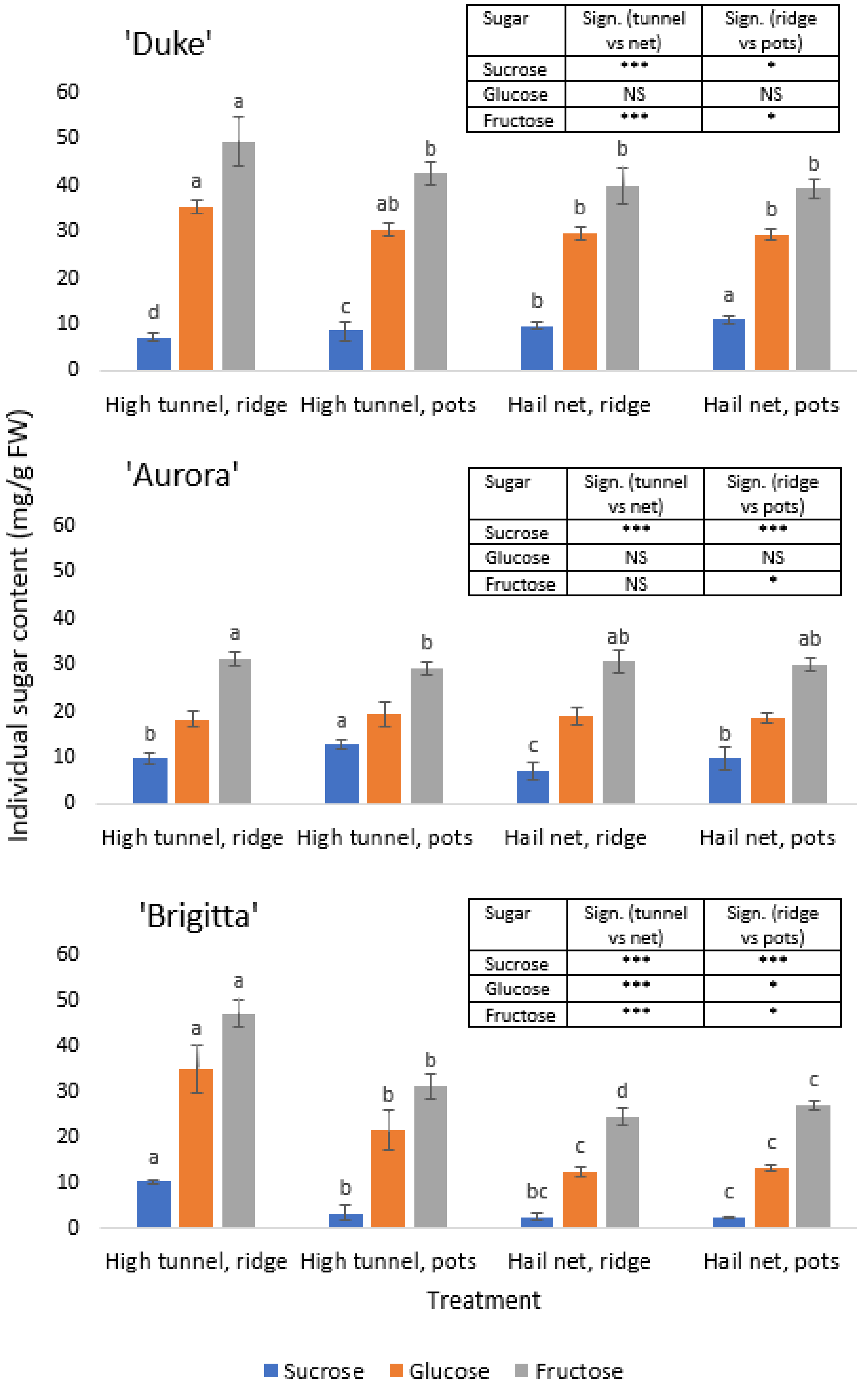
3.4. Total and Individual Sugar Contents
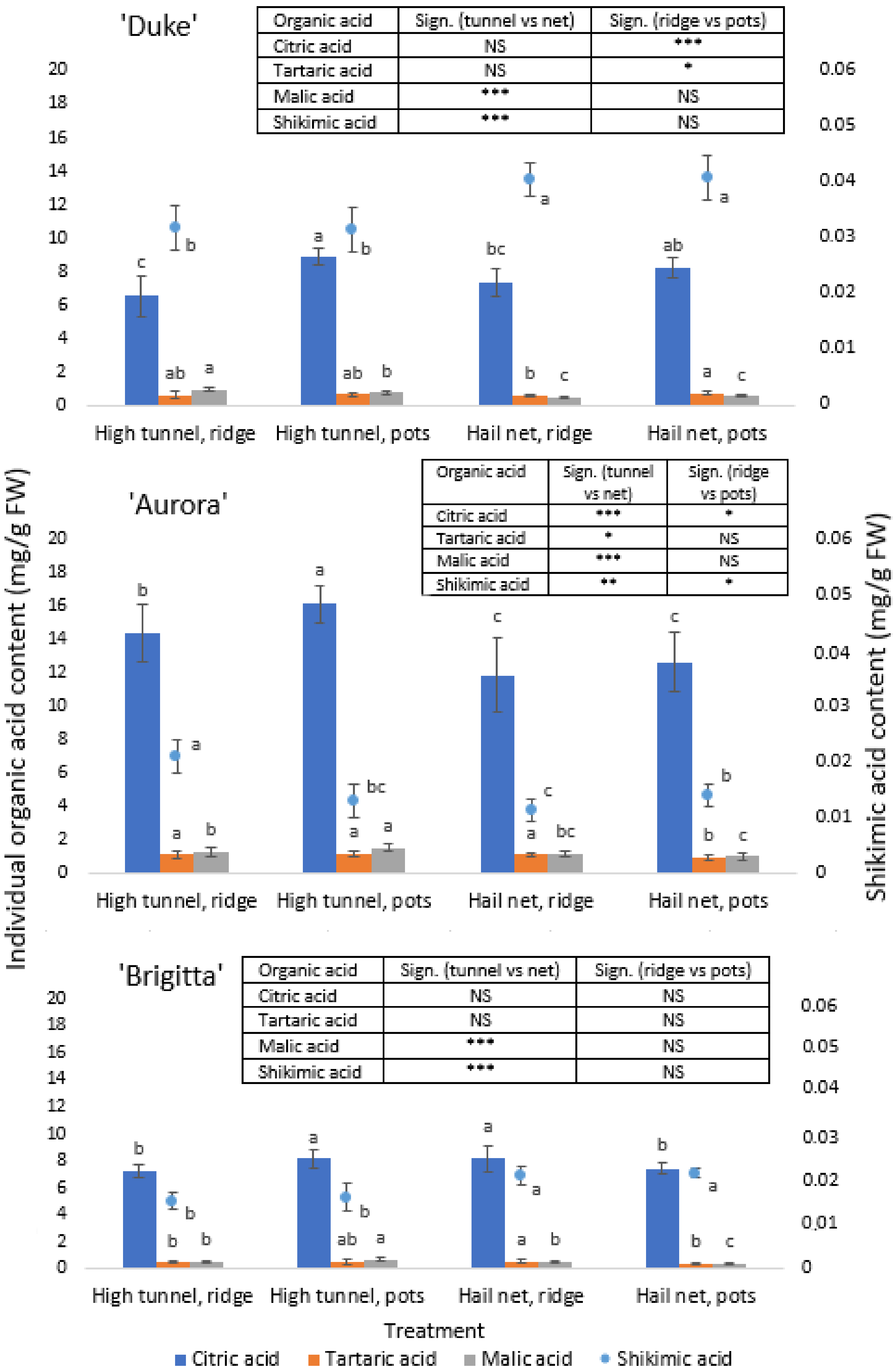
3.5. Total and Individual Organic Acid Contents
3.6. Sugar/Organic Acid Ratio
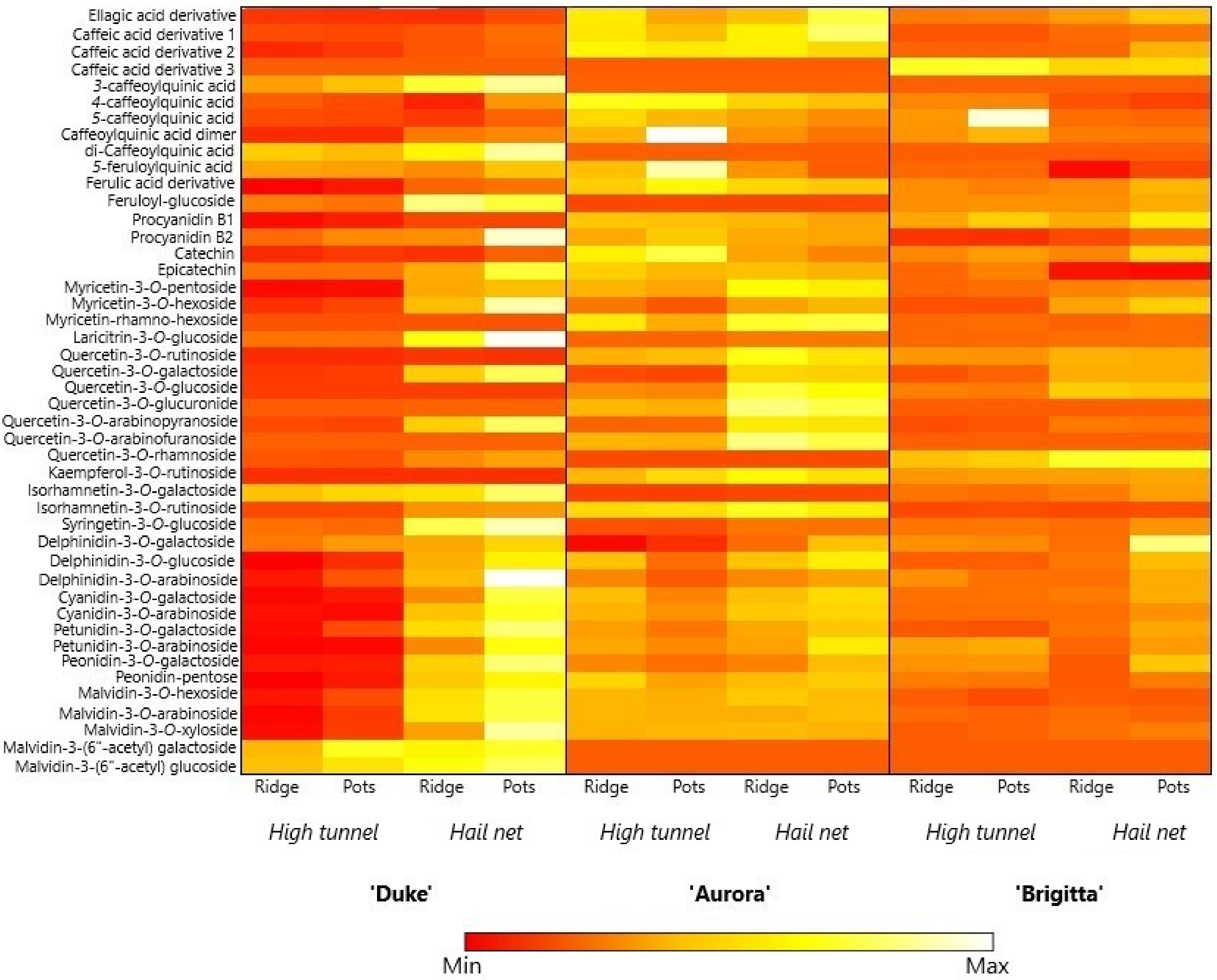
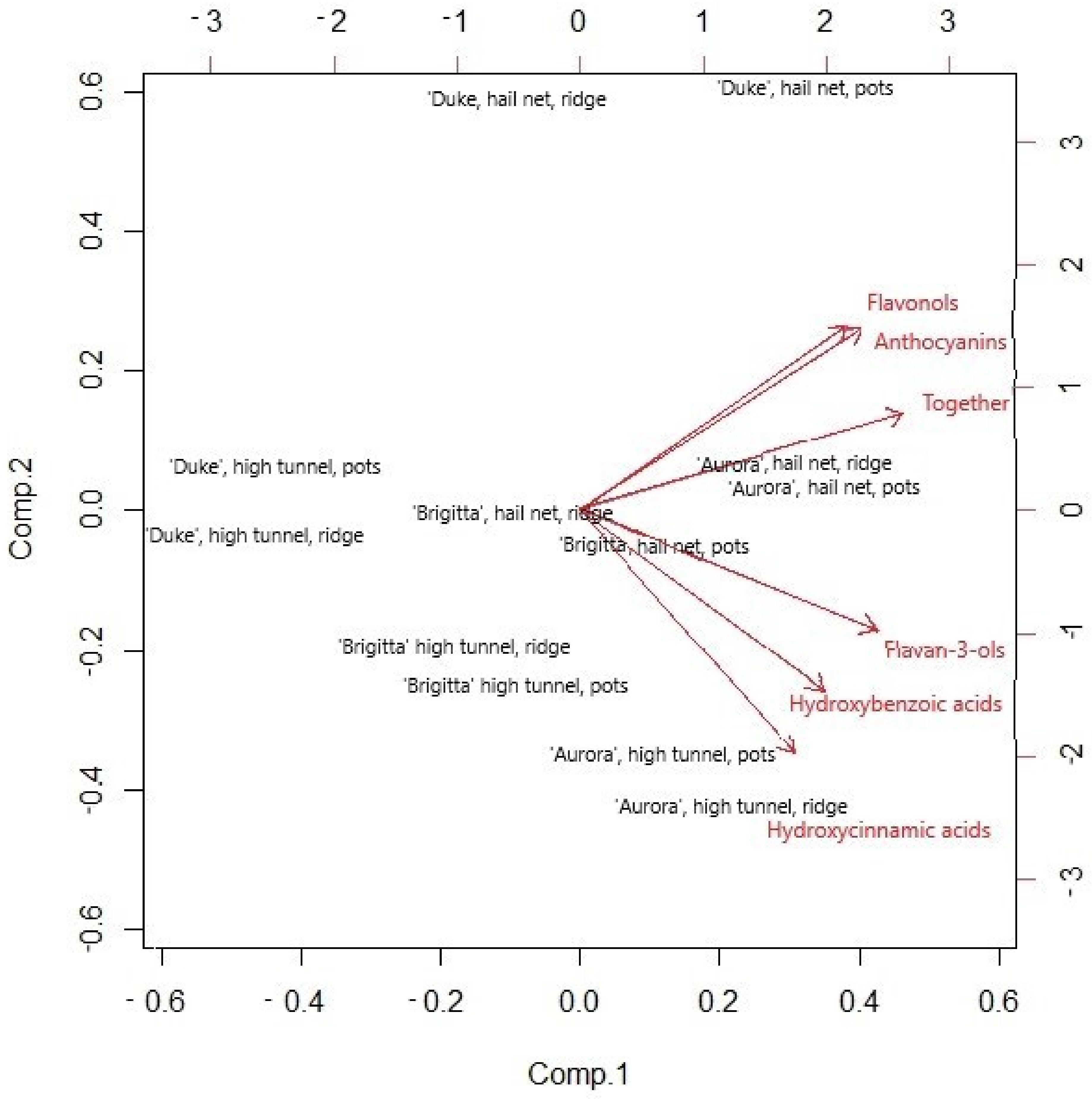
3.7. Phenolics Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; Cabi: Boston, MA, USA, 2018. [Google Scholar]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous amperometric detection of ascorbic acid and antioxidant capacity in orange, blueberry and kiwi juice, by a telemetric system coupled with a fullerene- or nanotubes-modified ascorbate subtractive biosensor. Biosens. Bioelectron. 2015, 67, 214–223. [Google Scholar] [CrossRef]
- Bunea, A.; Rugină, D.; Pintea, A.; Andrei, S.; Bunea, C.; Pop, R.; Bele, C. Carotenoid and fatty acid profiles of bilberries and cultivated blueberries from Romania. Chem. Pap. 2012, 66, 935–939. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, P.J.; Van Der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Bi, G. Container production of southern highbush blueberries using high tunnels. HortScience 2019, 54, 267–274. [Google Scholar] [CrossRef]
- Owen, G.W.; Hilligoss, A.; Lopez, R.G. Late-season high tunnel planting of specialty cut flowers in the midwestern United States influences yield and stem quality. Horttechnology 2016, 26, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Janke, R.R.; Altamimi, M.E.; Khan, M. The Use of High Tunnels to Produce Fruit and Vegetable Crops in North America. Agric. Sci. 2017, 08, 692–715. [Google Scholar] [CrossRef] [Green Version]
- Demchak, K.; Hanson, E.J. Small fruit production in high tunnels in the US. Acta Hortic. 2013, 987, 41–44. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Cobo, N.; del Pozo, A. Spectral irradiance, gas exchange characteristics and leaf traits of Vaccinium corymbosum L. “Elliott” grown under photo-selective nets. Environ. Exp. Bot. 2012, 75, 142–149. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M. The microclimate under coloured hailnets affects leaf and fruit temperature, leaf anatomy, vegetative and reproductive growth as well as fruit colouration in apple. Ann. Appl. Biol. 2010, 156, 121–136. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Romero-Bravo, S.; Del Pozo, A. Productivity and fruit quality of Vaccinium corymbosum cv. Elliott under photo-selective shading nets. Sci. Hortic. 2013, 153, 143–149. [Google Scholar] [CrossRef]
- Iglesias, I.; Alegre, S. The effect of anti-hail nets on fruit protection, radiation, temperature, quality and probability of Mondial Gala apples. J. Appl. Hortic. 2006, 08, 91–100. [Google Scholar] [CrossRef]
- Jakopic, J.; Veberic, R.; Stampar, F. The effect of reflective foil and hail nets on the lighting, color and anthocyanins of “Fuji” apple. Sci. Hortic. 2007, 115, 40–46. [Google Scholar] [CrossRef]
- Jakopic, J.; Stampar, F.; Veberic, R. Influence of hail net and reflective foil on cyanidin glycosides and quercetin glycosides in “Fuji” apple skin. HortScience 2010, 45, 1447–1452. [Google Scholar] [CrossRef]
- Kiprijanovski, M.; Gjamovski, V.; Arsov, T. The effects of anti-hail net in protection of pear orchard after hailstorm occurrence. Acta Hortic. 2016, 1139, 529–534. [Google Scholar] [CrossRef]
- Smrke, T.; Persic, M.; Veberic, R.; Sircelj, H.; Jakopic, J. Influence of reflective foil on persimmon (Diospyros kaki Thunb.) fruit peel colour and selected bioactive compounds. Sci. Rep. 2019, 9, 19069. [Google Scholar] [CrossRef] [Green Version]
- Basile, B.; Giaccone, M.; Cirillo, C.; Ritieni, A.; Graziani, G.; Shahak, Y.; Forlani, M. Photo-selective hail nets affect fruit size and quality in Hayward kiwifruit. Sci. Hortic. 2012, 141, 91–97. [Google Scholar] [CrossRef]
- Girona, J.; Behboudian, M.H.; Mata, M.; Del Campo, J.; Marsal, J. Effect of hail nets on the microclimate, irrigation requirements, tree growth, and fruit yield of peach orchards in Catalonia (Spain). J. Hortic. Sci. Biotechnol. 2012, 87, 545–550. [Google Scholar] [CrossRef]
