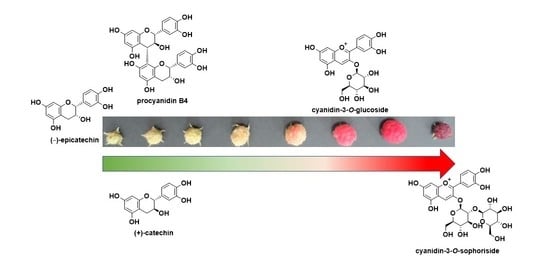
Changes in the Polyphenol Content of Red Raspberry Fruits during Ripening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Raspberry Cultivation Method and Sample Preparation for Analysis
2.3. Fruit Weight and Water Content
2.4. Total Polyphenol Analysis
2.5. Total Proanthocyanidin Analysis
2.6. Total Anthocyanin Analysis
2.7. DPPH Radical Scavenging Assay
2.8. ABTS Radical Scavenging Assay
2.9. Total Vitamin C Analysis
2.10. Total Glucose Analysis
2.11. LC-MS Analysis Conditions
2.12. Semi-Quantitative RT-PCR Analysis of Flavan-3-ol Derivative Biosynthetic-Enzyme-Related Gene Expression
3. Results and Discussion
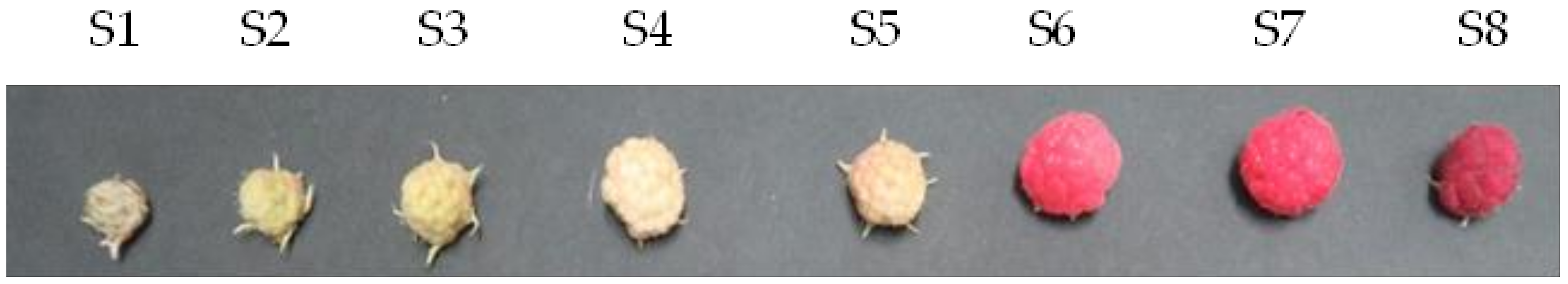
3.1. Raspberry Fruit Cultivation and Harvest Based on the Level of Maturity
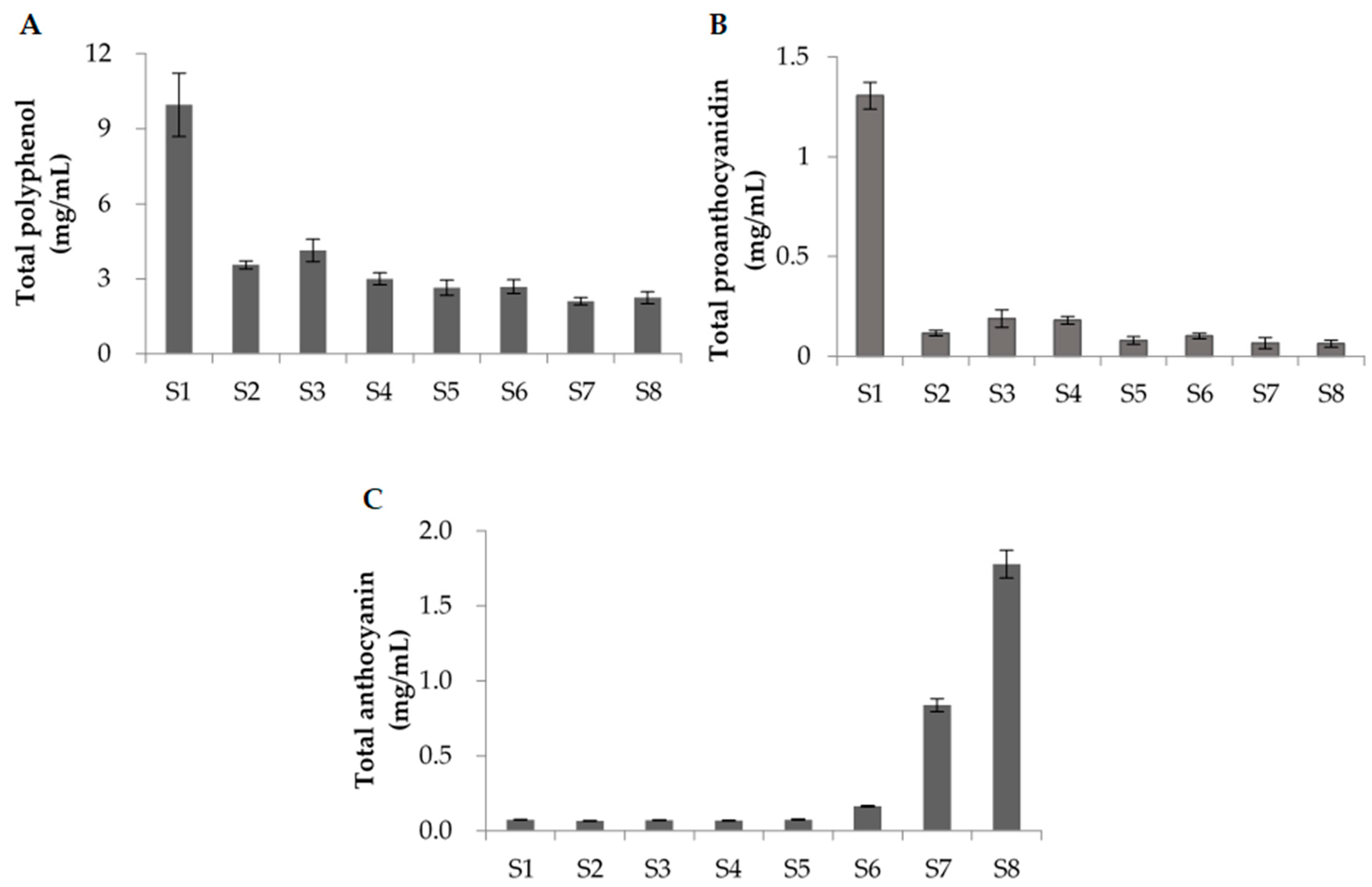
3.2. Analysis of the Polyphenol Content at Each Maturation Stage
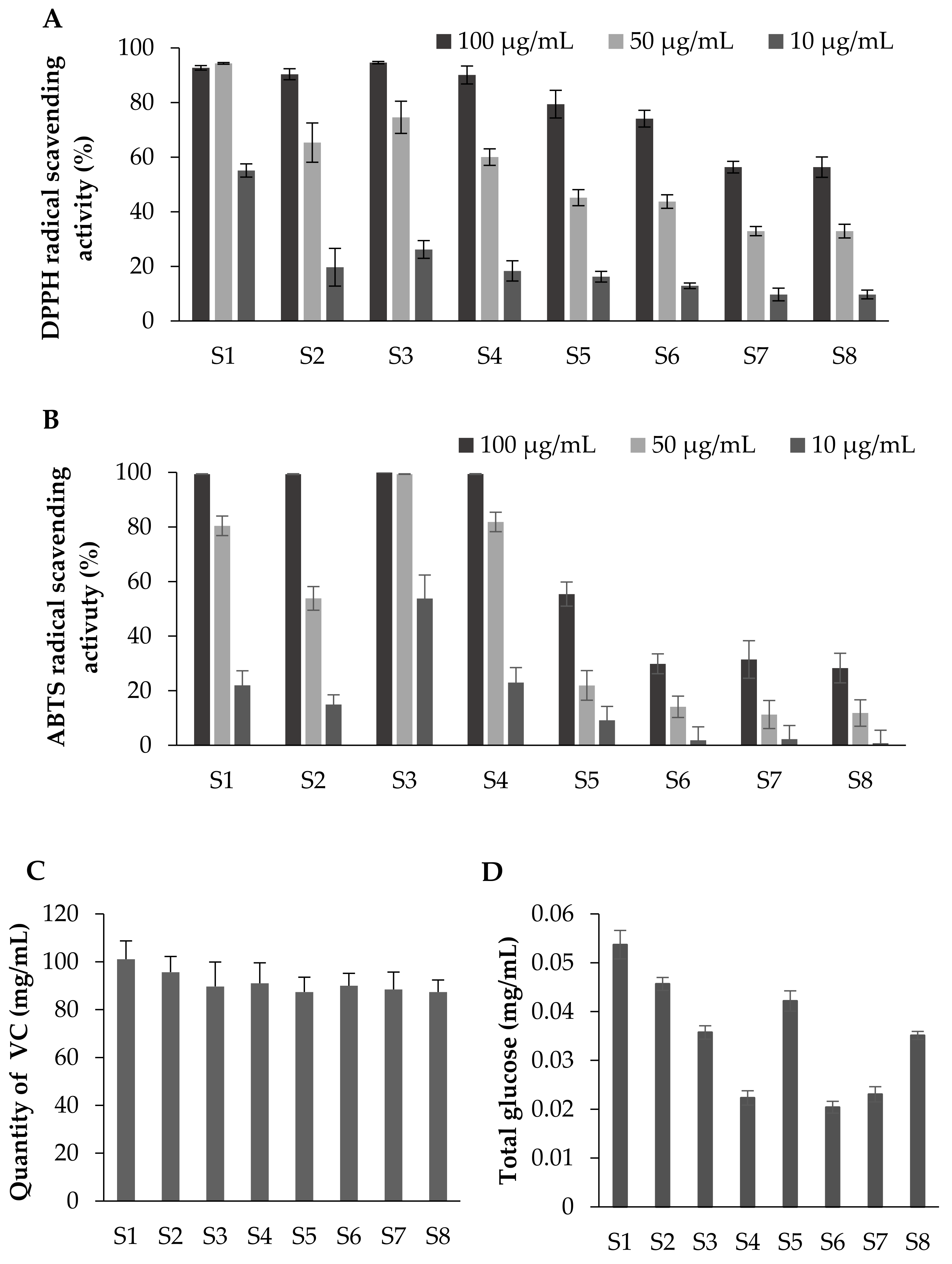
3.3. Radical Scavenging Activity at Each Maturation Stage
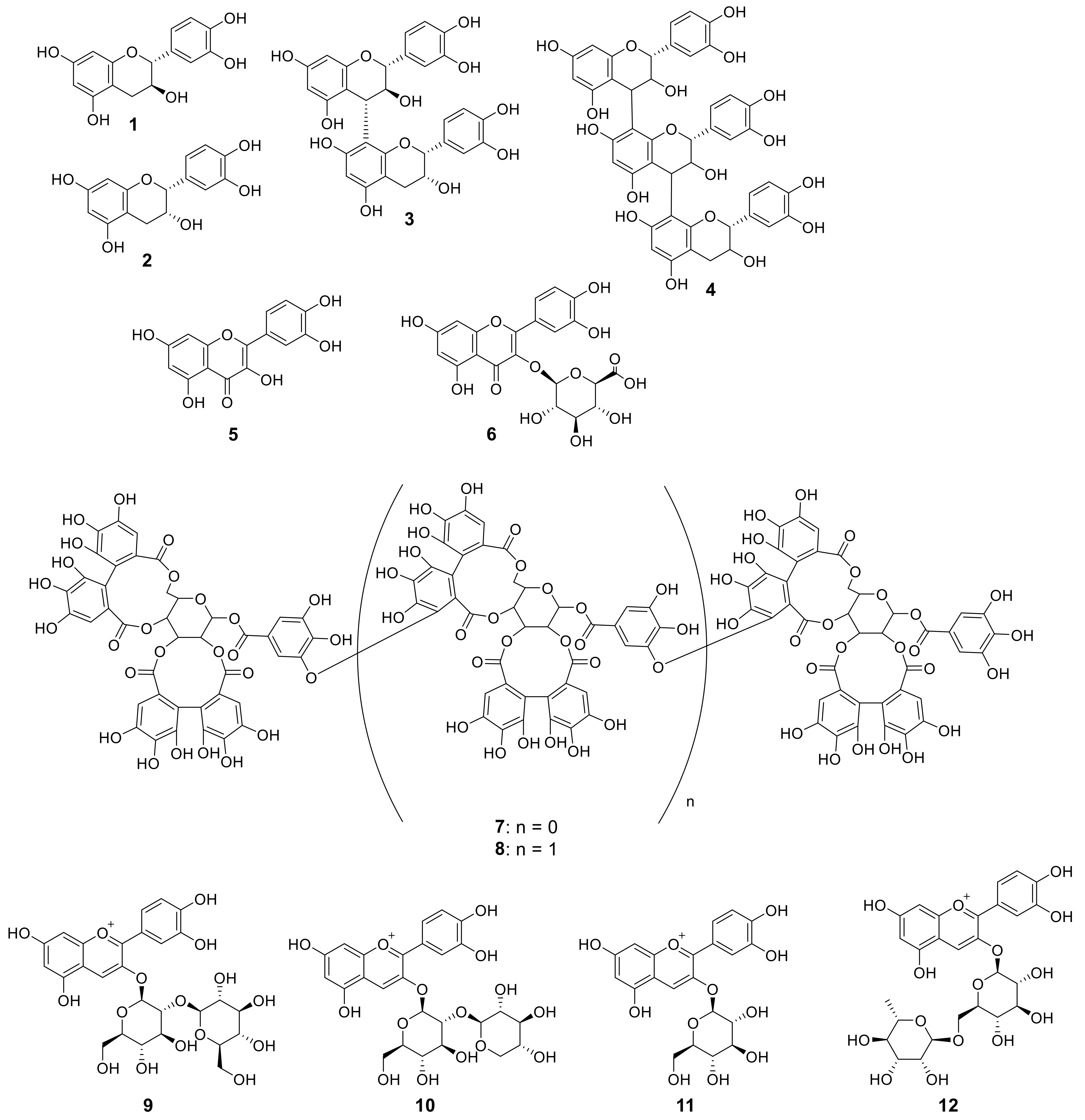
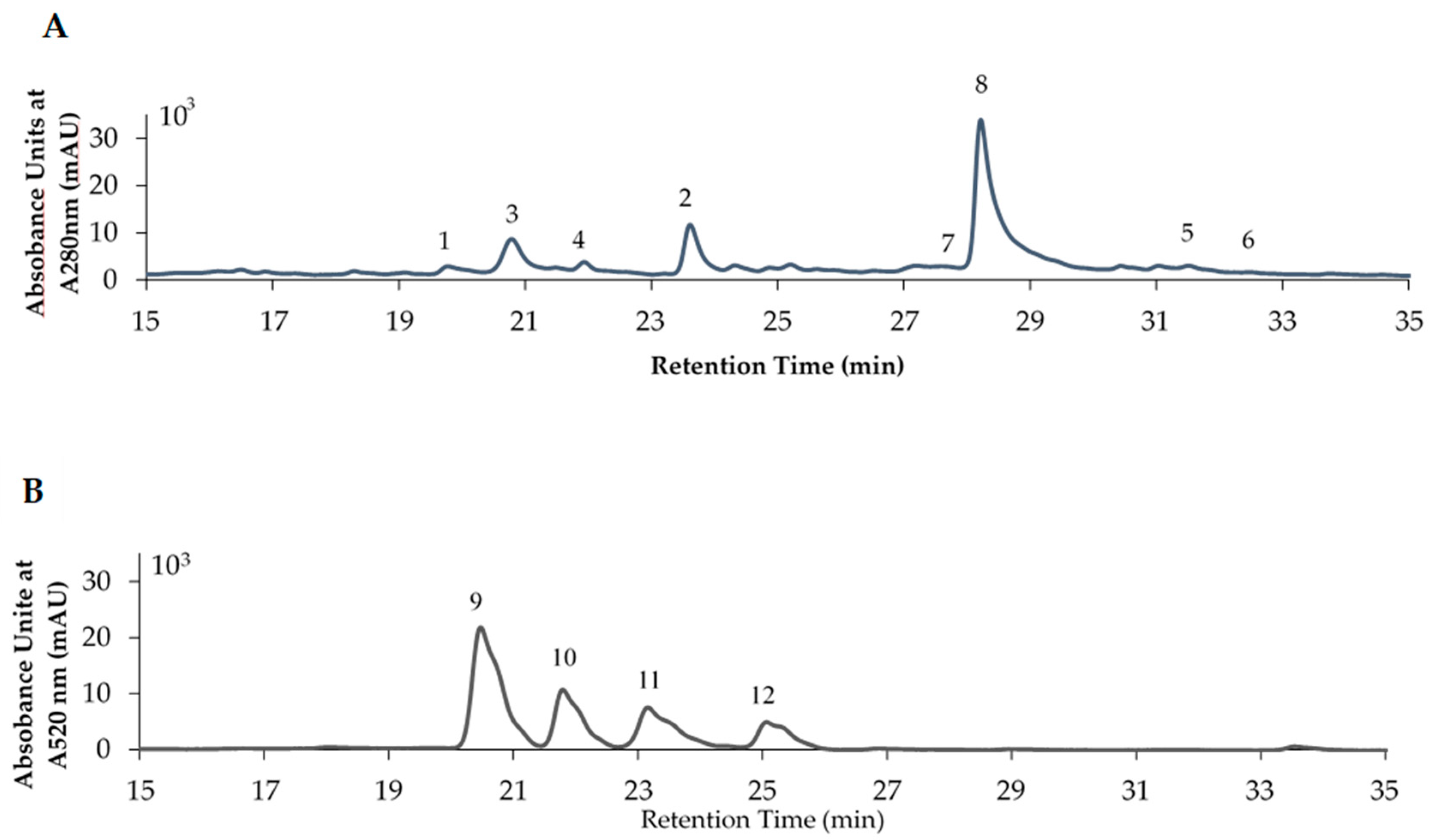
3.4. Identification and Quantification of the Polyphenol Compounds by HPLC Analysis
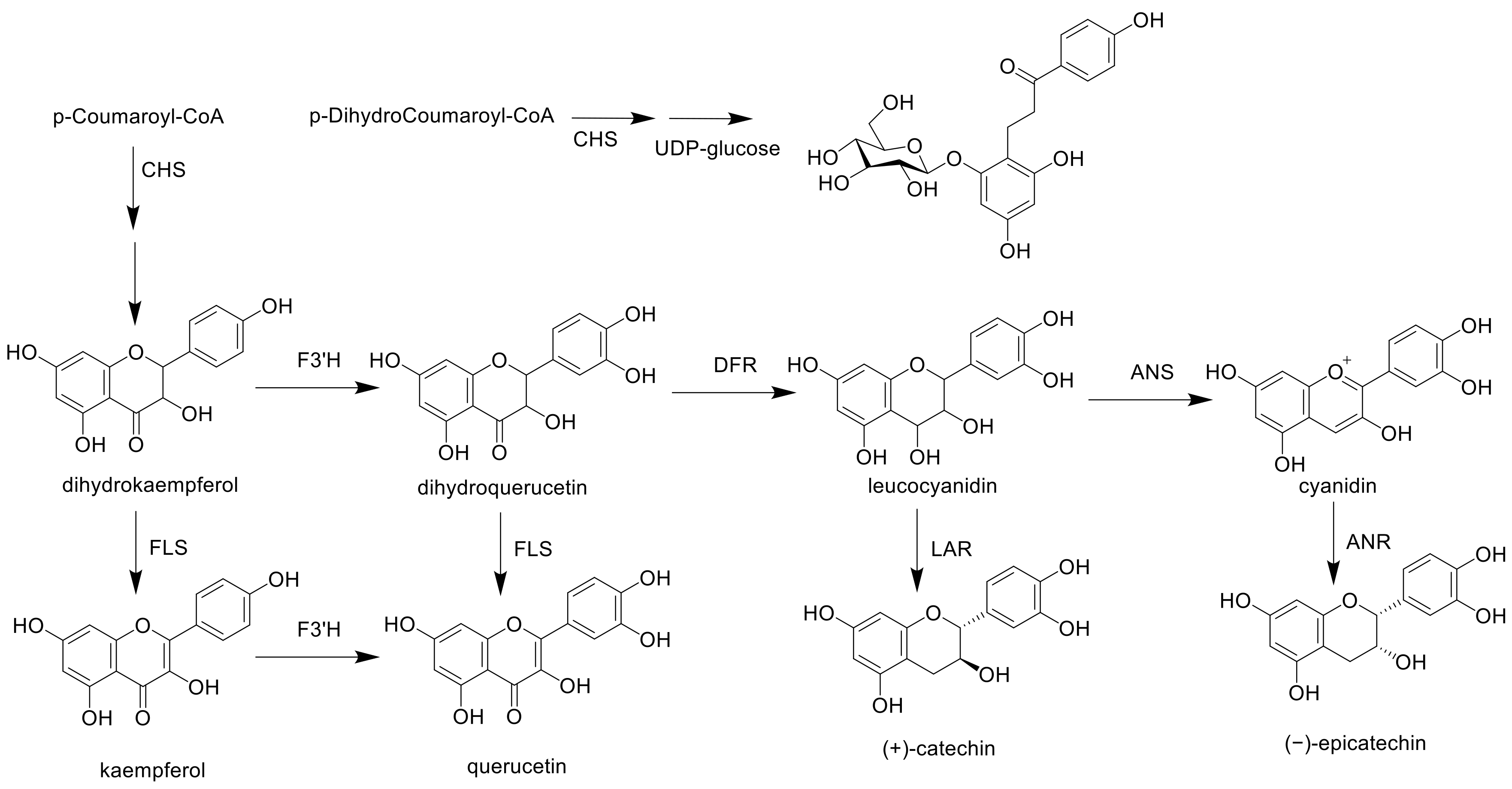
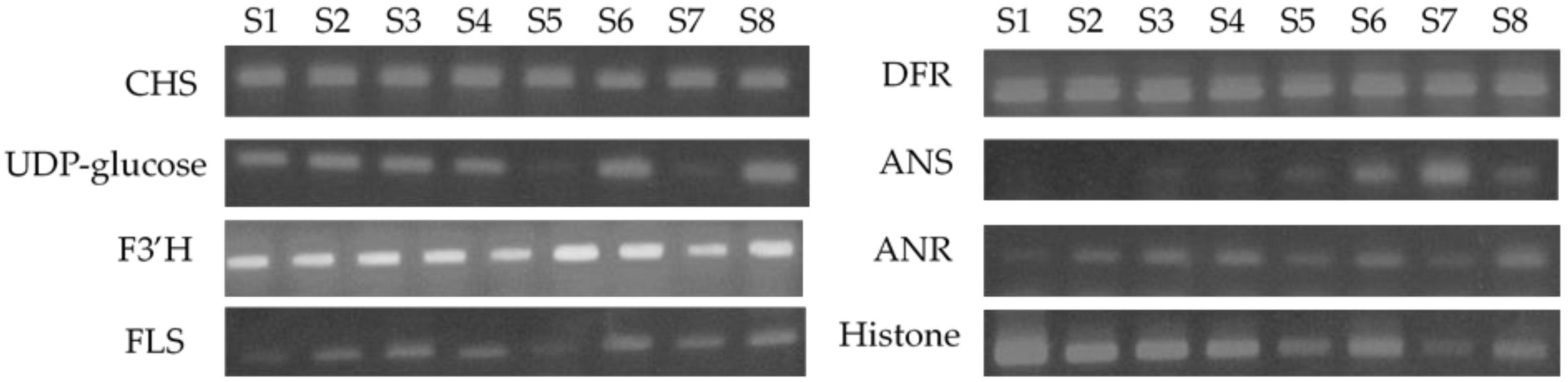
3.5. Semi-Quantitative RT-PCR Analysis of the Expression Levels of the Flavan-3-ol Biosynthetic Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Harborne, J.B. The Flavonoids: Advances in Research from 1986; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 2. Stereoselective gram-scale synthesis of procyanidin-B3. Tetrahedron 2002, 58, 7829–7837. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 4. The synthesis of procyanidin B1 and B4. TMSOTf-catalyzed cyclization of catechin and epicatechin condensation. Heterocycles 2003, 61, 287–298. [Google Scholar]
- Saito, A.; Doi, Y.; Tanaka, A.; Matsuura, N.; Ubukata, M.; Nakajima, N. Systematic synthesis of four epicatechin series procyanidin trimers and their inhibitory activity on the Maillard reaction and antioxidant activity. Bioorg. Med. Chem. 2004, 12, 4783–4790. [Google Scholar] [CrossRef]
