Rootstock-Mediated Transcriptional Changes Associated with Cold Tolerance in Prunus mume Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation Conditions
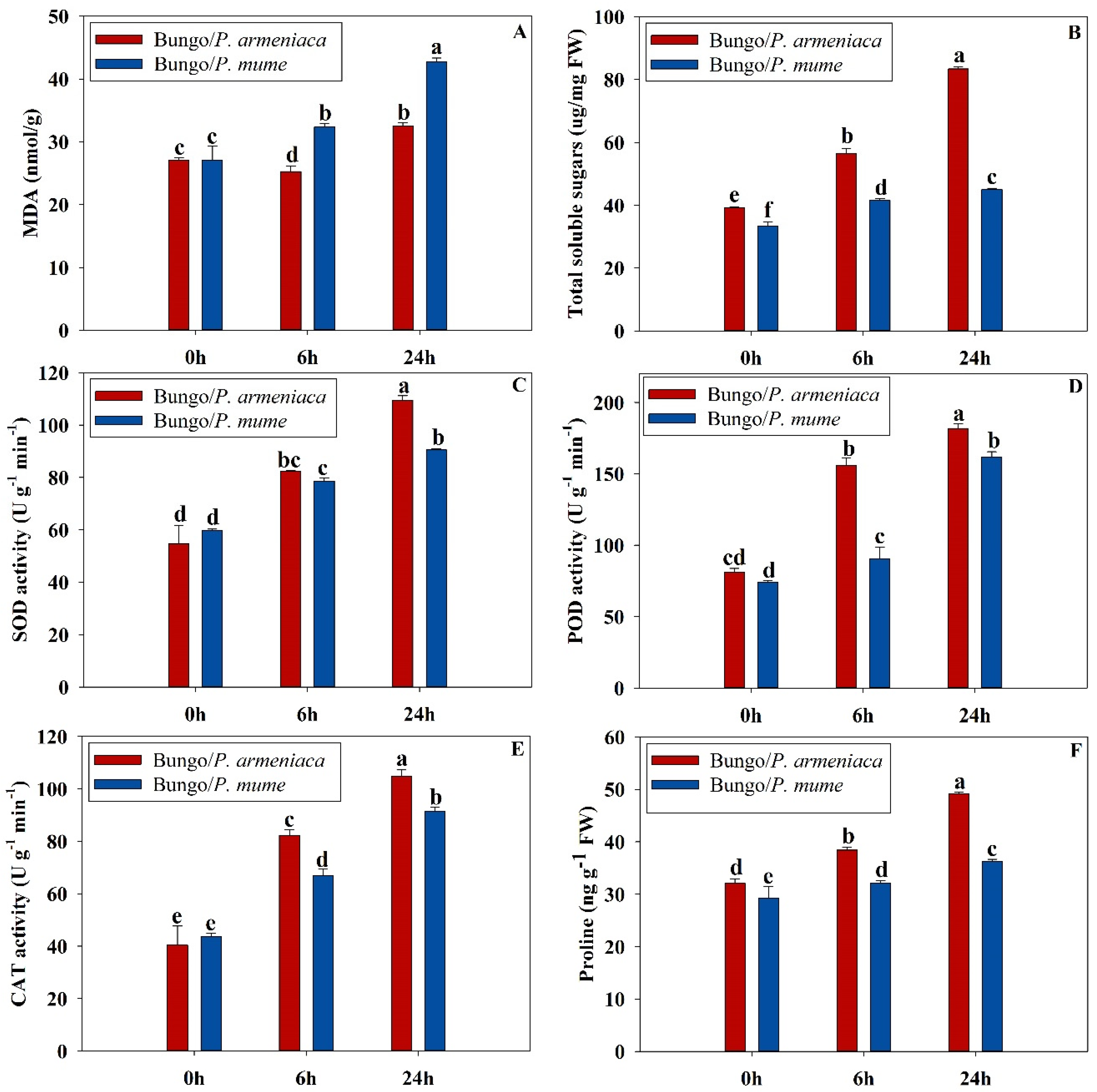
2.2. Determination of MDA, Soluble Sugars, and Proline Contents
2.3. Determination of Different Antioxidant Enzymatic Activities
2.4. RNA Extraction and Library Preparation
2.5. Analysis of Transcriptome Sequencing
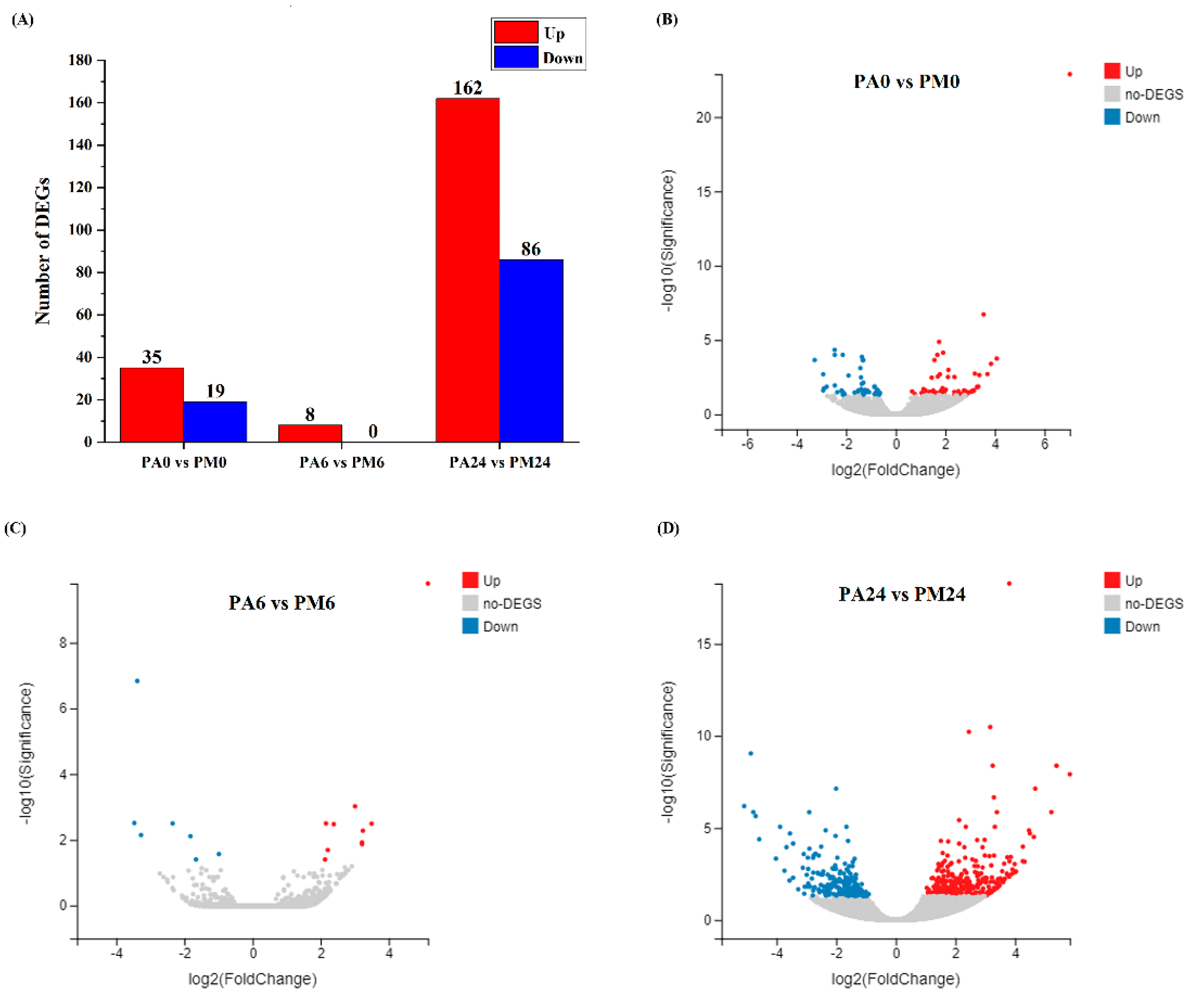
2.6. Screening of Differentially Expressed Genes (DEGs)
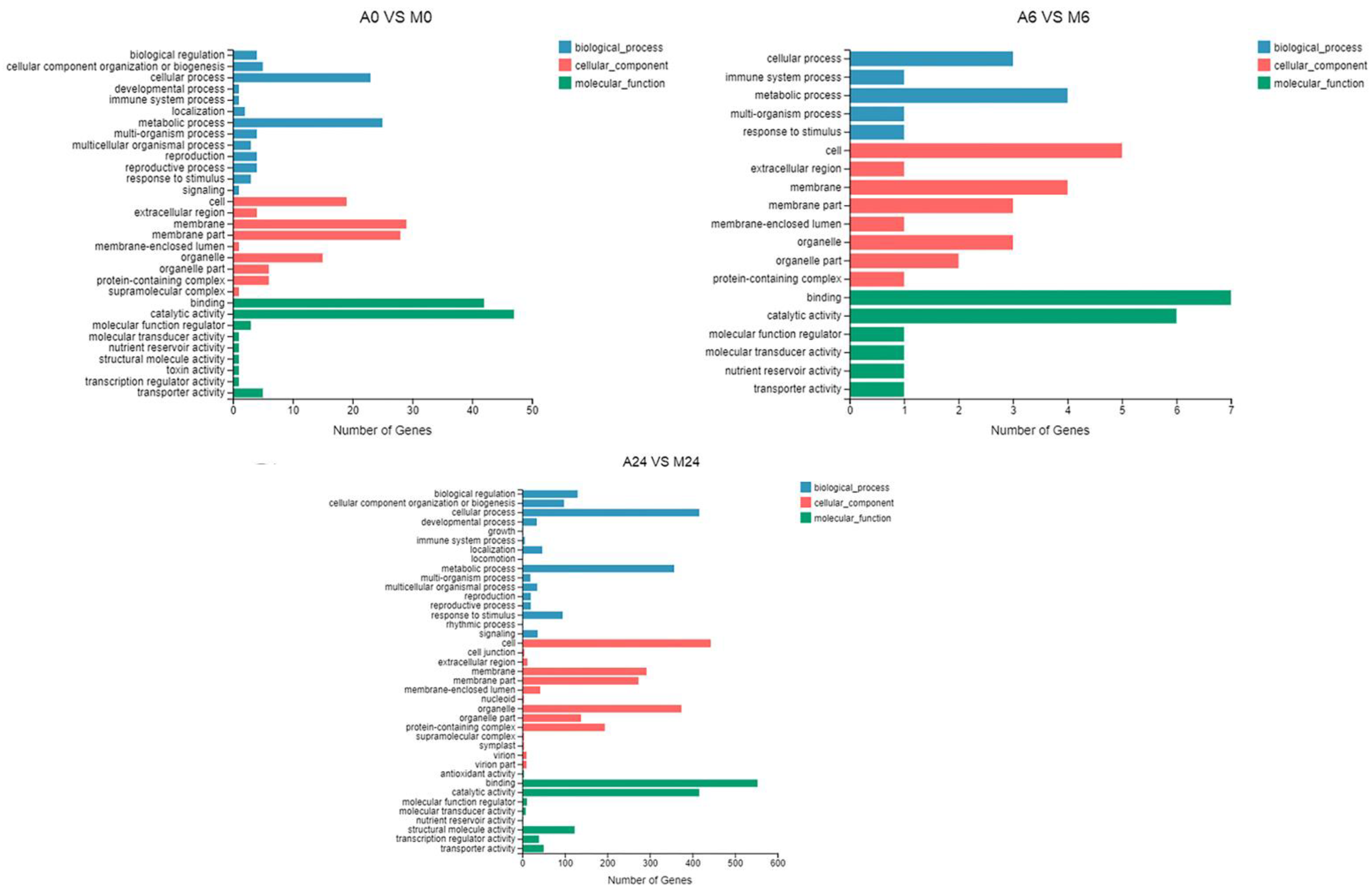
2.7. Gene Ontology and KEGG Pathway Analysis
2.8. Quantitative Real-Time PCR (qRT-PCR) for Expression Validation
2.9. Statistical Analysis
3. Results
3.1. Physiochemical Characteristics of Grafted Seedlings under Cold Stress
3.2. Overview of RNA Sequencing
3.3. Differentially Expressed Genes (DEGs) Analysis
3.4. GO and KEGG Enrichment Analysis
3.5. DEGs Related to Plant Hormone Signaling Transduction Pathway
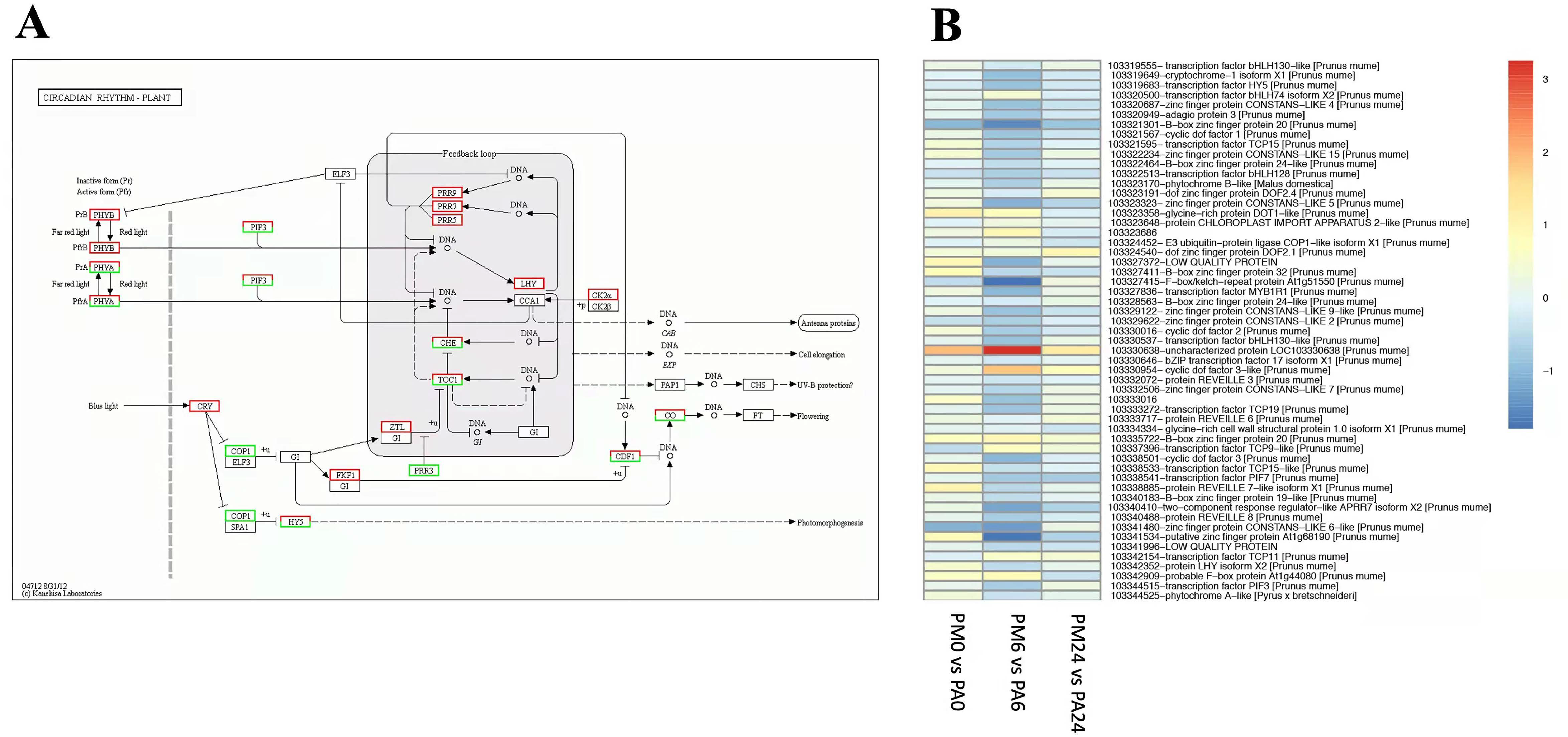
3.6. DEGs in Response to Circadian Rhythm–Plants Pathway
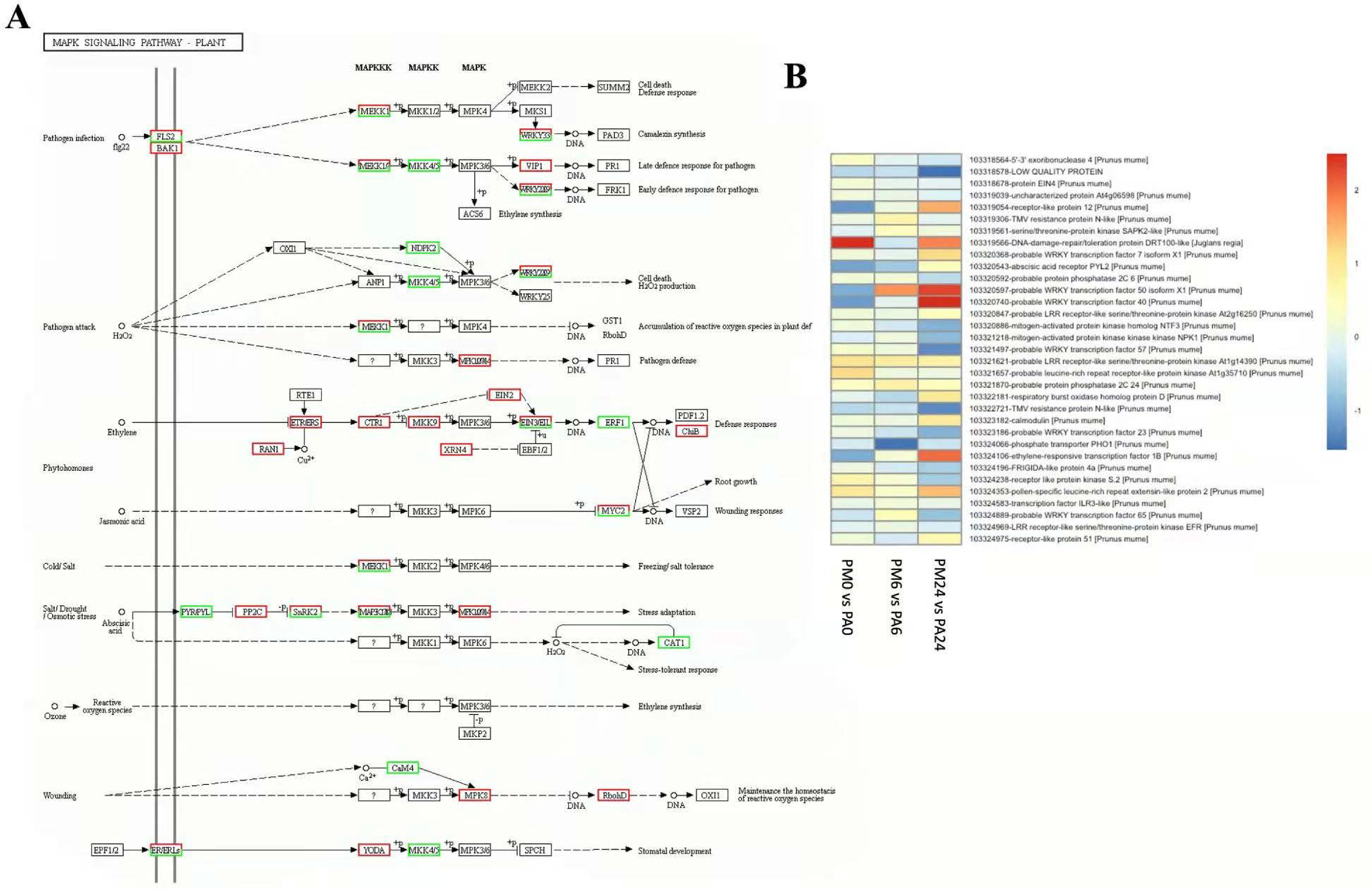
3.7. DEGs in Response to MAPK Signaling Pathway
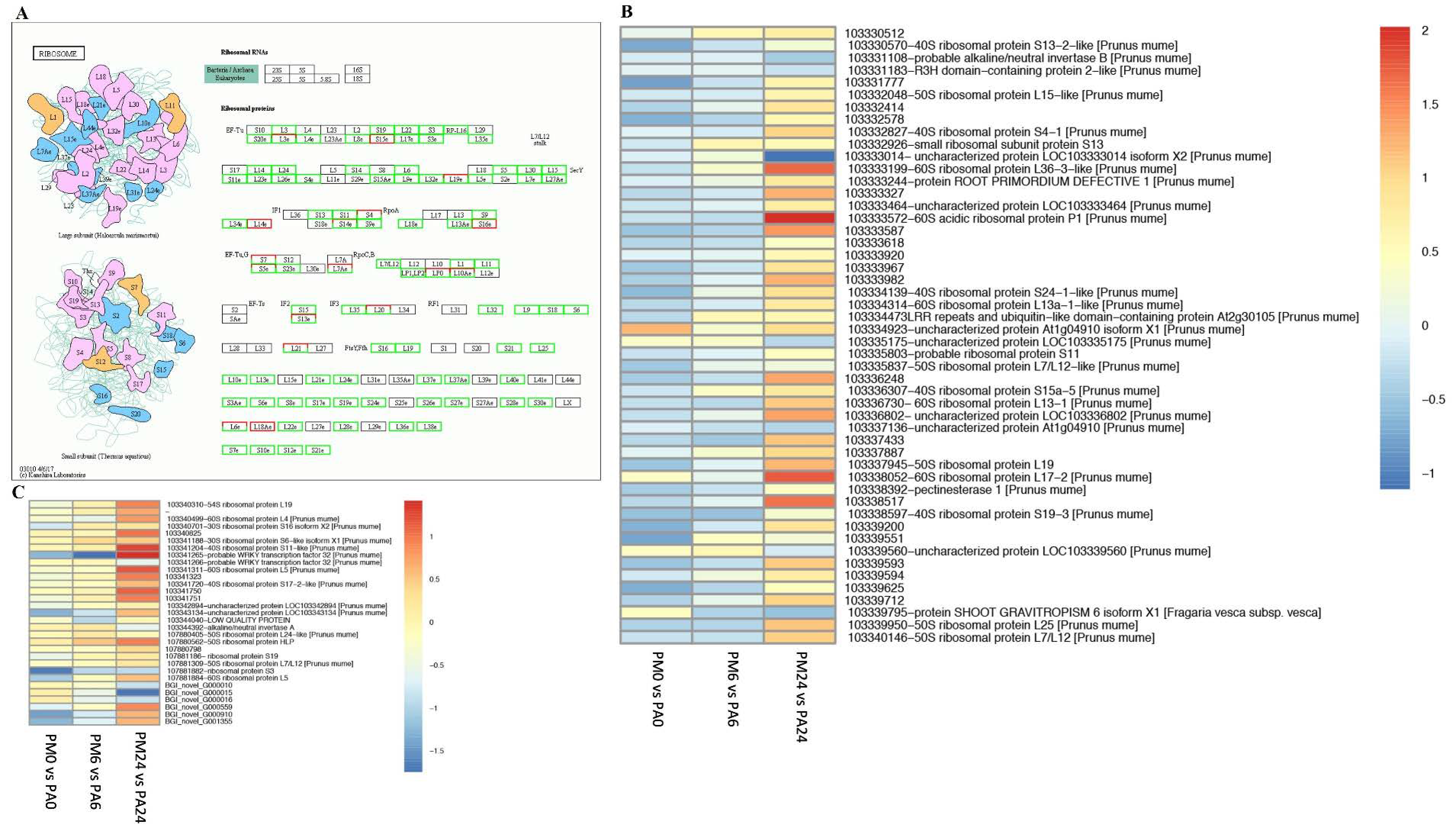
3.8. DEGs in Response to Ribosome Pathways
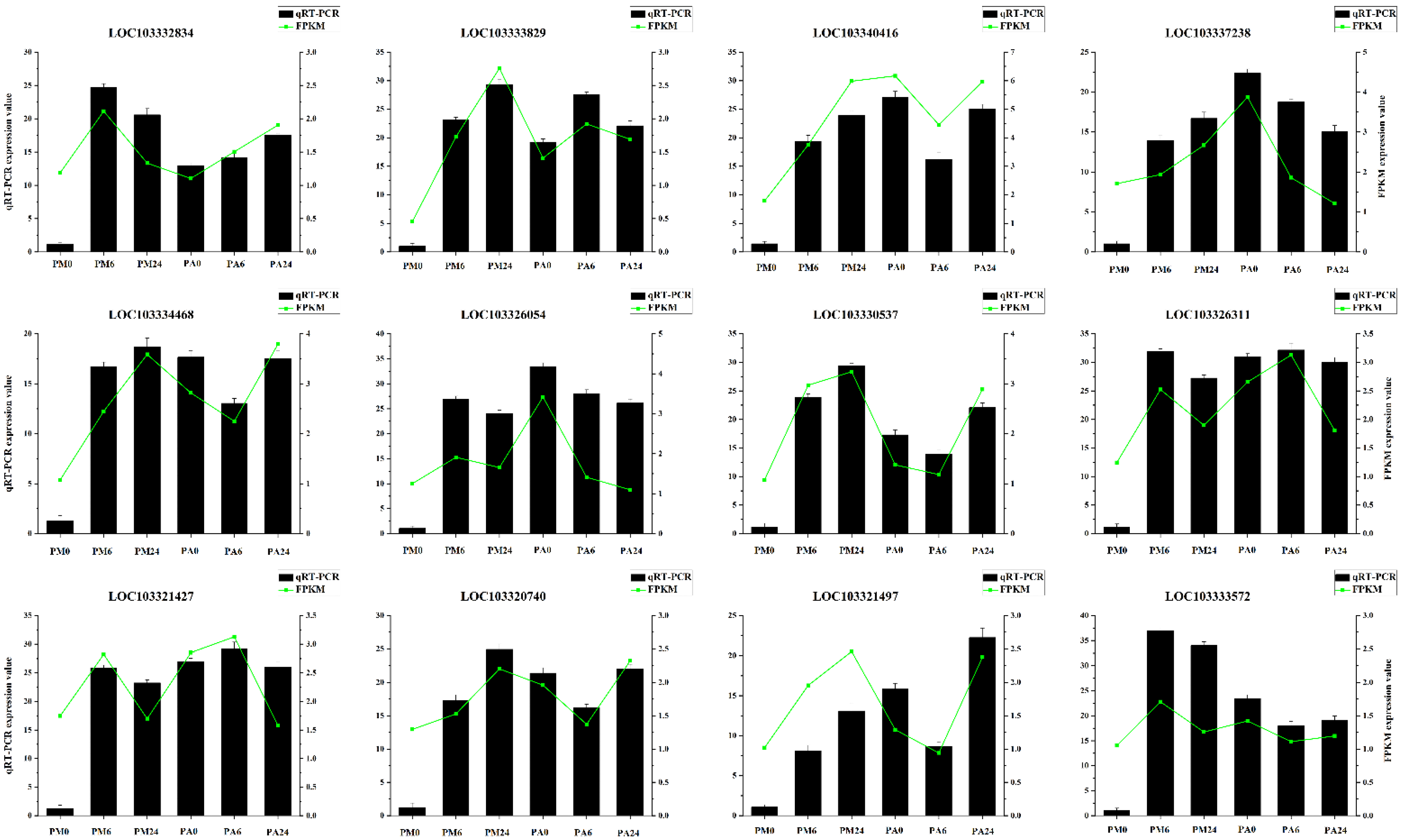
3.9. qRT-PCR Validation of Gene Expressions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| POD | peroxidase |
| CAT | catalase |
References
- Peng, T.; Guo, C.; Yang, J.; Xu, M.; Zuo, J.; Bao, M.; Zhang, J. Overexpression of a Mei (Prunus mume) CBF gene confers tolerance to freezing and oxidative stress in Arabidopsis. PCTOC 2016, 126, 373–385. [Google Scholar] [CrossRef]
- Iqbal, S.; Pan, Z.; Wu, X.; Shi, T.; Ni, X.; Bai, Y.; Gao, J.; Khalil-ur-Rehman, M.; Gao, Z. Genome-wide analysis of PmTCP4 transcription factor binding sites by ChIP-Seq during pistil abortion in Japanese apricot. Plant Genome 2020, 13, e20052. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Ding, A.; Cheng, T.; Wang, J.; Zhang, Q. Genome-wide analysis of members of the WRKY gene family and their cold stress response in Prunus mume. Genes 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, X.; Ban, Q.; Zhu, X.; Jiang, C.; Wei, C.; Bennetzen, J.L. Comparative transcriptomic analysis reveals gene expression associated with cold adaptation in the tea plant Camellia sinensis. BMC Genom. 2019, 20, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.A.; Wang, X.; Zafar, S.A.; Noor, M.A.; Hussain, H.A.; Azher Nawaz, M.; Farooq, M. Thermal Stresses in Maize: Effects and Management Strategies. Plants 2021, 10, 293. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, X.; Cui, Y.; Bi, Q.; Zhao, Y.; Li, D.; Yu, H.; Wang, L. Comparative transcriptome combined with morpho-physiological analyses revealed candidate genes potentially for differential cold tolerance in two contrasting apricot (Prunus armeniaca L.) cultivars. Trees 2020, 34, 1205–1217. [Google Scholar] [CrossRef]