- Milivojević, J.; Radivojević, D.; Ruml, M.; Dimitrijević, M.; Maksimović, J.D. Does microclimate under grey hail protection net affect biological and nutritional properties of “Duke” highbush blueberry (Vaccinium corymbosum L.)? Fruits 2016, 71, 161–170. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Wei, J.; Jiang, J.; Li, Y.; Chen, J.; Yu, H. Growth, fruit yield, photosynthetic characteristics, and leaf microelement concentration of two blueberry cultivars under different long-term soil pH treatments. Agronomy 2019, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Whidden, A. Commercial blueberry production methods in Hillsborough County. Proc. Fla. State Hort. Soc. 2008, 121, 36–37. [Google Scholar]
- Kingston, P.H.; Scagel, C.F.; Bryla, D.R. Suitability of sphagnum moss, coir, and douglas fir bark as soilless substrates for container production of highbush blueberry. HortScience 2017, 52, 1692–1699. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Parameters of inner quality of the apple scab resistant and susceptible apple cultivars (Malus domestica Borkh.). Sci. Hortic. 2007, 114, 37–44. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J. Sci. Food Agric. 2010, 90, 2366–2378. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Retamal-Salgado, J.; Bastías, R.M.; Wilckens, R.; Paulino, L. Influence of microclimatic conditions under high tunnels on the physiological and productive responses in blueberry ‘O’Neal’. Chil. J. Agric. Res. 2015, 75, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Ogden, A.B.; van Iersel, M.W. Southern highbush Blueberry production in High Tunnels: Temperatures, development, yield, and fruit quality during the establishment years. HortScience 2009, 44, 1850–1856. [Google Scholar] [CrossRef] [Green Version]
- Tamada, T.; Ozeki, M. Evaluation of Blueberry Types and Cultivars for Early Market Production in Japan Using Unheated Plastic House Culture. Int. J. Fruit Sci. 2012, 12, 83–91. [Google Scholar] [CrossRef]
- Moon, J.W., Jr.; Hancock, J.F., Jr.; Draper, A.D.; Flore, J.A. Genotypic differences in the effect of temperature on CO2 assimilation and water use efficiency in blueberry. J. Am. Soc. Hortic. Sci. 1987, 112, 170–173. [Google Scholar]