- Saito, A.; Mizushina, Y.; Tanaka, A.; Nakajima, N. Versatile synthesis of epicatechin series procyanidin oligomers, and their antioxidant and DNA polymerase inhibitory activity. Tetrahedron 2009, 65, 7422–7428. [Google Scholar] [CrossRef]
- Hamada, Y.; Takano, S.; Ayano, Y.; Tokunaga, M.; Koashi, T.; Okamoto, S.; Doi, S.; Ishida, M.; Kawasaki, T.; Hamada, M.; et al. Structure–Activity relationship of oligomeric flavan-3-ols: Importance of upper-unit B-ring hydroxyl groups in the dimeric structure for strong activities. Molecules 2015, 20, 18870–18885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashino, Y.; Okamoto, T.; Mori, K.; Kawasaki, T.; Hamada, M.; Nakajima, N.; Saito, A. Regioselective synthesis of procyanidin B6, a 4-6-condensed (+)-catechin dimer, by intramolecular condensation. Molecules 2018, 23, 205. [Google Scholar] [CrossRef] [Green Version]
- Saito, A.; Emoto, M.; Tanaka, A.; Doi, Y.; Shoji, K.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Matsuura, N.; Nakajima, N. Stereoselective synthesis of procyanidin B3-3-O-gallate and 3,3′′-di-O-gallate, and their abilities as antioxidant and DNA polymerase inhibitor. Tetrahedron 2004, 60, 12043–12049. [Google Scholar] [CrossRef]
- Saito, A.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Doi, Y.; Tanaka, A.; Nakajima, N. Systematic synthesis of galloyl-substituted procyanidin B1 and B2, and their ability of DPPH radical scavenging activity and inhibitory activity of DNA polymerases. Bioorg. Med. Chem. 2005, 13, 2759–2771. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, H.; Saito, A.; Mizushina, Y.; Yoshida, H.; Tanaka, A.; Nakajima, N. Synthesis of galloyl-substituted procyanidin B4 series, and their deep radical scavenging activity and DNA polymerase inhibitory activity. Heterocycles 2006, 67, 175–188. [Google Scholar]
- Mori, K.; Ayano, Y.; Hamada, Y.; Hojima, T.; Tanaka, R.; Higashino, Y.; Izuno, M.; Okamoto, T.; Kawasaki, T.; Hamada, M.; et al. Role of 2,3-cis structure of (–)-epicatechin-3,5-O-digallate in inhibition of HeLa S3 cell proliferation. Nat. Prod. Chem. Res. 2015, 3, 172. [Google Scholar]