- Janská, A.; Maršík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation—What is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Yin, H.; Fan, Z.; Li, X.; Ni, S.; He, L.; Li, J. Overexpression of CaAPX induces orchestrated reactive oxygen scavenging and enhances cold and heat tolerances in tobacco. BioMed Res. Int. 2017, 2017, 4049534. [Google Scholar]
- Lou, X.; Wang, H.; Ni, X.; Gao, Z.; Iqbal, S. Integrating proteomic and transcriptomic analyses of loquat (Eriobotrya japonica Lindl.) in response to cold stress. Gene 2018, 677, 57–65. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Fang, Z.; Wu, Y.; Tao, J. Physiological and transcriptomic analysis of tree peony (Paeonia section Moutan DC.) in response to drought stress. Forests 2019, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Riva-Roveda, L.; Escale, B.; Giauffret, C.; Périlleux, C. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biol. 2016, 16, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Xiaochuang, C.; Chu, Z.; Lianfeng, Z.; Junhua, Z.; Hussain, S.; Lianghuan, W.; Qianyu, J. Glycine increases cold tolerance in rice via the regulation of N uptake, physiological characteristics, and photosynthesis. Plant Physiol. Biochem. 2017, 112, 251–260. [Google Scholar] [CrossRef]
- Bustamante, C.A.; Monti, L.L.; Gabilondo, J.; Scossa, F.; Valentini, G.; Budde, C.O.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Differential metabolic rearrangements after cold storage are correlated with chilling injury resistance of peach fruits. Front. Plant Sci. 2016, 7, 1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, D.; Fowler, S.; Fiehn, O.; Thomashow, M.F. A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 15243–15248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomashow, M.F. Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensminger, I.; Busch, F.; Huner, N.P. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef]
- Eremina, M.; Rozhon, W.; Poppenberger, B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2016, 73, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhang, J.; Cao, J.; Cai, Q.; Li, X.; Sun, Y.; Li, S.; Li, Y.; Hu, G.; Cao, S. Leaf transcriptomic response mediated by cold stress in two maize inbred lines with contrasting tolerance levels. Genomics 2021, 113, 782–794. [Google Scholar] [CrossRef]
- Jia, Y.; Ding, Y.; Shi, Y.; Zhang, X.; Gong, Z.; Yang, S. The cbfs triple mutants reveal the essential functions of CBF s in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef] [Green Version]
