Effects of Different Oxygen Levels with High-Carbon Dioxide Atmosphere on Postharvest Quality of Fresh Fig under Palliflex Storage Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Weight Losses and Fruit Firmness
2.2. Total Soluble Solids (TSS) and Titratable Acidity (TA)
2.3. Visual Appearance and Taste
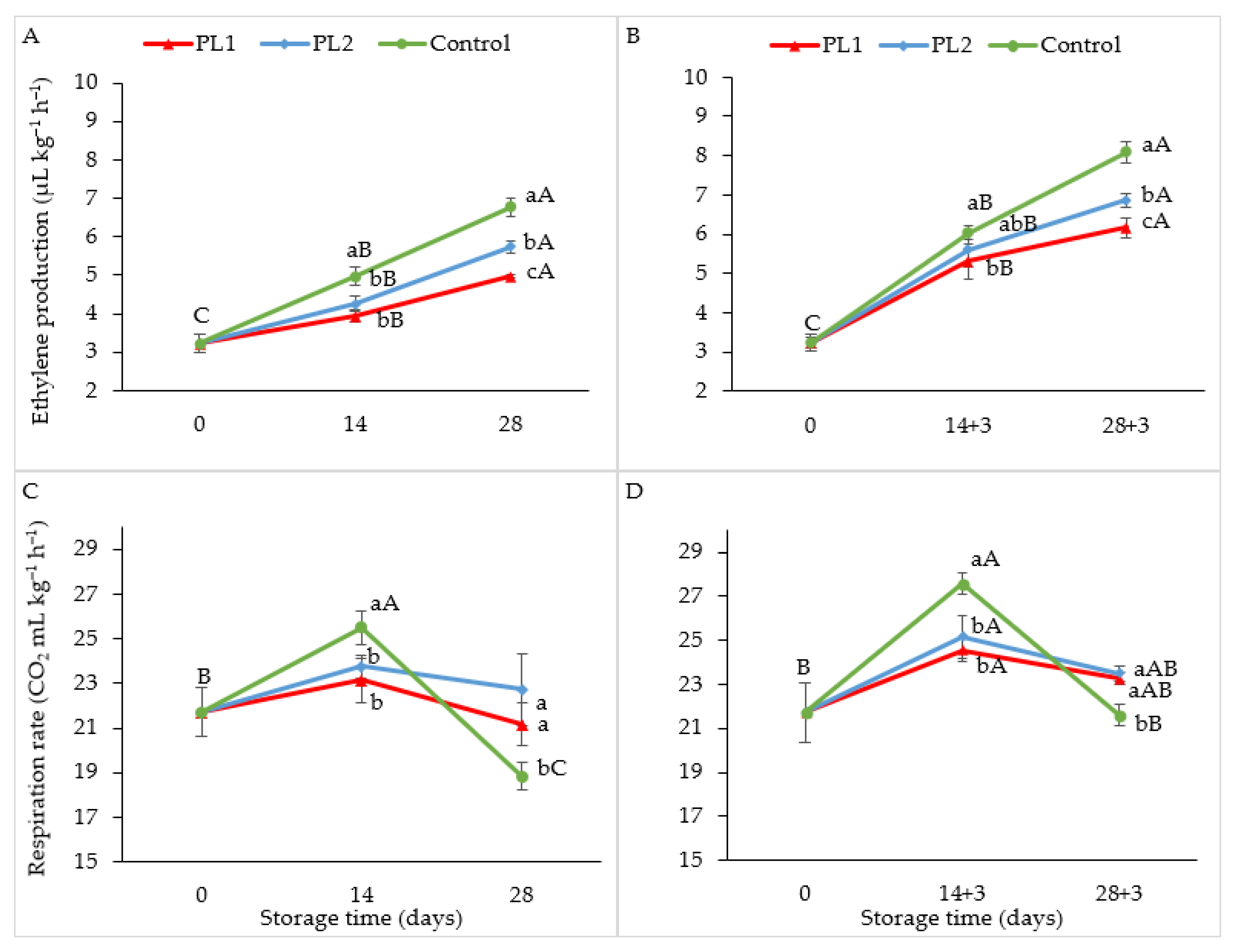
2.4. Respiration Rate and Ethylene Production
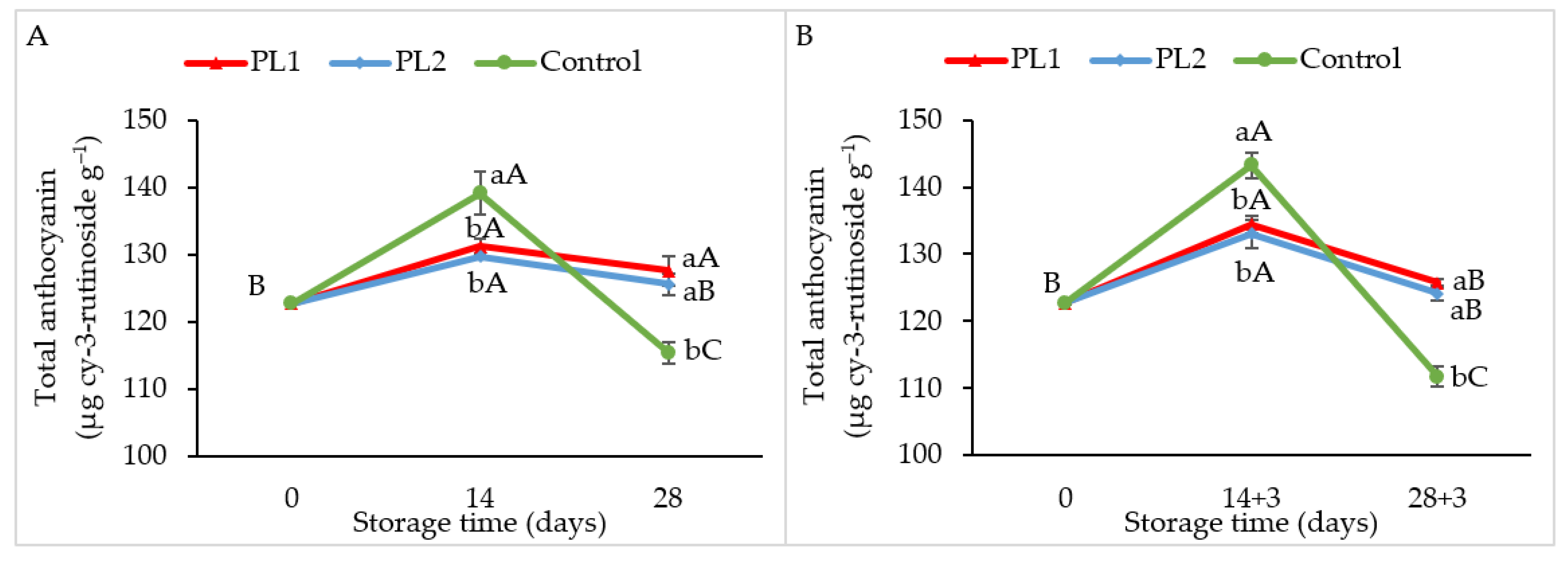
2.5. Total Antioxidant Activity and Anthocyanin Content
2.6. Decay Incidence, Decay Severity and Total Microorganisms
2.7. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. Firmness
3.3. Total Soluble Solids (TSS) and Titratable Acidity (TA)
3.4. Visual Appearance and Taste
3.5. Ethylene Production and Respiration Rate
3.6. Total Antioxidant Activity
3.7. Total Anthocyanin Content (TAC)
3.8. Decay Incidence, Decay Severity and Total Microorganisms
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food. Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Colaric, M.; Stampar, F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008, 106, 153–157. [Google Scholar] [CrossRef]
- Dueñas, M.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Escribano-Bailón, T. Anthocyanin composition in fig (Ficus carica L.). J. Food Compost. Anal. 2008, 21, 107–115. [Google Scholar] [CrossRef]
- Çalişkan, O.; Polat, A.A. Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Sci. Hortic. 2011, 128, 473–478. [Google Scholar] [CrossRef]
- Food and Agriculture Organization Statistics Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 January 2022).
- Uludag Exporters’ Association Trade Statistics. Available online: https://uib.org.tr/tr/kbfile/incir_raporu_mayis_2020 (accessed on 25 January 2022).