- Hojima, T.; Komeda, S.; Higashino, Y.; Hamada, M.; Nakajima, N.; Kawasaki, T.; Saito, A. Role of 3,5-digalloyl and 3′,4′-dihydroxyl structure of (–)-epicatechin-3,5-digallate in inhibition of HeLa S3 cell proliferation. Nat. Prod. Chem. Res. 2017, 5, 250. [Google Scholar] [CrossRef]
- Matsubara, K.; Saito, A.; Tanaka, A.; Nakajima, N.; Akagi, R.; Mori, M.; Mizushina, Y. Epicatechin conjugated with fatty acid is a potent inhibitor of DNA polymerase and angiogenesis. Life Sci. 2007, 80, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Doi, S.; Harui, K.; Okamoto, T.; Okamoto, S.; Uenishi, J.; Kawasaki, T.; Nakajima, N.; Saito, A. Development of a new synthetic strategy for procyanidin dimer condensation using peracetylated electrophiles. Heterocycles 2014, 88, 1595–1602. [Google Scholar]
- Okamoto, S.; Ishihara, S.; Okamoto, T.; Doi, S.; Harui, K.; Higashino, Y.; Kawasaki, T.; Nakajima, N.; Saito, A. Inhibitory activity of synthesized acetylated procyanidin B1 analogues against HeLa S3 cells proliferation. Molecules 2014, 19, 1775–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukuda, S.; Watashi, K.; Hojima, T.; Isogawa, M.; Iwamoto, M.; Omagari, K.; Suzuki, R.; Aizaki, H.; Kojima, S.; Sugiyama, M.; et al. A new class of hepatitis B and D virus entry inhibitors, proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology 2017, 65, 1104–1116. [Google Scholar] [CrossRef] [Green Version]
- Hummer, K.E. Rubus pharmacology: Antiquity to the present. HortScience 2010, 45, 1587–1591. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.V.; Synder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanidin-rich fractions from polana raspberry (Rubus ideaus L.) fruit and juice-in vitro study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.; Miranda-Garcia, O.; Sasaki, G.; Shay, N.F. Consumption of a single serving of red raspberries per day reduces metabolic syndrome parameters in high-fat fed mice. Food Funct. 2017, 8, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Olle, M.; Ngouajio, M.; Siomos, A. Vegetable quality and productivity as influenced by growing medium: A review. Zemdirb. Agric. 2012, 99, 399–408. [Google Scholar]
