Abstract
The short shelf-life and loss of bioactive compounds of strawberry fruit are the most important problems during strawberry refrigerated storage. This study was carried out to evaluate the effect of the pre-harvest foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) three times, 10 d apart, at fruit development and ripening stages on storage ability and bioactive compounds of strawberry fruit (cv. Festival) stored at 4 °C for 12 d. Our results showed that fruit obtained from both concentrations of ABA and 0.25 mM MeJA was firmer and had higher total soluble solids (TSS) than fruit from non-treated plants. However, all previous applications had no significant effect on weight loss, pH, or color. Applications of 4 mM SA and 0.25 mM MeJA conserved fruit from ascorbic acid (AsA) loss compared to control at the end of the storage period. In addition, all pre-harvest applications remained higher in total phenolic compounds (TPC) and anthocyanin contents compared to controls at the last storage period. Hence, the pre-harvest application of SA, ABA, and MeJA could be used to conserve TPC and anthocyanin as well as the quality of strawberry fruits during refrigerated storage.
1. Introduction
Strawberry (Fragaria × anannasa Duch) is the most important cultivated crop belonging to the Rosaceae family. Moreover, it is a highly nutritious and health-promoting food due to its high vitamin (C and E), mineral, and phytochemical content, including anthocyanins and phenolic compounds [1]. Previous studies reported that the regular consumption of strawberry fruit could minimize some human vital diseases such as malignant (cancerous) tumors, cardiovascular disease, and type II diabetes [2]. However, the storability of strawberry fruit is very limited due to its high water content, high respiration rate, and water loss rate [3]. Additionally, strawberry fruit is classified as a non-climacteric and highly perishable fruit. Therefore, the fruits should be harvested at the fully mature stage to obtain the best visual and nutritional quality. As a result, high fruit loss percentages were recorded during post-harvest operations and refrigerated storage. Thus, pre and/or postharvest treatments are required to preserve quality and nutritional value after the fruit harvest.
Many previous postharvest treatments have been applied to extend the shelf-life and retard the deterioration of strawberry fruit such as melatonin application [1], hot air and essential oils treatments such as tea tree [4] and citrus oils [5], edible coating with ascorbic acid carried on aloe vera [6], plant growth regulator applications [7], ethylene action inhibitor (1-MCP) treatment [8], coating with nano-chitosan and calcium chloride [9], and UV-C treatment [10]. However, some of these postharvest applications are unrealistic due to the difficulty of practical application, high economic value, or low customer acceptance. Thus, the pre-harvest application of eco-friendly compounds to extend the shelf-life and preserve the bioactive compounds of fruits and vegetables could be a suitable alternative.
Various pre-harvest treatments have been used to prolong the storage period, extend the shelf-life, and maintain the quality of vegetable crops, such as supplementation using different mineral fertilizers and bio-stimulants [11,12,13], the application of oxalic acid [14], and chitosan oligosaccharide treatment [15]. To our knowledge, the influence of the pre-harvest application of plant growth regulators, such as SA, ABA, and MeJA, has not been previously studied, particularly on strawberry plants.
SA is a simple phenolic compound and is recognized as a plant growth regulator [16]. It has been known that SA controls some important physiological processes in the plant, including ethylene production [17]. Previous studies have been conducted to investigate the effect of pre-harvest SA application on storage ability and fruit quality of strawberry fruits. For example, Shafiee et al. [18] reported that adding SA to the nutrient solution during the growing season of strawberry plants resulted in less weight loss and decay as well as higher firmness. In addition, it was confirmed that the ethylene production and fungal decay of strawberry fruit were reduced by three applications of SA together (plant foliar application, growth media application, and fruit dipping) [19]. It was also reported that these three combined applications retained the overall quality of strawberry fruits. In addition, pre-harvest foliar applications of SA positively enhanced the TSS and AsA content of strawberry fruits [20].
ABA is classified as an inhibitor of organ growth. ABA showed a role for fruit ripening in both climacteric, such as tomatoes and non-climacteric fruits including strawberry [21,22]. To our knowledge, there are no previous studies on the effect of the pre-harvest application of ABA on strawberry fruits’ storage ability and quality.
MeJA is a plant growth regulator and has a role in the defense of the plant against some plant pathogens [23]. It was shown that the post-harvest MeJA application has a positive effect on maintaining quality, reducing decay, and enhancing antioxidant compounds [24]. In addition, it was observed that foliar applications of MeJA control the gene expression responsible for fruit crops ripening [25]. However, the effect of the pre-harvest MeJA treatment on strawberry fruits’ storage ability and quality has yet to be studied.
Our hypothesis is that the pre-harvest application of SA, ABA, and MeJA could enhance the quality of strawberry fruits after harvest and during cold storage. Thus, the current study was carried out to investigate the effect of the pre-harvest application of strawberry plants with SA, ABA, and MeJA on weight loss, firmness, pH, titratable acidity, total soluble solids, color intensity, AsA, total phenolic compounds (TPC), and the anthocyanin content of strawberry fruits (cv. Festival).
2. Materials and Methods
2.1. Plants and Treatments
Strawberry (Fragaria × ananassa) transplants of cv. Festival were transplanted on 30 October 2018 in a plastic greenhouse at the Agricultural Experimental and Research Station, Faculty of Agriculture, Cairo University, Giza, Egypt. The experimental plot was 2.1 m2 and the space between plants was 50 cm. Furthermore, there were 10–12 plants/plot and the experimental soil was loam clay soil. The study used a randomized complete block design (RCBD), which included seven treatments and four replicates. Treatments were pre-harvest-sprayed on whole strawberry plants. The treatments included: SA-2 (2 mmol SA), SA-4 (4 mmol SA), MeJA-0.25 (0.25 mmol MeJA), MeJA-0.50 (0.50 mmol MeJA), ABA-0.25 (0.25 mmol ABA), and ABA-0.50 (0.50 mmol ABA). The control (CON “seventh”) group was sprayed with tap water. Tween-20 was employed as a surfactant in all treatments. SA and ABA were dissolved in 70.0% ethanol, MeJA in 95.0% methanol, and all solutions were prepared using distilled water. At 10-day intervals, applications were sprayed three times at different stages of strawberry plant development: full flowering, production of green fruits, and the beginning of the pink stage, respectively.