- Pagter, M.; Alpers, J.; Erban, A.; Kopka, J.; Zuther, E.; Hincha, D.K. Rapid transcriptional and metabolic regulation of the deacclimation process in cold acclimated Arabidopsis thaliana. BMC Genom. 2017, 18, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, J.T.; Zarka, D.G.; Van Buskirk, H.A.; Fowler, S.G.; Thomashow, M.F. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2005, 41, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, S.; Thomashow, M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar] [CrossRef] [Green Version]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.; Shi, T.; Gao, Z. An insight into dwarfing mechanism: Contribution of scion-rootstock interactions toward fruit crop improvement. Fruit Res. 2021, 1, 1–11. [Google Scholar]
- Kumar, S.; Kaur, G.; Nayyar, H. Exogenous application of abscisic acid improves cold tolerance in chickpea (Cicer arietinum L.). J. Agron. Crop. Sci. 2008, 194, 449–456. [Google Scholar]
- Xue-Xuan, X.; Hong-Bo, S.; Yuan-Yuan, M.; Gang, X.; Jun-Na, S.; Dong-Gang, G.; Cheng-Jiang, R. Biotechnological implications from abscisic acid (ABA) roles in cold stress and leaf senescence as an important signal for improving plant sustainable survival under abiotic-stressed conditions. Crit. Rev. Biotechnol. 2010, 30, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. Auxin: A regulator of cold stress response. Physiol. Plant. 2013, 147, 28–35. [Google Scholar] [CrossRef]
- Barrero-Gil, J.; Salinas, J. CBFs at the crossroads of plant hormone signaling in cold stress response. Mol. Plant 2017, 10, 542–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, E5982–E5991. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.-J.; Wang, X.-L.; Shang, Q.-M. Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci. Hortic. 2011, 129, 629–636. [Google Scholar] [CrossRef]
- Iqbal, S.; Ni, X.; Bilal, M.S.; Shi, T.; Khalil-ur-Rehman, M.; Zhenpeng, P.; Jie, G.; Usman, M.; Gao, Z. Identification and expression profiling of sugar transporter genes during sugar accumulation at different stages of fruit development in apricot. Gene 2020, 742, 144584. [Google Scholar] [CrossRef]
- Ljung, K.; Nemhauser, J.L.; Perata, P. New mechanistic links between sugar and hormone signalling networks. Curr. Opin. Plant Biol. 2015, 25, 130–137. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Iqbal, S.; Ayaz, A.; Bai, Y.; Pan, Z.; Ni, X.; Hayat, F.; Saqib Bilal, M.; Khuram Razzaq, M.; Gao, Z. Analyzing Differentially Expressed Genes and Pathways Associated with Pistil Abortion in Japanese Apricot via RNA-Seq. Genes 2020, 11, 1079. [Google Scholar] [CrossRef]
- Iqbal, S.; Pan, Z.; Hayat, F.; Bai, Y.; Coulibaly, D.; Ali, S.; Ni, X.; Shi, T.; Gao, Z. Comprehensive transcriptome profiling to identify genes involved in pistil abortion of Japanese apricot. Physiol. Mol. Biol. Plants 2021, 27, 1191–1204. [Google Scholar] [CrossRef]