- Shukitt-Hale, B.; Thangthaeng, N.; Kelly, M.E.; Smith, D.E.; Miller, M.G. Raspberry differentially improves age-related declines in psychomotor function dependent on baseline motor ability. Food Funct. 2017, 8, 4752–4759. [Google Scholar] [CrossRef]
- Nowak, A.; Sojka, M.; Klewicka, E.; Lipinska, L.; Klewicki, R.; Kolodziejczyk, K. Ellagitannins from Rubus ideaus L. exert geno- and cytotoxic effects against human colon adenocarcinoma cell line Caco-2. J. Agric. Food Chem. 2017, 65, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yao, G.D.; Song, X.Y.; Wang, B.; Lin, B.; Wang, X.B.; Huang, X.X.; Song, S.J. Neuroprotective effects of 1,2-diarylpropane type phenylpropanoid enantiomers from red raspberry against H2O2-induced oxidative stress in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2018, 66, 331–338. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Luan, G.; Zhang, S.; Zhuoma, Y.; Xie, J.; Zhou, W. Quantitative analysis of nine phenolic compounds and their antioxidant activities from thirty-seven varieties of raspberry grown in the Qinghai-Tibetan plateau region. Molecules 2019, 24, 3932. [Google Scholar] [CrossRef] [Green Version]
- Ponder, A.; Hallmann, E. The effects of organic and conventional farm management and harvest time on the polyphenol content in different raspberry cultivars. Food Chem. 2019, 301, 125295. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-qTOF-MS/MS characterization, antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobori, R.; Hashimoto, S.; Koshimizu, H.; Yakami, S.; Hirai, M.; Noro, K.; Kawasaki, T.; Saito, A. Flavan-3-ols content in red raspberry leaves increases under blue LED-light irradiation. Metabolites 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- LI, Q.; Chang, X.X.; Wang, H.; Brennan, C.S.; Guo, X.B. Phytochemicals accumulation in Sanhua Plum (Prunus salicina L.) during fruit development and their potential use as antioxidants. J. Agric. Food Chem. 2019, 67, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Ndou, A.; Tinyani, P.P.