2.2. Storage Experiment Design
The fruits were harvested at the 75% red color stage and were transferred to the postharvest laboratory within 1 h. Next, the fruits were randomly separated into seven groups based on size homogeneity and the absence of any visual flaws. Each treatment’s fruit was packaged in clamshells (each having about 250 g) and preserved for 12 days at 4 °C and 95% RH. Clamshells were split into two groups for each treatment. To evaluate weight loss, the first group was stored continuously during the experiment storage period. Fruit quality measures (firmness, TSS, and color changes) and chemical parameters (pH, titratable acidity (TA), AsA, anthocyanin, and TPC) were determined in the second group at time intervals of 0, 4, 8, and 12 days from harvest. Each treatment was replicated three times. Each replicate had ten clamshells. Fresh fruits were used for all physical and chemical evaluations on the day of the assay.
2.3. Chemicals
Abscisic acid, methyl jasmonate, salicylic acid, ethanol (95.0%), methanol, standard gallic acid, Folin–Ciocalteu reagent, potassium acetate, sodium carbonate, and HCl concentrated were purchased from Sigma-Aldrich (St. Louis, Missouri, MS, USA).
2.4. Physico-Chemical Quality
Weight loss % (WL%) was measured by weighing each replicate at day zero and at 4, 8, and 12 days, which was represented as a percentage of the weight at day zero. Firmness was measured by randomly selecting ten strawberry fruits from each treatment and assessing firmness at two parts; the two parts examined were on opposite sides of the fruits, in the center zone. A firmness meter (FT011 Fruit Firmness Tester; Wagner Instruments, Italy) was used to measure firmness, and firmness values were recorded in Newtons (N). On five strawberry fruits of each duplicate, the surface color was measured using a colorimeter (CR-400 Chroma meter, Konica Minolta, Inc., Tokyo, Japan). L*, a*, b* (L*: lightness, a*: positive values—redness, negative values—greenness; b*: positive values—yellowness, negative values —blueness), chroma (C*), and hue angle (h°) were used to establish color characteristics for fruits. Each fruit’s surface color was assessed at three different parts of the fruit [26]. When calibrating the colorimeter, a standard white plate was used.
Ten strawberry fruits were ground in a blinder for juice extraction. A digital refractometer (model PR101, Co. Ltd., Tokyo, Japan) was used to determine the TSS% in the extracted strawberry juice [27]. The pH of extracted strawberry juice was measured using a digital pH meter (HI 9219, Smithfield, RI, USA). Using distilled water, 10 g of fruit was homogenized and blended up to 100 mL. Then, in triplicates, 10 mL of the aliquot was titrated with 0.1 N NaOH in the presence of phenolphthalein as an indicator, and the titratable acidity percentage was estimated from the titter as the percentage of citric acid in the juice [7].
2.5. Phytochemicals Profile
The titrimetric 2,6-dichlorophenol indophenol technique was used to evaluate the ascorbic acid concentration [28]. The amount of ascorbic acid in the fruit was measured in mg/100 g FW. Total phenolic compounds (TPC) in the fruit juice extract were quantified using the Folin–Ciocalteu reagent and standard gallic acid, as reported previously [29], with minor modifications. In brief, strawberry aliquot puree was centrifuged at 8000× g at 25 °C for 20 min. To obtain a clear juice, the homogenate was filtered using filter paper. One milliliter of clear juice was blended with 5 mL of a 1/10 dilution of the Folin–Ciocalteau reagent. Sodium carbonate (7.5% w/v) was then added in 4 mL increments. Distilled water was used to dilute the previous mixture solution to 100 mL. Then, the previous solution was incubated at room temperature in the dark. A spectrophotometer was used to measure the absorbance of the solution at 765 nm after 2 h of incubation (model UV-2401 PC, Shimadzu, Milano, Italy). Using the gallic acid standard curve, the TPC value was calculated as mg of gallic acid equivalent mg/100 g of fresh fruit (FW) (mg GAE/100 g FW). Four grams of fruit puree was extracted with 40 mL of ethanol: 0.1 MHCl (85:15%, v:v) to quantitatively evaluate anthocyanin content. The mixture was then centrifuged for 20 min at 6000× g. The supernatant was then filtered before being collected and was used to determine anthocyanin levels. The extracts (3 mL) were diluted in 5 mL of pH = 1.0 and pH = 4.5 buffers. After 30 min of incubation at room temperature, the absorption (A) was measured at 510 nm and 700 nm [30]. The following formula was used to determine the absorbance values of the diluted samples (A):
A = (A510 − A700) pH1.0 − (A510 − A700) pH4.5.
Total anthocyanin content was calculated according to the following equation:
where A is the difference of absorbance between pH 1 and pH 4.5 solutions, MW is the molecular weight (MW) 484.82 gmol−1 for C3G, DF is the dilution factor, ε is the molar absorptivity (ε of 24,825 M−1 cm−1 (at 510 nm), λ is the cuvette optical path length (1 cm), and m is the weight of the sample (g). The total anthocyanin content was calculated as mg cyanidin-3-glucoside equivalent per kg dry extract (mg C3GE/kg).
TAC = (A × MW × DF × 1000)/(ε × λ × m),
2.6. Statistical Analyses
The experiment was performed twice, and the results were the same. To determine the contributions of pre-harvest treatments, SPSS statistics software (SPSS for Windows, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The data were submitted for analysis of variance (ANOVA). The storage time (days) and treatments were the main sources of variance. To compare means between treatments, Duncan’s test was performed at a p < 0.05 level of probability.