- Hao, L.; Guo, L.; Li, R.; Cheng, Y.; Huang, L.; Zhou, H.; Xu, M.; Li, F.; Zhang, X.; Zheng, Y. Responses of photosynthesis to high temperature stress associated with changes in leaf structure and biochemistry of blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2019, 246, 251–264. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Belanger, R.R. Biological control in greenhouse systems. Annu. Rev. Phytopathol. 2001, 39, 103–133. [Google Scholar] [CrossRef]
- Milivojević, J.; Radivojević, D.; Nikolić, M.; Maksimović, J.D. Changes in fruit quality of highbush blueberries (Vaccinium corymbosum) during the ripening season. Acta Hortic. 2016, 1139, 657–664. [Google Scholar] [CrossRef]
- Cantliffe, D.J. Pre- and Postharvest Practices for Improved Vegetable Transplant Quality. Horttechnology 2018, 3, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Liu, E.; Fu, Y.; Yuan, F.; Zhang, T.; Peng, S. High temperatures during flowering reduce fruit set in rabbiteye blueberry. J. Am. Soc. Hortic. Sci. 2019, 144, 339–351. [Google Scholar] [CrossRef]
- Lang, A. Xylem, phloem and transpiration flows in developing apple fruits. J. Exp. Bot. 1990, 41, 645–651. [Google Scholar] [CrossRef]
- Santos, B.M.; Salame-Donoso, T.P. Performance of southern highbush blueberry cultivars under high tunnels in Florida. Horttechnology 2012, 22, 700–704. [Google Scholar] [CrossRef] [Green Version]
- Retamales, J.B.; Montecino, J.M.; Lobos, G.A.; Rojas, L.A. Colored shading nets increase yields and profitability of highbush blueberries. Acta Hortic. 2008, 770, 193–197. [Google Scholar] [CrossRef]
- Duarte, C.; Guerra, M.; Daniel, P.; Camelo, A.L.; Yommi, A. Quality changes of highbush blueberries fruit stored in CA with different CO2 levels. J. Food Sci. 2009, 74, S154–S159. [Google Scholar] [CrossRef] [PubMed]
- Ehlenfeldt, M.K.; Martin, R.B. A survey of fruit firmness in highbush blueberry and species-introgressed blueberry cultivars. HortScience 2002, 37, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. White versus blue: Does the wild “albino” bilberry (Vaccinium myrtillus L.) differ in fruit quality compared to the blue one? Food Chem. 2016, 211, 876–882. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, C.; Sun, J.; Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef] [PubMed]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk. J. Agric. For. 2016, 40, 855–864. [Google Scholar] [CrossRef]