- Ni, X.; Xue, S.; Iqbal, S.; Wang, W.; Ni, Z.; Khalil-ur-Rehman, M.; Gao, Z. Candidate genes associated with red colour formation revealed by comparative genomic variant analysis of red-and green-skinned fruits of Japanese apricot (Prunus mume). PeerJ 2018, 6, e4625. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.Y.; Bian, X.; Lee, C.-J.; Kim, H.S.; Kim, S.-E.; Park, S.-C.; Xie, Y.; Guo, X.; Kwak, S.-S. De novo transcriptome sequencing and gene expression profiling of sweet potato leaves during low temperature stress and recovery. Gene 2019, 700, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.A.; Uzair, M.; Khan, M.R.; Patil, S.B.; Fang, J.; Zhao, J.; Singla-Pareek, S.L.; Pareek, A.; Li, X. DPS1 regulates cuticle development and leaf senescence in rice. Food Energy Secur. 2021, 10, e273. [Google Scholar] [CrossRef]
- Zafar, S.A.; Patil, S.B.; Uzair, M.; Fang, J.; Zhao, J.; Guo, T.; Yuan, S.; Uzair, M.; Luo, Q.; Shi, J.; et al. Degenerated panicle and partial sterility 1 (DPS1) encodes a cystathionine β-synthase domain containing protein required for anther cuticle and panicle development in rice. New Phytol. 2020, 225, 356–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanyam, K.; Du Laing, G.; Van Damme, E.J. Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front. Plant Sci. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Li, H.; Zhou, W.; Takeuchi, Y.; Yoneyama, K. Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul. 2006, 49, 27–34. [Google Scholar]
- Zafar, S.A.; Hameed, A.; Ashraf, M.; Khan, A.S.; Qamar, Z.U.; Li, X.; Siddique, K.H.M. Agronomic, physiological and molecular characterisation of rice mutants revealed the key role of reactive oxygen species and catalase in high-temperature stress tolerance. FPB 2020, 47, 440–453. [Google Scholar] [CrossRef]
- Wang, S.Q.; Tang, J.; Hu, K.D.; Huang, Z.Q.; Yang, F.; Zhang, H.Y.; Hu, L.Y.; Li, Y.H.; Yao, G.F.; Zhang, H. Antioxidative system in sweet potato root is activated by low-temperature storage. J. Sci. Food Agric. 2019, 99, 3824–3833. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Iqbal, S.; Ma, R.; Song, J.; Yu, M.; Gao, Z. High-density genetic map construction and quantitative trait loci analysis of the stony hard phenotype in peach based on restriction-site associated DNA sequencing. BMC Genom. 2018, 19, 612. [Google Scholar] [CrossRef]
- Wu, X.; Shi, T.; Iqbal, S.; Zhang, Y.; Liu, L.; Gao, Z. Genome-wide discovery and characterization of flower development related long non-coding RNAs in Prunus mume. BMC Plant Biol. 2019, 19, 1–17. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hayat, F.; Asghar, S.; Yanmin, Z.; Xue, T.; Nawaz, M.A.; Xu, X.; Wang, Y.; Wu, T.; Zhang, X.; Qiu, C. Rootstock Induced Vigour is Associated with Physiological, Biochemical and Molecular Changes in ‘Red Fuji’Apple. Int. J. Agric. Biol. 2020, 24, 1823–1834. [Google Scholar]