3. Results
3.1. Influence of Treatments on Weight Loss, Firmness, and Color
The impact of foliar treatments of SA, ABA, or MeJA on weight loss and firmness of strawberry fruits (stored at 4 °C for 12 d) is shown in Figure 1 and Supplementary Table S1. There was incremental weight loss by increasing storage time (Supplementary Table S1). After 4 d of refrigerated storage, 4 SA and 0.25 MeJA treatments significantly (p < 0.05) induced weight loss as compared to untreated fruits and all other treatments (Figure 1A). Moreover, after 8 and 12 d of storage, insignificant differences were detected between all treated fruits and the control.
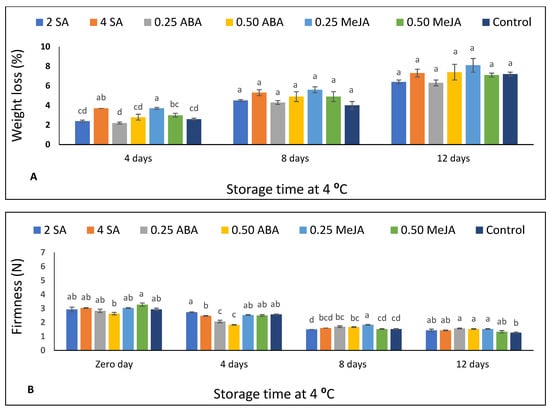
Figure 1.
The effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on (A) weight loss and (B) firmness of strawberry fruits stored at 4 °C for 12 days. Different letters for every storage point indicate significant differences (Duncan’s test, p < 0.05).
The firmness of strawberry fruit decreased with increasing storage time (Supplementary Table S1), ranging from 2.63 N to 3.27 N and from 1.27 to 1.57 N at harvest time and after 12 d of storage, respectively. No clear significant differences were obtained between treatments and the control at harvest time after 4 d of storage (Figure 1B). Strawberry plants applied with 0.25 mM MeJA had significantly higher fruit firmness than untreated plants after 8 d of storage, whereas after 12 d of storage, both concentrations of ABA and 0.25 mM MeJA recorded significantly higher firmness values as compared to untreated fruits.
The impact of SA, ABA, and MeJA foliar treatments on strawberry fruit surface color (stored at 4 °C for 12 d) is shown in Table 1. Insignificant differences were detected among all treatments in color lightness (L*), redness (a*), blueness (b*), chroma (C*) and hue angle (h°) of strawberry fruit across all storage periods (Supplementary Table S2).

Table 1.
Effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on surface color of strawberry fruits stored at 4 °C for 12 days.
3.2. Influence of Treatments on TSS, pH, and TA
The impact of SA, ABA, and MeJA foliar treatment on TSS and pH of strawberry fruits (stored at 4 °C for 12 d) is shown in Figure 2. The pre-harvest treatments significantly affected TSS values (p < 0.05) (Figure 2A). At 0 d, fruits treated with 0.25 ABA and 0.25 MeJA obtained significantly higher TSS values than the untreated fruits (control). After 4 d of storage, the treatments recorded no significant differences as compared to untreated fruits.
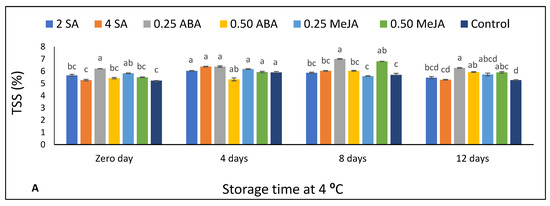
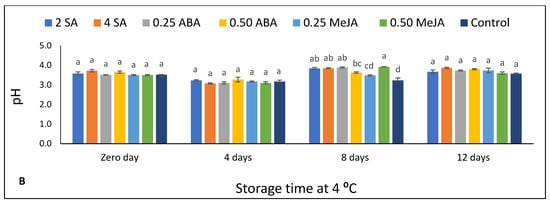
Figure 2.
The effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on (A) total soulble solids TSS (%) and (B) pH of strawberry fruits stored at 4 °C for 12 days. Different letters for every storage point indicate significant differences (Duncan’s test, p < 0.05).
However, after 8 d of storage, the lowest ABA concentration (0.25 mM) showed the highest TSS compared with the control and most of the treated fruits—except MeJA0.50. By the end of the storage period, ABA (0.25 mM) obtained the highest TSS compared with applications by both concentrations of SA and untreated fruits. TSS values increased in the control, and most of the treated fruits—by increasing the storage period to 4 d—subsequently decreased after 8 d of storage especially at both concentrations of ABA and 0.50 MeJA. After 12 d of refrigerated storage, TSS values declined significantly with the application of the highest concentrations of SA, ABA, or MeJA compared with the values after 8 d of storage (Supplementary Table S3).
Figure 2B shows that no significant differences were detected between untreated fruits and all treated fruits at 0 d and after 4 d of storage. Moreover, it can be observed that the pH values decreased significantly in the control and all treated fruits after 4 d of storage (Supplementary Table S3). However, after 8 d of storage, the pH of untreated fruits was significantly lower as compared with all treated fruits—except 0.25 MeJA. After 12 d of storage, there were no significant pH changes among all treatments. The pH of untreated fruits increased by increasing the days of storage from 8 to 12 d (Supplementary Table S3). On the contrary, the remaining treatments did not record significant differences by increasing the storage period to 12 d—except with 0.50 MeJA.
The impact of foliar application of SA, ABA, and MeJA on TA of strawberry fruits stored at 4 °C for 12 d is shown in Figure 3A. TA, at harvest time, was the highest in the fruits treated by 2 SA, 0.25, and 0.50 MeJA with significant differences compared to untreated fruits. Moreover, the treated fruits with 0.25 MeJA obtained the highest TA content after 8 d of storage in comparison with all treatments. After 8 d of storage, the TA content decreased compared to the harvest day values or after 4 d of storage, and this decrement continued until the end of the refrigerated storage (Supplementary Table S3).