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. An integrated approach for harvesting Natal plum (Carissa macrocarpa) for quality and functional compounds related to maturity stages. Food Chem. 2019, 293, 499–510. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Giuffrida, D.; Mangraviti, D.; Rigano, F.; Mondello, L.; Arlorio, M.; Coïsson, J.D. Characterization of peel and pulp proanthocyanidins and carotenoids during ripening in persimmon “Kaki Tipo” cv, cultivated in Italy. Food Res. Int. 2019, 120, 800–899. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Chen, D.; Fan, M.; Young, Q.S. UPLC-QqQ-MS/MS-based phenolic quantification and antioxidant activity assessment for thinned young kiwifruits. Food Chem. 2019, 281, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Bombai, G.; Pasini, F.; Verardo, V.; Sevindik, O.; Di Foggia, M.; Tessarin, P.; Bregoli, A.M.; Taddei, P.; Cabone, M.F.; Rombola, A.D. Monitoring of compositional changes during berry ripening in grape seed extracts of cv. sagiovese (Vitis vinifera L.). J. Sci. Food Agric. 2017, 97, 3058–3064. [Google Scholar] [CrossRef]
- Zorenc, Z.; Veberic, R.; Koron, D.; Miosic, S.; Hutabarat, O.S.; Halbwirth, H.; Mikulic-Petkovsek, M. Polyphenol metabolism in different colored cultivars of red currant (Ribes rubrum L.) through fruit ripening. Planta 2017, 246, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Johnson-Cicalese, J.; Singh, A.P.; Vorsa, N. Characterization and quantification of flavonoids and organic acid over fruit development in American cranberry (Vaccinium macrocarpon) cultivars using HPLC and APCI-MS/MS. Plant Sci. 2017, 262, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Nagpala, E.G.; Guidarelli, M.; Gasperotti, M.; Masuero, D.; Bertolini, P.; Vrhovsek, U.; Baraldi, E. Polyphenols variation in fruits of the susceptible strawberry cultivar alba during-ripening and upon fungal pathogen interaction and possible involvement in unripe fruit tolerance. J. Agric. Food Chem. 2016, 64, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, J.; Chen, Z.; Jiang, J.; Jacson, A. Characterization of carotenoids and phenolics during fruit ripening of Chinese raspberry (Rubus chingii Hu). RSC Adv. 2021, 11, 10804–10813. [Google Scholar] [CrossRef]