- Cerruti, E.; Gisbert, C.; Drost, H.-G.; Valentino, D.; Portis, E.; Barchi, L.; Prohens, J.; Lanteri, S.; Comino, C.; Catoni, M. Grafting vigour is associated with DNA de-methylation in eggplant. Hortic. Res. 2021, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.-K.; Sunkar, R. Gene regulation during cold stress acclimation in plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 39–55. [Google Scholar]
- Zafar, S.A.; Noor, M.A.; Waqas, M.A.; Wang, X.; Shaheen, T.; Raza, M.; Rahman, M. Temperature extremes in cotton production and mitigation strategies. In Past Present Future Trends in Cotton Breeding; IntechOpen: London, UK, 2018; pp. 65–91. [Google Scholar]
- Li, J.; Liu, H.; Xia, W.; Mu, J.; Feng, Y.; Liu, R.; Yan, P.; Wang, A.; Lin, Z.; Guo, Y. De novo transcriptome sequencing and the hypothetical cold response mode of Saussurea involucrata in extreme cold environments. Int. J. Mol. Sci. 2017, 18, 1155. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Zeng, X.; Ling, Z.; Wei, Z.; Wang, Y.; Zhuang, Z.; Xu, Q.; Tang, Y.; Tashi, N. Transcriptome profiles reveal cold acclimation and freezing tolerance of susceptible and tolerant hulless barley genotypes. Acta Physiol. Plant. 2017, 39, 275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Lin, Y.; Luo, Y.; Wang, X.; Chen, Q.; Sun, B.; Wang, Y.; Li, M.; Tang, H. A transcriptomic analysis reveals diverse regulatory networks that respond to cold stress in strawberry (Fragaria× ananassa). Int. J. Genom. 2019, 2019, 7106092. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.; Feng, X.; Chen, J.; Zhang, Y.; Wei, X.; Chen, Y.; Cheng, C.; Wu, R. De novo transcriptome assembly and comparative transcriptomic analysis provide molecular insights into low temperature stress response of Canarium album. Sci. Rep. 2021, 11, 10561. [Google Scholar] [CrossRef]
- Kim, S.-I.; Tai, T.H. Evaluation of seedling cold tolerance in rice cultivars: A comparison of visual ratings and quantitative indicators of physiological changes. Euphytica 2011, 178, 437–447. [Google Scholar] [CrossRef]
- Venekamp, J. Regulation of cytosol acidity in plants under conditions of drought. Physiol. Plant. 1989, 76, 112–117. [Google Scholar] [CrossRef]
- Yuanyuan, M.; Yali, Z.; Jiang, L.; Hongbo, S. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Morsy, M.R.; Almutairi, A.M.; Gibbons, J.; Yun, S.J.; Benildo, G. The OsLti6 genes encoding low-molecular-weight membrane proteins are differentially expressed in rice cultivars with contrasting sensitivity to low temperature. Gene 2005, 344, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Chang-Quan, W.; Rui-Chang, L. Enhancement of superoxide dismutase activity in the leaves of white clover (Trifolium repens L.) in response to polyethylene glycol-induced water stress. Acta Physiol. Plant. 2008, 30, 841–847. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [Green Version]