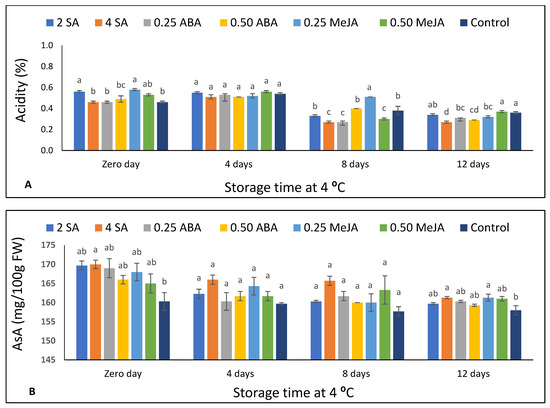
Figure 3.
The effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on (A) titratable acidity and (B) ascorbic acid (AsA) of strawberry fruits stored at 4 °C for 12 days. Different letters for every storage point indicate significant differences (Duncan’s test, p < 0.05).
3.3. Influence of Treatments on Ascorbic Acid, Total Phenolic, and Anthocyanin Content
Ascorbic acid content (AsA) decreased by increasing the storage time (Figure 3B). However, the loss of AsA—by increasing the storage period—was not significant either in treated fruits with both concentrations of MeJA or untreated fruits (Supplementary Table S4). At harvest time, the treated fruits with 4 SA obtained significantly higher AsA content than the untreated fruits (Figure 3B). In contrast, insignificant differences were observed in AsA amongst all treated fruits and the controls, after either 4 or 8 d of storage. However, at the end of refrigerated storage, the treated fruits with 4 mM SA and 0.25 mM MeJA obtained an AsA content significantly higher than in the control. The application of 4 mM SA enhanced the AsA content at harvest time and at the end of storage. However, no significant difference was obtained between the two SA concentrations.
Total phenolic compounds (TPC) were enhanced with storage time in all treatments (Supplementary Table S4). Treatment with concentrations of SA, ABA, and MeJA significantly increased TPC content in the fruits compared to untreated fruits from the beginning until the end of storage (Figure 4A).
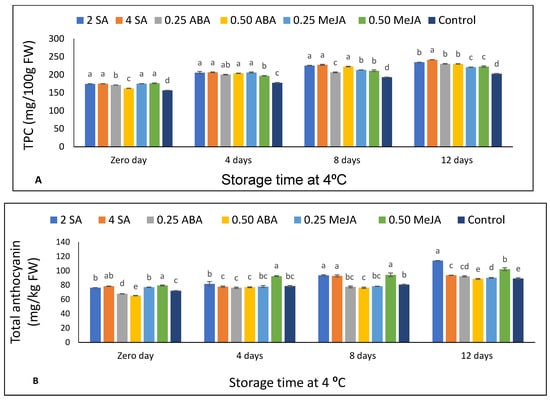
Figure 4.
The effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on (A) total phenolic compounds and (B) total anthocyanin of strawberry fruits stored at 4 °C for 12 days. Different letters for every storage point indicate significant differences (Duncan’s test, p < 0.05).
Anthocyanin content of strawberry fruits increased significantly (p < 0.05) with storage time (Supplementary Table S4). At harvest time, strawberry plants treated with both concentrations of SA and MeJA showed the highest anthocyanin content compared to the control. Moreover, both concentrations of ABA showed lower values of anthocyanin content compared to the control and other treatments (Figure 4B). After 4 d of storage, strawberry plants treated with 0.50 mM MeJA showed the highest anthocyanin values compared to other treatments and the control. Strawberry plants treated with both concentrations of SA and a high concentration of MeJA (0.50 mM) maintained their superior anthocyanin content in fruits after 8 and 12 d of storage compared to the control.
3.4. Correlation Study among Weight Loss (WL), Firmness, TSS, pH, Titratable Acidity (TA), Ascorbic Acid Content (ASA), Phenolic Compounds, and Anthocyanin Content
A strong negative correlation (Table 2 and Figure 5) was found between weight loss and other measured parameters such as firmness, TA, and AsA. In contrast, a positive correlation was found between weight loss and chemical compositions of fruits such as TPC, pH, and anthocyanin content.

Table 2.
Correlation study among weight loss (WL), firmness, TSS, pH, titratable acidity (TA), ascorbic acid content (ASA), phenolic compounds (TPC), and anthocyanin content.
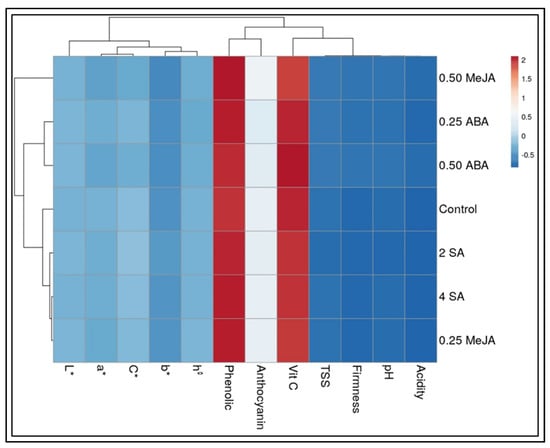
Figure 5.
Correlation heat map between physiochemical quality of strawberry fruits and pre-harvest treatments. Positive relationships (red color) and negative relationships (blue color).
In contrast, a positive correlation was recorded between firmness from one side and TA and AsA from another side of the plant. On the other hand, a negative correlation was found between firmness and some bioactive compounds such as TPC, pH, and anthocyanin content.