- Boeges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of Flavonoid and Phenolic Antioxidants in Black Currants, Blueberries, Raspberry, Red Currants, and Cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar]
- Folin, O.; Denis, W. A Colorimetric method for the determination of phenols (and derivatives) in urine. J. Biol. Chem. 1915, 22, 305–308. [Google Scholar] [CrossRef]
- Juilcunen-Tiittoo, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Harbertrson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in skins and seed of cabernet sauvignon, syrah, and pinot noir berries during ripening. Am. J. Enol. Vitic. 2002, 52, 54–59. [Google Scholar]
- Cáceres-Mella, Á.; Peña-Neira, Á.; Narváez-Bastias, J.; Jara-Campos, C.; López-Solís, R.; Canals, J.M. Comparison of analysis methods for measuring proanthocyanidins in wines and their relationship with perceived astringency. Int. J. Food Sci. Technol. 2013, 48, 2588–2594. [Google Scholar] [CrossRef]
- Gao, L.; Mazz, G. Quantitation and distribution of simple and acylated anthocyanins and other phenolics in blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kishida, E.; Nishimoto, Y.; Kojo, S. Specific determination of ascorbic acid with chemical derivatization and high-performance liquid chromatography. Anal. Chem. 1992, 64, 1505–1507. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substance. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zheng, D.; Schröder, G.; Schröder, J.; Hrazdina, G. Molecular and biochemical characterization of three aromatic polyketide synthase genes from Rubus idaeus. Plant Mol. Biol. 2001, 46, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McCallum, S.; Woodhead, M.; Hackett, C.A.; Kassim, A.; Paterson, A.; Graham, J. Genetic and environmental, effects influencing fruit color and QTL analysis in raspberry. Theor. Appl. Genet. 2010, 121, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Rafique, M.Z.; Carvalho, E.; Stracke, R.; Palmieri, L.; Harrera, L.; Feller, A.; Malnoy, M.; Martens, S. Martens. Nonsense mutation inside anthocyanidin synthase gene controls pigmentation in yellow raspberry (Rubus idaeus L.). Front. Plant Sci. 2016, 7, 1892. [Google Scholar] [CrossRef] [PubMed]