- de Freitas, G.M.; Thomas, J.; Liyanage, R.; Lay, J.O.; Basu, S.; Ramegowda, V.; do Amaral, M.N.; Benitez, L.C.; Bolacel Braga, E.J.; Pereira, A. Cold tolerance response mechanisms revealed through comparative analysis of gene and protein expression in multiple rice genotypes. PLoS ONE 2019, 14, e0218019. [Google Scholar]
- Liscum, E.; Reed, J. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002, 49, 387–400. [Google Scholar] [CrossRef]
- Shibasaki, K.; Uemura, M.; Tsurumi, S.; Rahman, A. Auxin response in Arabidopsis under cold stress: Underlying molecular mechanisms. Plant Cell 2009, 21, 3823–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, E.J.; Estelle, M. Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 2009, 43, 265–285. [Google Scholar] [CrossRef] [Green Version]
- Paterlini, A. Uncharted routes: Exploring the relevance of auxin movement via plasmodesmata. Biol. Open 2020, 9, bio055541. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T. The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 2003, 15, 533–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.N.; Verslues, P.E. Stress physiology functions of the Arabidopsis histidine kinase cytokinin receptors. Physiol. Plant. 2015, 154, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000, 3, 217–223. [Google Scholar] [CrossRef]
- Verma, R.K.; Santosh Kumar, V.V.; Yadav, S.K.; Pushkar, S.; Rao, M.V.; Chinnusamy, V. Overexpression of ABA receptor PYL10 gene confers drought and cold tolerance to indica rice. Front. Plant Sci. 2019, 10, 1488. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Xie, J.; Yu, J. Study on signal induced expression of cold tolerance in edible lily in alpine environment. Appl. Ecol. Environ. Res. 2020, 18, 2687–2701. [Google Scholar] [CrossRef]
- Maruyama, K.; Urano, K.; Yoshiwara, K.; Morishita, Y.; Sakurai, N.; Suzuki, H.; Kojima, M.; Sakakibara, H.; Shibata, D.; Saito, K. Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiol. 2014, 164, 1759–1771. [Google Scholar] [CrossRef] [Green Version]
- McClung, C.R. Plant circadian rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, C.; Bieniawska, Z.; Hincha, D.K.; Hannah, M.A. Interactions between the circadian clock and cold-response in Arabidopsis. Plant Signal. Behav. 2008, 3, 593–594. [Google Scholar] [CrossRef] [Green Version]
- Nemchenko, A.; Kunze, S.; Feussner, I.; Kolomiets, M. Duplicate maize 13-lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. J. Exp. Bot. 2006, 57, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Huang, P.; Yuan, X.; Chen, H.; Huang, J.; Zhang, H. CMYB1 encoding a MYB transcriptional activator is involved in abiotic stress and circadian rhythm in rice. Sci. World J. 2014, 2014, 178038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washio, K. Functional dissections between GAMYB and Dof transcription factors suggest a role for protein-protein associations in the gibberellin-mediated expression of the RAmy1A gene in the rice aleurone. Plant Physiol. 2003, 133, 850–863. [Google Scholar] [CrossRef] [Green Version]