4. Discussion
4.1. Influence of Treatments on Weight Loss, Firmness, and Color
Weight loss is considered one of the most important quality parameters for fresh vegetables such as strawberries. Our results showed that 4 SA and 0.25 MeJA increased the weight loss of fruits after 4 d of storage. However, no significant differences were detected among all treatments for the two other periods. These results are in accordance with Baninaiem et al. [31], who found that the pre-harvest SA treatment had no significant effect on the weight loss of tomatoes during refrigerated storage. However, other pre-harvest SA application methods, such as the addition of nutrient solutions, might be significant for reducing weight loss [18]. The differences in responses to previous research may be related to the application method for plant growth regulators (foliar application or adding nutrient solutions). In contrast, postharvest applications with SA and MeJA were more effective in weight loss reduction in strawberries [7].
Our results showed that fruits treated with 0.25 mM MeJA had a positive role in conserving firmness after 8 and 12 d of refrigerated storage. It has been reported that exogenous MeJA application reduced the activity of some degradation enzymes such as polygalacturonase, delayed cell wall degradation, and therefore, conserved firmness [32]. Our results are in agreement with Zuñiga et al. [33], who found that pre-harvest application of MeJA had little or no effect on strawberry (Camarosa) firmness at harvest time. Moreover, El-Mogy et al. [7] reported that postharvest treatment with MeJA obtained high fruit firmness during 12 d of refrigerated storage, whereas at the last storage time point, both ABA concentrations showed significantly higher firmness values compared to the control. Previous work has indicated that two pre-harvest ABA applications had no effect on firmness during refrigerated storage (9 d) of two strawberry cultivars (Jewel and Wendy) fruits [34]. The difference observed from our results could be due to the fact that our ABA application was carried out three times prior to harvest and on a different cultivar (Festival). From our results, it is clear that ABA application had little or no effect on strawberry firmness loss during refrigerated storage. However, ABA was reported to promote fruit ripening. This may be attributed to the ripening process of the fruit; development is not only governed by the production of one phytohormone but also seems to be the result of a controlled hormonal balance [35,36,37]. Hence, all ripening attributes may not simultaneously appear by a single hormone application. Moreover, some studies have reported that the fruit ripening mechanisms of non-climacteric fruits, that is, strawberries, remain unclear [38].
Strawberry fruit color is the most important trait for visual quality, which is typically accompanied by anthocyanin content [39]. No effects were recorded among all treatments including the control of all fruit surface color parameters: lightness (L*), redness (a*), blueness (b*), chroma (C*), and hue angle (h°) across all storage periods. Our results are in agreement with Aghaeifard et al. [40], who reported that pre-harvest SA foliar application had no significant effect on the lightness (L*) of strawberry fruits during storage. Moreover, previous work reported that SA-treated tomato plants had no significant effects on changes in a* values during storage [31]. In this study, MeJA did not affect L*, a*, b*, C*, or h°. However, our results (shown in Figure 4B) showed that anthocyanin content increased via pre-harvest MeJA application.
4.2. Influence of Treatments on TSS, pH and TA
ABA foliar application substantially enhanced the TSS content of strawberry fruits. Importantly, Ayub et al. [41] agree with our findings. However, this contradicts previous work [42]. In these studies, the authors reported that ABA application did not affect the TSS of strawberries or grapefruits, respectively. Fruit ripening is accelerated by the combined effects of different parameters, that is, color, TSS, firmness, and the accumulation of anthocyanin. Here, the fruits treated with ABA obtained high firmness and total anthocyanin, especially at the last storage period. ABA application to fruit enhanced ethylene synthesis and accelerated fruit ripening [42], which is in agreement with Lopes et al. [43]. Here, ABA treatment showed the same effects on ripening parameters. Variation among the different studies may be the result of differences in ripening stages or genotypes. In our study, fruits were picked at 0.75% red color stage; Fan et al. [44] reported that the TSS of strawberry fruits—especially at the de-greening stage (18 days after anthesis)—was not affected by ABA treatment, in addition to varietal differences in each study.
MeJA application obtained a higher TSS value than untreated fruits. This result is in accordance with Lolaei et al. [45], who reported that strawberry fruits treated with MeJA had a higher soluble solids content than both tested cultivars. In our study, SA treatment did not significantly affect the TSS content. This is in agreement with previous work [18], which reported that pre-harvest SA treatment did not change fruit total soluble solids. Moreover, El-Mogy et al. [7] found that postharvest SA treatment did not affect the TSS content of strawberry fruits. In addition, Karlidag et al. [20] found that SA application enhanced the TSS content of strawberry fruits compared to the control in both tested growing seasons.
Application with 2 SA showed a high TA content at 0 d compared to untreated fruits. This is in agreement with Lolaei et al. [46], who reported that pre-harvest SA application enhanced the rate of TA in strawberry fruits as compared to the control. In contrast, in our experiment, SA foliar application did not change TA in comparison with the control for the other storage periods. Our result agrees with previous studies on strawberry [18,20] and pineapple fruit [47].
4.3. Influence of Treatments on Ascorbic Acid, Total Phenolic, and Anthocyanin Content
AsA was higher in SA-treated strawberry fruits. The highest SA concentration recorded an AsA content higher than the lower concentration. Similar results have been previously reported [20], in which SA significantly enhanced AsA content in strawberries and in which this enhancement was improved by high SA concentrations. In addition, Aghaeifard et al. [40] and Baninaiem et al. [31] reported that pre-harvest SA application significantly increased the AsA of strawberry and tomatoes, respectively. Moreover, Pérez-Llorca et al. [48] found that pre- and post-harvest SA application enhanced the accumulation of bioactive compounds, which led to improved antioxidant activity in both climacteric and non-climacteric fruits. In addition, Martínez-Esplá et al. [49] reported that SA-treated plums obtained significantly higher ascorbic acid, anthocyanins, and phenolic compounds content both at harvest and after prolonged cold storage. In fruits, SA application activates ascorbate peroxidase, which enhances ascorbic acid and antioxidant ability [50]. Other previous work [51] suggests that SA treatment may regulate AsA synthesis under stress conditions or senescence. The obvious effect of SA on AsA content could be enhanced by continuous SA application or increasing the SA concentration. Conversely, Lolaei et al. [46] found that the AsA content in strawberry fruits was not affected by pre-harvest SA application. In addition, Shafiee et al. [18] found that the AsA content of strawberry fruit was unchanged in response to SA addition. It can be observed that ABA application did not affect the AsA content in fruits. This is in agreement with Ayub et al. [41], who did not find any changes in AsA content related to ABA application. The fruits treated with 0.25 mM MeJA obtained significantly higher AsA than the control at the end of the refrigerated storage. This is in agreement with Lolaei et al. [45], who reported a significant increase in AsA content by increasing the MeJA concentration in both tested cultivars (Selva and Queen Elisa). In our study, the highest MeJA dose was 0.50 mM; therefore, increasing the dose above 0.50 mM would indeed show an obvious difference. Importantly, the differences among tested genotypes in each study must be taken into consideration.