- Lobos, G.A.; Callow, P.; Hancock, J.F. The effect of delaying harvest date on fruit quality and storage of late highbush blueberry cultivars (Vaccinium corymbosum L.). Postharvest Biol. Technol. 2014, 87, 133–139. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Bryla, D.R.; Strik, B.C. Potential of deficit irrigation, irrigation cutoffs, and crop thinning to maintain yield and fruit quality with less water in northern highbush blueberry. HortScience 2017, 52, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Lobos, T.E.; Retamales, J.B.; Ortega-Farías, S.; Hanson, E.J.; López-Olivari, R.; Mora, M.L. Regulated deficit irrigation effects on physiological parameters, yield, fruit quality and antioxidants of Vaccinium corymbosum plants cv. Brigitta. Irrig. Sci. 2018, 36, 49–60. [Google Scholar] [CrossRef]
- Jakopic, J.; Stampar, F.; Veberic, R. The influence of exposure to light on the phenolic content of “Fuji” apple. Sci. Hortic. 2009, 123, 234–239. [Google Scholar] [CrossRef]
- Smrke, T.; Veberic, R.; Hudina, M.; Stamic, D.; Jakopic, J. Comparison of Highbush Blueberry (Vaccinium corymbosum L.) under Ridge and Pot Production. Agriculture 2021, 11, 929. [Google Scholar] [CrossRef]
- Spiers, J.M. Substrate temperatures influence root and shoot growth of southern highbush and rabbiteye blueberries. HortScience 1995, 30, 1029–1030. [Google Scholar] [CrossRef] [Green Version]
| Cultivar | Protected | Planting | Plant Volume | Fruit Weight | Fruit Number | Yield | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environment | System | (dm3) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs. Pots) | (g) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs. Pots) | (plant−1) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs. Pots) | (g plant−1) | Sign. (Tunnel vs. Net) | Sign. (ridge vs. Pots) | |
| ‘Duke’ | High tunnel | Ridge | 96.4 ± 7.4 ab | NS | NS | 1.13 ± 0.07 | NS | NS | 81.3 ± 7.6 b | *** | *** | 91.1 ± 10.1 b | *** | *** |