- Gualberti, G.; Papi, M.; Bellucci, L.; Ricci, I.; Bouchez, D.; Camilleri, C.; Costantino, P.; Vittorioso, P. Mutations in the Dof zinc finger genes DAG2 and DAG1 influence with opposite effects the germination of Arabidopsis seeds. Plant Cell 2002, 14, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Carbajosa, J.; Moose, S.P.; Parsons, R.L.; Schmidt, R.J. A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc. Natl. Acad. Sci. USA 1997, 94, 7685–7690. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef] [Green Version]
- Corrales, A.-R.; Nebauer, S.G.; Carrillo, L.; Fernández-Nohales, P.; Marqués, J.; Renau-Morata, B.; Granell, A.; Pollmann, S.; Vicente-Carbajosa, J.; Molina, R.-V. Characterization of tomato Cycling Dof Factors reveals conserved and new functions in the control of flowering time and abiotic stress responses. J. Exp. Bot. 2014, 65, 995–1012. [Google Scholar] [CrossRef] [Green Version]
- Grundy, J.; Stoker, C.; Carré, I.A. Circadian regulation of abiotic stress tolerance in plants. Front. Plant Sci. 2015, 6, 648. [Google Scholar] [CrossRef] [PubMed]
- Abeynayake, S.W.; Byrne, S.; Nagy, I.; Jonavičienė, K.; Etzerodt, T.P.; Boelt, B.; Asp, T. Changes in Lolium perenne transcriptome during cold acclimation in two genotypes adapted to different climatic conditions. BMC Plant Biol. 2015, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Hussain, I.; Afzal, S.; Singh, A.; Singh, N. Circadian regulation of abiotic stress tolerance in legumes. In Abiotic Stress and Legumes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 105–136. [Google Scholar]
- Fu, J.; Miao, Y.; Shao, L.; Hu, T.; Yang, P. De novo transcriptome sequencing and gene expression profiling of Elymus nutans under cold stress. BMC Genom. 2016, 17, 870. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Chen, X.; Chai, X.; Qiu, Y.; Gong, C.; Zhang, Z.; Wang, T.; Zhang, Y.; Li, J.; Wang, A. Effects of low temperature on mRNA and small RNA transcriptomes in Solanum lycopersicoides leaf revealed by RNA-Seq. Biochem. Biophys. Res. Commun. 2015, 464, 768–773. [Google Scholar] [CrossRef]
- Smékalová, V.; Doskočilová, A.; Komis, G.; Šamaj, J. Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signalling in plants. Biotechnol. Adv. 2014, 32, 2–11. [Google Scholar] [CrossRef]
- de Zelicourt, A.; Colcombet, J.; Hirt, H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016, 21, 677–685. [Google Scholar] [CrossRef]