A similar TPC increase was observed in strawberry [52] and grape [53], which were pre-harvest-treated with SA compared to the control. SA may interact as a modulator of phenylpropanoid metabolism, resulting in the formation of phenolics and the stimulation of enzymes involved in phenylpropanoid metabolism, such as PAL [54]. It has been demonstrated that SA elicitation can cause PAL activity in apple fruits [51], resulting in the formation of plant secondary metabolites. Furthermore, as observed in lemon fruit, MeJA applied as a post- or pre-harvest treatment has significant effects on boosting bioactive fruit components with antioxidant capacity, contributing to health benefits [55].
The total anthocyanin results are in accordance with Zuñiga et al. [33], who reported that the pre-harvest application of 250 mmol/L MeJA improved the accumulation of anthocyanin in postharvest strawberry fruit. The application of MeJA has been shown to increase anthocyanin accumulation in F. chiloensis and F. ananassa cv. ‘Camarosa’ and cv. ‘Aromas’ in in vitro ripening systems [23,56], due to a stimulatory effect on its biosynthesis via JA signaling activation. This regulates the FaMYC and FaJAZs genes, leading to an increase in bioactive jasmonoylisoleucine and JA-Ile biosynthesis [57]. As a result, the regulatory (FaMYB10) and structural (FaANS, FaUFGT) genes involved in the strawberry (Fananassa ‘Aromas’) anthocyanin production pathway were upregulated [23,56]. Furthermore, Hadian-Deljou et al. [17] demonstrated that pre-harvest SA treatment (1 and 2 mM) showed an increasing tendency of anthocyanin content in apples until the 60th day of storage, after which it began to decline. Although the role of SA in anthocyanin synthesis is unclear, it is possible that SA might activate the main enzyme in the anthocyanin biosynthetic pathway [54]. ABA also promotes color development in strawberries [58] and mangoes [59] by increasing anthocyanin production during storage. Furthermore, the anthocyanin synthesis pathway is more likely to be induced by ABA [38,59]. The accumulation of ABA-induced anthocyanin enhances fruit color and also participates in the activation of plant tissue defense against potential damage [38].
5. Conclusions
The pre-harvest of ABA (at both concentrations) and 0.25 mM MeJA applications prevented fruit softening and conserved TSS at the end of storage. All pre-harvest applications had no significant effect on weight loss, color, or pH during refrigerated storage. The application of 2 mM SA and 0.25 mM MeJA had a positive effect on TA for some storage periods. Some applications, such as 4 mM SA and 0.25 mM MeJA, conserved fruits from AsA loss compared to the control at the end of the storage period. In addition, all pre-harvest applications showed higher TPC and anthocyanin content than the control at the end of storage. Our results conclude that the pre-harvest application of SA, ABA, and MeJA could be more effective for conserving bioactive compounds. Moreover, due to the scarcity of available literature on strawberries, further studies are required to support our hypothesis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae7120568/s1. Table S1: Effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on weight loss and firmness (N) of strawberry fruits stored at 4 °C for 12 days. Table S2: Effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on surface colour of strawberry fruits stored at 4 °C for 12 days. Table S3: Effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on TSS, pH, and TA of strawberry fruits stored at 4 °C for 12 days. Table S4: Effect of foliar application of salicylic acid (SA) (2 and 4 mM), abscisic acid (ABA) (0.25 and 0.50 mM), and methyl jasmonate (MeJA) (0.25 and 0.50 mM) on ascorbic acid (AsA), total phenolic, and anthocyanin of strawberry fruits stored at 4 °C for 12 days.