- Beekwilder, J.; Jonker, H.; Meesters, P.; Hall, R.D.; Van der Meer, I.M.; Ric de Vos, C.H. Antioxidants in Raspberry: On line analysis links antioxidant activity to diversity of individual metabolites. J. Agric. Food Chem. 2005, 53, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Glod, D.; Kula, M.; Majdam, M.; Halasa, R.; Matkowski, A.; Kozlowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus ideaus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef] [PubMed]
| Gene | Accession No. of Sequence Used for Primer Design | Primer Sequence |
|---|---|---|
| CHS | AF292367 | F: AAC CCT TGT TTC TTC GTA CCA TTA |
| R: GAT GGG TAG CTA GTA CTT ACA CAT | ||
| UDP-glucose | AWT04749 | F: CCATGTTTTCTTGGTTTCCTT |
| R: ATGAAAGGGTTGTTAATGAGG | ||
| F3′H | GT029980 | F: TGA TGA AGC TTT ATA AGC ATG TGA GC |
| R: GGG TCC ACT CTC TTG GTG AA | ||
| FLS | GT029981 | F: AGG TGA ACAGGT GGA GTT GG |
| R: TGA AGA CCA TCA TCG AAT GC | ||
| DFR | GT029979 | F: ATG CGA AAC AAC TTG CAT TT |
| R: GCT ACG ATT CAC GAC ATT GC | ||
| ANS | KX950789.1 | F: ATC GTA ATG CAC ATA GGC GAC ACC |
| R: CCT TGG GCG GCT CAG AGA AAA | ||
| ANR | AMP19723 | F: ATC TCA AAC AAG ACT GCT TGT G |
| R: GAG AGT ATT GAC AGT CAC TGC AG | ||
| Histone | AF301365.1 | F: CAA GGA AGC AAT TGG CTA CCA AGG |
| R: AGT TGG ATA TCC TTG GGC ATA ATA |
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | |
|---|---|---|---|---|---|---|---|---|
| Fruit Color | green | green | green | white | white | red | red | red |
| Fruit Size (cm) | 0.80 ± 0.05 | 1.05 ± 0.03 | 1.15 ± 0.05 | 1.37 ± 0.05 | 1.37 ± 0.03 | 1.48 ± 0.05 | 1.53 ± 0.06 | 1.19 ± 0.11 |
| Fruit Weight (g) | 0.19 ± 0.03 | 0.47 ± 0.01 | 0.68 ± 0.04 | 1.05 ± 0.16 | 1.11 ± 0.27 | 1.39 ± 0.03 | 1.45 ± 0.06 | 0.68 ± 0.22 |
| Water Content (%) | 70.6 ± 5.35 | 77.2 ± 3.00 | 80.8 ± 0.49 | 84.4 ± 1.53 | 83.7 ± 2.34 | 85.4 ± 0.30 | 85.2 ± 0.44 | 78.2 ± 3.70 |