| Pots | 100.2 ± 5.3 a | 1.06 ± 0.09 | 54.3 ± 8.5 c | 57.6 ± 12.0 c | ||||||||||
| Hail net | Ridge | 99.1 ± 5.2 ab | 1.17 ± 0.06 | 86.6 ± 7.3 b | 101.1 ± 10.1 b | |||||||||
| Pots | 94.5 ± 5.0 b | 1.04 ± 0.39 | 144.1 ± 33.8 a | 166.6 ± 59.9 a | ||||||||||
| Sign. (within cultivar) | * | NS | *** | *** | ||||||||||
| ‘Aurora’ | High tunnel | Ridge | 76.0 ± 14.0 c | *** | * | 1.15 ± 0.08 c | *** | NS | 40.5 ± 13.0 b | *** | * | 46.7 ± 15.8 c | *** | * |
| Pots | 52.8 ± 17.7 d | 1.07 ± 0.07 c | 9.1 ± 2.8 c | 9.7 ± 2.7 d | ||||||||||
| Hail net | Ridge | 118.2 ± 18.5 a | 1.63 ± 0.20 a | 51.0 ± 5.8 b | 83.8 ± 16.2 b | |||||||||
| Pots | 101.7 ± 19.6 b | 1.45 ± 0.03 b | 141.8 ± 20.6 a | 205.7 ± 31.5 a | ||||||||||
| Sign. (within cultivar) | *** | *** | *** | *** | ||||||||||
| ‘Brigitta’ | High tunnel | Ridge | 66.5 ± 14.8 c | *** | * | 1.11 ± 0.07 d | *** | ** | 38.4 ± 6.0 c | *** | * | 42.5 ± 7.0 b | *** | NS |
| Pots | 99.2 ± 18.9 b | 1.34 ± 0.05 c | 34.4 ± 8.9 c | 45.9 ± 11.7 b | ||||||||||
| Hail net | Ridge | 141.4 ± 15.8 a | 1.47 ± 0.09 b | 60.9 ± 13.4 a | 89.3 ± 17.9 a | |||||||||
| Pots | 155.5 ± 17.3 a | 1.61 ± 0.06 a | 49.9 ± 10.0 b | 80.1 ± 14.8 a | ||||||||||
| Sign. (within cultivar) | *** | *** | *** | *** | ||||||||||
| Cultivar | Protected | Planting | Harvest | ||
|---|---|---|---|---|---|
| Environment | System | First | Peak | End | |
| ‘Duke’ | High tunnel | Ridge | 8 June | 27 June | 8 July |
| Pots | |||||
| Hail net | Ridge | 17 June | 2 July | 19 July | |
| Pots | |||||
| ‘Aurora’ | High tunnel | Ridge | 8 July | 5 August | 5 September |
| Pots | |||||
| Hail net | Ridge | 26 July | 5 August | 5 September | |
| Pots | |||||
| ‘Brigitta’ | High tunnel | Ridge | 21 June | 27 June | 5 August |
| Pots | 15 July | ||||
| Hail net | Ridge | 27 June | 15 July | 5 August | |
| Pots | 26 July | ||||
| Cultivar | Protected | Planting | Firmness | L* | C* | h° | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environment | System | (N) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs Pots) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs Pots) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs Pots) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs Pots) | ||||