Author Contributions
Conceptualization, O.S.D., M.M.E.-M. and M.R.A.; methodology, O.S.D., M.M.E.-M. and M.R.A.; software, M.M.E.-M. and E.K.; validation, O.S.D.; formal analysis, B.N.S.; investigation, M.M.E.-M. and M.R.A.; resources, M.M.E.-M. and K.M.A.R.; data curation, O.S.D. and E.K.; writing—original draft preparation, O.S.D., M.M.E.-M. and M.R.A.; writing—review and editing, O.S.D., M.M.E.-M., B.N.S. and K.M.A.R.; visualization, M.R.A. and E.K.; supervision, M.M.E.-M. and O.S.D.; project administration, O.S.D.; funding acquisition, O.S.D., M.M.E.-M. and M.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to Taif University, for funding the current study by Taif University Research Supporting Project number (TURSP-2020/307), Taif University, Taif, Saudi Arabia. Also, the authors acknowledge Cairo University, Faculty of Agriculture for supporting facilities, equipment’s, and other research tools for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Mogy, M.M.; Ludlow, R.A.; Roberts, C.; Müller, C.T.; Rogers, H.J. Postharvest exogenous melatonin treatment of strawberry reduces postharvest spoilage but affects components of the aroma profile. J. Berry Res. 2019, 9, 297–307. [Google Scholar] [CrossRef]
- Hadi, A.; Askarpour, M.; Miraghajani, M.; Symonds, M.E.; Sheikhi, A.; Ghaedi, E. Effects of strawberry supplementation on cardiovascular risk factors: A comprehensive systematic review and meta-analysis of randomized controlled trials. Food Funct. 2019, 10, 6987–6998. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Alsanius, B.W. Cassia oil for controlling plant and human pathogens on fresh strawberries. Food Control 2012, 28, 157–162. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Y.; Xu, F.; Shao, X. The combined effects of tea tree oil and hot air treatment on the quality and sensory characteristics and decay of strawberry. Postharvest Biol. Technol. 2018, 136, 139–144. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Ali, M.R.; Darwish, O.S.; Rogers, H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Res. 2019, 9, 333–348. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Chalupowicz, D.; Gamrasni, D.; Goldberg, T.; Nerya, O.; Ben-Arie, R.; Porat, R. Effects of the ethylene-action inhibitor 1-methylcyclopropene on postharvest quality of non-climacteric fruit crops. Postharvest Biol. Technol. 2016, 111, 322–329. [Google Scholar] [CrossRef]
- Nguyen, V.T.B.; Nguyen, D.H.H.; Nguyen, H.V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria × ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. UV-C treatment maintains quality and enhances antioxidant capacity of fresh-cut strawberries. Postharvest Biol. Technol. 2019, 156, 110945. [Google Scholar] [CrossRef]
- Shehata, S.A.; El-Mogy, M.M.; Mohamed, H.F.Y. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2019, 25, 196–209. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Mahmoud, A.W.M.; El-Sawy, M.B.I.; Parmar, A. Pre-Harvest Foliar Application of Mineral Nutrients to Retard Chlorophyll Degradation and Preserve Bio-Active Compounds in Broccoli. Agronomy 2019, 9, 711. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelgawad, K.F.; El-Mogy, M.M. Quality and Shelf-Life of Onion Bulbs Influenced by Biostimulants. Int. J. Veg. Sci. 2017, 23, 362–371. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; García-Pastor, M.E.; Zapata, P.J.; Guillén, F.; Serrano, M.; Valero, D.; Gironés-Vilaplana, A. Preharvest application of oxalic acid improves quality and phytochemical content of artichoke (Cynara scolymus L.) at harvest and during storage. Food Chem. 2017, 230, 343–349. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-Harvest Treatment of Chitosan Oligosaccharides Improved Strawberry Fruit Quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bi, Y. The role of signal production and transduction in induced resistance of harvested fruits and vegetables. Food Qual. Saf. 2021, 5, fyab011. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.S.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E.; Turan, M. Exogenous applications of salicylic acid affect quality and yield of strawberry grown under antifrost heated greenhouse conditions. J. Plant Nutr. Soil Sci. 2009, 172, 270–276. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef]
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.A.; Schwab, W.; Figueroa, C.R. Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 2013, 70, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Blanch, G.P.; del Castillo, M.L.R. Changes in strawberry volatile constituents after pre-harvest treatment with natural hormonal compounds. Flavour Fragr. J. 2012, 27, 180–187. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef]
- Awad, A.H.R.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef]
- Ali, M.R.; Mohamed, R.M.; Abedelmaksoud, T.G. Functional strawberry and red beetroot jelly candies rich in fibers and phenolic compounds. Food Syst. 2021, 4, 12–18. [Google Scholar] [CrossRef]
- Abdelgawad, K.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Abdallah, I.S.; Abdelgawad, K.F.; El-Mogy, M.M.; El-Sawy, M.B.I.; Mahmoud, H.A.; Fahmy, M.A.M. Weed Control, Growth, Nodulation, Quality and Storability of Peas as Affected by Pre- and Postemergence Herbicides. Horticulturae 2021, 7, 307. [Google Scholar] [CrossRef]
- Tonu, T.; Ulvi, M.; Lech, S. Strawberry anthocyanin determination by pH differential spectroscopic method- how to get true results? Acta Sci. Pol. Hortorum Cultus 2014, 13, 35–47. Available online: https://czasopisma.up.lublin.pl/index.php/asphc/article/view/2729 (accessed on 1 December 2021).
- Baninaiem, E.; Mirzaaliandastjerdi, A.M.; Rastegar, S.; Kh, A. Effect of pre- and postharvest salicylic acid treatment on quality characteristics of tomato during cold storage. Adv. Hortic. Sci. 2017, 30, 183–192. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Lobos, T.; Cardemil, L.; Nunes-Nesi, A.; Retamales, J.; Jaakola, L.; Alberdi, M.; Ribera-Fonseca, A. Methyl Jasmonate: An Alternative for Improving the Quality and Health Properties of Fresh Fruits. Molecules 2016, 21, 567. [Google Scholar] [CrossRef]