- Sun, S.; Lin, M.; Qi, X.; Chen, J.; Gu, H.; Zhong, Y.; Sun, L.; Muhammad, A.; Bai, D.; Hu, C. Full-length transcriptome profiling reveals insight into the cold response of two kiwifruit genotypes (A. arguta) with contrasting freezing tolerances. BMC Plant Biol. 2021, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Tang, A.Y. Overexpression of Arabidopsis thaliana malonyl-CoA synthetase gene enhances cold stress tolerance by activating mitogen-activated protein kinases in plant cells. J. For. Res. 2021, 32, 741–753. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.-C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.A. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell 2017, 43, 618–629.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3-and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e4. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Dane, F.; Si, Y.; Ebel, R.; Zhang, C. Gene expression analysis of cold treated versus cold acclimated Poncirus trifoliata. Euphytica 2008, 164, 209–219. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. WRKY proteins: Signaling and regulation of expression during abiotic stress responses. Sci. World J. 2015, 2015, 807560. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Total Raw Reads (M) | Total Clean Reads (M) | Clean Reads Q20 (%) | Clean Reads Q30 (%) | Clean Reads Ratio (%) | Total Mapping (%) | Uniquely Mapping (%) |
|---|---|---|---|---|---|---|---|
| PA0h | 46.16 | 42.65 | 97.75 | 93.80 | 92.41 | 88.03 | 56.19 |
| PA6h | 45.57 | 42.47 | 97.63 | 93.48 | 93.18 | 87.62 | 54.40 |
| PA24h | 46.16 | 42.68 | 97.78 | 93.87 | 92.46 | 87.11 | 55.28 |
| PM0h | 46.16 | 42.71 | 97.77 | 93.85 | 92.54 | 89.26 | 58.07 |
| PM6h | 45.57 | 42.54 | 97.72 | 93.70 | 93.35 | 89.45 | 57.81 |
| PM24h | 45.57 | 42.45 | 97.63 | 93.46 | 93.15 | 89.45 | 57.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, F.; Ma, C.; Iqbal, S.; Huang, X.; Omondi, O.K.; Ni, Z.; Shi, T.; Tariq, R.; Khan, U.; Gao, Z. Rootstock-Mediated Transcriptional Changes Associated with Cold Tolerance in Prunus mume Leaves. Horticulturae 2021, 7, 572. https://doi.org/10.3390/horticulturae7120572
Hayat F, Ma C, Iqbal S, Huang X, Omondi OK, Ni Z, Shi T, Tariq R, Khan U, Gao Z. Rootstock-Mediated Transcriptional Changes Associated with Cold Tolerance in Prunus mume Leaves. Horticulturae. 2021; 7(12):572. https://doi.org/10.3390/horticulturae7120572
Chicago/Turabian StyleHayat, Faisal, Chengdong Ma, Shahid Iqbal, Xiao Huang, Ouma Kenneth Omondi, Zhaojun Ni, Ting Shi, Rezwan Tariq, Ummara Khan, and Zhihong Gao. 2021. "Rootstock-Mediated Transcriptional Changes Associated with Cold Tolerance in Prunus mume Leaves" Horticulturae 7, no. 12: 572. https://doi.org/10.3390/horticulturae7120572
APA StyleHayat, F., Ma, C., Iqbal, S., Huang, X., Omondi, O. K., Ni, Z., Shi, T., Tariq, R., Khan, U., & Gao, Z. (2021). Rootstock-Mediated Transcriptional Changes Associated with Cold Tolerance in Prunus mume Leaves. Horticulturae, 7(12), 572. https://doi.org/10.3390/horticulturae7120572