| ‘Duke’ | High tunnel | Ridge | 0.35 ± 0.12 | NS | NS | 37.32 ± 2.05 ab | * | NS | 6.55 ± 0.61 bc | * | NS | 253.85 ± 1.72 b | * | * |
| Pots | 0.34 ± 0.12 | 36.54 ± 2.83 b | 6.07 ± 0.61 c | 253.09 ± 1.84 b | ||||||||||
| Hail net | Ridge | 0.30 ± 0.12 | 39.06 ± 3.01 a | 6.87 ± 0.84 ab | 255.87 ± 2.99 a | |||||||||
| Pots | 0.34 ± 0.12 | 39.09 ± 3.03 a | 7.23 ± 1.07 a | 253.70 ± 1.25 b | ||||||||||
| Sign. (within cultivar) | NS | * | ** | ** | ||||||||||
| ‘Aurora’ | High tunnel | Ridge | 0.17 ± 0.04 | NS | NS | 33.24 ± 2.17 | NS | NS | 5.11 ± 1.41 | NS | NS | 258.95 ± 8.23 ab | * | NS |
| Pots | 0.20 ± 0.05 | 34.15 ± 2.92 | 5.61 ± 1.29 | 260.83 ± 6.39 a | ||||||||||
| Hail net | Ridge | 0.18 ± 0.04 | 32.47 ± 2.57 | 4.73 ± 1.55 | 255.77 ± 8.01 b | |||||||||
| Pots | 0.17 ± 0.04 | 32.69 ± 3.86 | 4.87 ± 1.98 | 254.09 ± 2.73 b | ||||||||||
| Sign. (within cultivar) | NS | NS | NS | * | ||||||||||
| ‘Brigitta’ | High tunnel | Ridge | 0.30 ± 0.13 a | * | NS | 34.56 ± 2.83 | * | NS | 6.49 ± 1.09 a | ** | NS | 259.29 ± 4.04 ab | * | NS |
| Pots | 0.23 ± 0.08 b | 34.95 ± 3.19 | 6.40 ± 1.31 ab | 259.93 ± 7.95 a | ||||||||||
| Hail net | Ridge | 0.21 ± 0.07 b | 32.85 ± 3.75 | 5.47 ± 1.16 b | 257.20 ± 3.41 ab | |||||||||
| Pots | 0.22 ± 0.07 b | 32.44 ± 3.36 | 5.49 ± 1.62 ab | 255.93 ± 2.27 b | ||||||||||
| Sign. (within cultivar) | * | NS | * | * | ||||||||||
| Cultivar | Protected | Planting | Total Sugar Content | Total Organic Acid Content | Sugar/Organic Acid Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Environment | System | (mg g−1) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs. Pots) | (mg g−1) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs. Pots) | Sign. (Tunnel vs. Net) | Sign. (Ridge vs. Pots) | ||
| ‘Duke’ | High tunnel | Ridge | 92.12 ± 7.92 a | * | * | 8.15 ± 1.84 b | NS | *** | 11.96 ± 3.48 a | NS | *** |
| Pots | 81.81 ± 10.83 b | 10.34 ± 0.56 a | 7.95 ± 1.25 b | ||||||||
| Hail net | Ridge | 79.47 ± 7.72 b | 8.47 ± 0.87 b | 9.45 ± 1.25 b | |||||||
| Pots | 79.85 ± 2.67 b | 9.61 ± 0.60 a | 8.34 ± 0.67 b | ||||||||