- Zuñiga, P.E.; Castañeda, Y.; Arrey-Salas, O.; Fuentes, L.; Aburto, F.; Figueroa, C.R. Methyl Jasmonate Applications From Flowering to Ripe Fruit Stages of Strawberry (Fragaria × ananassa ‘Camarosa’) Reinforce the Fruit Antioxidant Response at Post-harvest. Front. Plant Sci. 2020, 11, 538. [Google Scholar] [CrossRef]
- El Kayal, W.; El-Sharkawy, I.; Dowling, C.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Effect of preharvest application of hexanal and growth regulators in enhancing shelf life and regulation of membrane-associated genes in strawberry. Can. J. Plant Sci. 2017, 97, 1109–1120. [Google Scholar] [CrossRef]
- Symons, G.M.; Chua, Y.J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar] [CrossRef]
- Teribia, N.; Tijero, V.; Munné-Bosch, S. Linking hormonal profiles with variations in sugar and anthocyanin contents during the natural development and ripening of sweet cherries. N. Biotechnol. 2016, 33, 824–833. [Google Scholar] [CrossRef]
- Li, Y.; Lua, Y.; Lia, L.; Chu, Z.; Zhang, H.; Li, H. Impairment of hormone pathways results in a general disturbance of fruit primary metabolism in tomato. Food Chem. 2019, 274, 170–179. [Google Scholar] [CrossRef]
- Leng, P.; Yuan, B.; Guo, Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lara, R.; Gordillo, B.; Rodríguez-Pulido, F.J.; Lourdes González-Miret, M.; del Villar-Martínez, A.A.; Dávila-Ortiz, G.; Heredia, F.J. Assessment of the differences in the phenolic composition and color characteristics of new strawberry (Fragaria × ananassa Duch.) cultivars by HPLC–MS and Imaging Tristimulus Colorimetry. Food Res. Int. 2015, 76, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Aghaeifard, F.; Babalar, M.; Fallahi, E.; Ahmadi, A. Influence of humic acid and salicylic acid on yield, fruit quality, and leaf mineral elements of strawberry (Fragaria × Ananassa duch.) cv. Camarosa. J. Plant Nutr. 2016, 39, 1821–1829. [Google Scholar] [CrossRef]
- Ayub, R.A.; Bosetto, L.; Galvão, C.W.; Etto, R.M.; Inaba, J.; Lopes, P.Z. Abscisic acid involvement on expression of related gene and phytochemicals during ripening in strawberry fruit Fragaria × ananassa cv. Camino Real. Sci. Hortic. 2016, 203, 178–184. [Google Scholar] [CrossRef]
- Ren, J.; Sun, L.; Wang, C.; Zhao, S.; Leng, P. Expression analysis of the cDNA for magnesium chelatase H subunit (CHLH) during sweet cherry fruit ripening and under stress conditions. Plant Growth Regul. 2011, 63, 301–307. [Google Scholar] [CrossRef]
- Lopes, P.Z.; Fornazzari, I.M.; Almeida, A.T.; Galvão, C.W.; Etto, R.M.; Inaba, J.; Ayub, R.A. Effect of ethylene treatment on phytochemical and ethylene-related gene expression during ripening in strawberry fruit Fragaria × ananassa cv. Camino Real. Genet. Mol. Res. 2015, 14, 16113–16125. [Google Scholar] [CrossRef]
- Fan, M.; Cong, G.; Yanling, L.; Hao-Ru, T.; Qing, C.; Bo, S.; Yong, Z.; Ya, L. Abscisic Acid Affects Strawberry Fruit Quality. In Proceedings of the 2018 International Conference on Management, Economics, Education, Arts and Humanities (MEEAH 2018), Shenzhen, China, 14–15 July 2018; December 2018; pp. 24–29. [Google Scholar]
- Lolaei, A.; Zamani, S.; Ahmadian, E.; Mobasheri, S. Effect of methyl jasmonate on the composition of yield and growth of strawberry (Selva and Queen Elisa). Int. J. Agric. Crop. Sci. 2013, 5, 200–206. [Google Scholar]
- Lolaei, A.; Kaviani, B.; Rezaei, M.A.; Raad, M.K.; Mohammadipour, R. Effect of pre- and postharvest treatment of salicylic acid on ripening of fruit and overall quality of strawberry (Fragaria ananasa Duch cv.Camarosa) fruit. Ann. Biol. Res. 2012, 3, 4680–4684. [Google Scholar]
- Lu, X.; Sun, D.; Li, Y.; Shi, W.; Sun, G. Pre- and post-harvest salicylic acid treatments alleviate internal browning and maintain quality of winter pineapple fruit. Sci. Hortic. 2011, 130, 97–101. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Müller, M.; Munné-Bosch, S. Biosynthesis, Metabolism and Function of Auxin, Salicylic Acid and Melatonin in Climacteric and Non-climacteric Fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Preharvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2017, 98, 2742–2750. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Kong, W.; Li, S.; Archbold, D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006, 41, 244–251. [Google Scholar] [CrossRef]
- Hadian-Deljou, M.; Esna-Ashari, M.; Sarikhani, H. Effect of pre- and post-harvest salicylic acid treatments on quality and antioxidant properties of ‘Red Delicious’ apples during cold storage. Adv. Hortic. Sci. 2017, 31, 31–38. [Google Scholar]
- Geransayeh, M.; Sepahvand, S.; Abdossi, V. Extending Postharvest Longevity and Improving Quality of Strawberry (Fragaria Ananasa Duch Cv. ‘Gaviota’) Fruit by Postharvest Salicylic Acid Treatment. J. Agric. Stud. 2015, 3, 17. [Google Scholar] [CrossRef][Green Version]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Technol. 2015, 52, 3607–3616. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.; Loyola-Vargas, V.M. Effect of acetylsalicylic acid on secondary metabolism ofCatharanthus roseus tumor suspension cultures. Plant Cell Rep. 1997, 16, 287–290. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef]
- Delgado, L.D.; Zúñiga, P.E.; Figueroa, N.E.; Pastene, E.; Escobar-Sepúlveda, H.F.; Figueroa, P.M.; Garrido-Bigotes, A.; Figueroa, C.R. Application of a JA-Ile Biosynthesis Inhibitor to Methyl Jasmonate-Treated Strawberry Fruit Induces Upregulation of Specific MBW Complex-Related Genes and Accumulation of Proanthocyanidins. Molecules 2018, 23, 1433. [Google Scholar] [CrossRef]
- Garrido-Bigotes, A.; Figueroa, P.M.; Figueroa, C.R. Jasmonate Metabolism and Its Relationship with Abscisic Acid During Strawberry Fruit Development and Ripening. J. Plant Growth Regul. 2018, 37, 101–113. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gray, D.J.; Lu, J.; Gu, L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2011, 126, 982–988. [Google Scholar] [CrossRef]
- Kumar, S.P.; Maurer, D.; Feygenberg, O.; Love, C.; Alkan, N. Improving the Red Color and Fruit Quality of ‘Kent’ Mango Fruit by Pruning and Preharvest Spraying of Prohydrojasmon or Abscisic Acid. Agronomy 2020, 10, 944. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).