| Mg/ML | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|
| (+)-catechin (1) | 2.29 ± 0.005 | 2.29 ± 0.004 | 2.29 ± 0.006 | 2.18 ± 0.009 | 2.04 ± 0.0006 | 2.02 ± 0.005 | 2.00 ± 0.008 | 2.00 ± 0.003 |
| (−)-epicatechin (2) | 3.12 ± 0.01 | 3.33 ± 0.009 | 3.07 ± 0.03 | 2.70 ± 0.02 | 2.74 ± 0.02 | 2.70 ± 0.006 | 2.71 ± 0.007 | 2.70 ± 0.008 |
| procyanidin B4 (3) | 0.23 ± 0.002 | 0.22 ± 0.0001 | 0.21 ± 0.001 | 0.11 ± 0.002 | 0.09 ± 0.001 | 0.08 ± 0.001 | 0.07 ± 0.002 | 0.07 ± 0.001 |
| flavan-3-ol trimer (4) | 0.35 ± 0.0006 | 0.35 ± 0.0001 | 0.33 ± 0.0001 | 0.31 ± 0.0005 | 0.31 ± 0.0003 | 0.31 ± 0.0005 | 0.31 ± 0.0001 | 0.31 ± 0.0003 |
| sauguiin H-6 (8) | 1.98 ± 0.007 | 1.77 ± 0.04 | 2.05 ± 0.03 | 0.85 ± 0.12 | 0.70 ± 0.009 | 0.76 ± 0.021 | 0.72 ± 0.009 | 0.97 ± 0.01 |
| cyanidin-3-O-sophoroside (9) | - | - | - | - | 0.69 ± 0.001 | 0.68 ± 0.005 | 1.96 ± 0.002 | 1.59 ± 0.01 |
| cyanidin-3-O-sambubiside (10) | - | - | - | - | 0.27 ± 0.002 | 0.24 ± 0.05 | 0.65 ± 0.007 | 0.67 ± 0.009 |
| cyanidin-3-O-glucoside (11) | - | - | 0.26 ± 0.005 | 0.21 ± 0.003 | 0.49 ± 0.003 | 0.50 ± 0.009 | 0.88 ± 0.02 | 0.83 ± 0.02 |
| cyanidin-3-O-rutinoside (12) | - | - | - | - | - | 0.21 ± 0.001 | 0.35 ± 0.008 | 0.42 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobori, R.; Yakami, S.; Kawasaki, T.; Saito, A. Changes in the Polyphenol Content of Red Raspberry Fruits during Ripening. Horticulturae 2021, 7, 569. https://doi.org/10.3390/horticulturae7120569
Kobori R, Yakami S, Kawasaki T, Saito A. Changes in the Polyphenol Content of Red Raspberry Fruits during Ripening. Horticulturae. 2021; 7(12):569. https://doi.org/10.3390/horticulturae7120569
Chicago/Turabian StyleKobori, Ryo, Syuichi Yakami, Takashi Kawasaki, and Akiko Saito. 2021. "Changes in the Polyphenol Content of Red Raspberry Fruits during Ripening" Horticulturae 7, no. 12: 569. https://doi.org/10.3390/horticulturae7120569
APA StyleKobori, R., Yakami, S., Kawasaki, T., & Saito, A. (2021). Changes in the Polyphenol Content of Red Raspberry Fruits during Ripening. Horticulturae, 7(12), 569. https://doi.org/10.3390/horticulturae7120569