| Sign. (within cultivar) | ** | *** | *** | ||||||||
| ‘Aurora’ | High tunnel | Ridge | 59.43 ± 2.62 ab | ** | NS | 16.83 ± 1.79 b | *** | NS | 3.57 ± 0.40 b | *** | NS |
| Pots | 61.28 ± 3.58 a | 18.84 ± 1.24 a | 3.27 ± 0.28 b | ||||||||
| Hail net | Ridge | 56.93 ± 2.48 b | 14.16 ± 2.29 c | 4.11 ± 0.65 a | |||||||
| Pots | 58.44 ± 3.44 ab | 14.59 ± 1.93 c | 4.06 ± 0.47 a | ||||||||
| Sign. (within cultivar) | * | *** | *** | ||||||||
| ‘Brigitta’ | High tunnel | Ridge | 92.50 ± 6.33 a | *** | * | 8.22 ± 0.53 b | NS | NS | 11.30 ± 1.14 a | *** | * |
| Pots | 56.24 ± 7.76 b | 9.29 ± 0.78 a | 6.11 ± 1.08 b | ||||||||
| Hail net | Ridge | 39.77 ± 2.98 c | 9.19 ± 1.00 a | 4.38 ± 0.62 d | |||||||
| Pots | 42.84 ± 1.02 c | 8.19 ± 0.40 b | 5.24 ± 0.26 c | ||||||||
| Sign. (within cultivar) | *** | *** | *** | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smrke, T.; Veberic, R.; Hudina, M.; Zitko, V.; Ferlan, M.; Jakopic, J. Fruit Quality and Yield of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars Grown in Two Planting Systems under Different Protected Environments. Horticulturae 2021, 7, 591. https://doi.org/10.3390/horticulturae7120591
Smrke T, Veberic R, Hudina M, Zitko V, Ferlan M, Jakopic J. Fruit Quality and Yield of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars Grown in Two Planting Systems under Different Protected Environments. Horticulturae. 2021; 7(12):591. https://doi.org/10.3390/horticulturae7120591
Chicago/Turabian StyleSmrke, Tina, Robert Veberic, Metka Hudina, Vid Zitko, Mitja Ferlan, and Jerneja Jakopic. 2021. "Fruit Quality and Yield of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars Grown in Two Planting Systems under Different Protected Environments" Horticulturae 7, no. 12: 591. https://doi.org/10.3390/horticulturae7120591
APA StyleSmrke, T., Veberic, R., Hudina, M., Zitko, V., Ferlan, M., & Jakopic, J. (2021). Fruit Quality and Yield of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars Grown in Two Planting Systems under Different Protected Environments. Horticulturae, 7(12), 591. https://doi.org/10.3390/horticulturae7120591