- Dogan, A.; Ali, Q.; Erkan, M. Comparison of quality attributes of fig fruit ‘Bursa Siyahi’ harvested at two different maturity stages. Acta Hortic. 2020, 1275, 311–316. [Google Scholar] [CrossRef]
- Dogan, A.; Cat, A.; Catal, M.; Erkan, M. First report of Alternaria alternata causing postharvest decay in fig (Ficus carica L. cv. Bursa Siyahi) fruit in Turkey. J. Biotechnol. 2018, 280, S32–S91. [Google Scholar] [CrossRef]
- Colelli, G.; Mitchell, F.G.; Kader, A.A. Extension of postharvest life of Mission figs by CO2 enriched atmospheres. Hortscience 1991, 26, 1193–1195. [Google Scholar] [CrossRef]
- Tsantil, E.; Karaiskos, G.; Pontikis, C. Storage of fresh figs in low oxygen atmosphere. J. Hortic. Sci. Biotechnol. 2003, 78, 56–60. [Google Scholar] [CrossRef]
- Mathooko, F.M.; Sotokawa, T.; Kubo, Y.; Inaba, A.; Nakamura, R. Retention of freshness in fig fruit by CO2 enriched atmosphere treatment or modified atmosphere packaging under ambient temperature. J. Jpn. Soc. Hortic. Sci. 1993, 62, 661–667. [Google Scholar] [CrossRef][Green Version]
- Ayhan, Z.; Karaçay, E. Preservation of the Bursa Siyahı fresh fig under modified atmosphere packaging (MAP) and cold storage. Int. J. AgriSci. 2011, 1, 1–9. [Google Scholar]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Pereira, C.; Córdoba, M.G. Use of equilibrium modified atmosphere packaging for preservation of ‘San Antonio’ and ‘Banane’ breba crops (Ficus carica L.). Postharvest Biol. Technol. 2014, 98, 14–22. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; López, C.M.; Pereira, C.; Córdoba, M.D.G. Preservation of different fig cultivars (Ficus carica L.) under modified atmosphere packaging during cold storage. J. Sci. Food Agric. 2016, 96, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Türk, R.; Eriş, A.; Özer, M.H.; Tuncelli, E.; Henze, J. Research on the CA storage of Fig cv. Bursa Siyahı. Acta Hortic. 1994, 368, 830–839. [Google Scholar] [CrossRef]
- Bahar, A.; Lichter, A. Effect of controlled atmosphere on the storage potential of Ottomanit fig fruit. Sci. Hortic. 2018, 227, 196–201. [Google Scholar] [CrossRef]
- Özkaya, O.; Çömlekçioğlu, S.; Demircioğlu, H. Assessment of the potential of 1-Methylcyclopropene treatments to maintain fruit quality of the common fig (Ficus carica L. cv. Bursa Siyahi) during refrigerated storage. Not. Bot. Horti. Agrobot. Cluj-Napoca 2014, 42, 516–522. [Google Scholar] [CrossRef]
- Bal, E. Effect of postharvest UV-C treatments on quality attributes of fresh fig. Bulg. J. Agric. Sci. 2012, 18, 191–196. [Google Scholar]
- Cantín, C.M.; Palou, L.; Bremer, V.; Michailides, T.J.; Crisosto, C.H. Evaluation of the use of sulfur dioxide to reduce postharvest losses on dark and green figs. Postharvest Biol. Technol. 2011, 59, 150–158. [Google Scholar] [CrossRef]
- Irfan, P.K.; Vanjakshi, V.; Prakash, M.K.; Ravi, R.; Kudachikar, V.B. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 2013, 82, 70–75. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Ilhan, K.; Arslan, U.; Vardar, C. Evaluation of the use of chlorine dioxide by fogging for decreasing postharvest decay of fig. Postharvest Biol Technol. 2009, 52, 313–315. [Google Scholar] [CrossRef]
- Venditti, T.; Molinu, M.G.; Dore, A.; D’Hallewin, G.; Fiori, P.; Tedde, M.; Agabbio, M. Treatments with gras compounds to keep fig fruit (Ficus carica L.) quality during cold storage. Commun. Agric. Appl. Biol. Sci. 2005, 70, 339–343. [Google Scholar]
- Marpudi, S.L.; Ramachandran, P.; Srividya, N. Aloe vera gel coating for postharvest quality maintenance of fresh fig fruits. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 878–887. [Google Scholar]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Watkins, C.B. Responses of horticultural commodities to high carbon dioxide as related to modified atmosphere packaging. HortTechnology 2000, 10, 501–506. [Google Scholar] [CrossRef]
- Beaudry, R.M. Responses of horticultural commodities to low oxygen: Limits to the expanded use of modified atmosphere packaging. HortTechnology 2000, 10, 491–500. [Google Scholar] [CrossRef]
- Dogan, A.; Erkan, M. A new storage technology: Palistore (palliflex) storage system for horticultural crops. Fruit Sci. 2014, 1, 1–6. [Google Scholar]
- Selcuk, N.; Erkan, M. The effects of modified and palliflex controlled atmosphere storage on postharvest quality and composition of ‘Istanbul’ medlar fruit. Postharvest Biol. Technol. 2015, 99, 9–19. [Google Scholar] [CrossRef]
- Dogan, A.; Selcuk, N.; Erkan, M. Comparison of pesticide-free and conventional production systems on postharvest quality and nutritional parameters of peppers in different storage conditions. Sci. Hortic. 2016, 207, 104–116. [Google Scholar] [CrossRef]
- Fernández-León, M.F.; Fernández-León, A.M.; Lozano, M.; Ayuso, M.C.; Amodio, M.L.; Colelli, G.; González-Gómez, D. Retention of quality and functional values of broccoli ‘Parthenon’ stored in modified atmosphere packaging. Food Control 2013, 31, 302–313. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Salerno, M. Effect of short hypobaric treatments on postharvest rots of sweet cherries, strawberries, and table grapes. Postharvest Biol. Technol. 2001, 22, 1–6. [Google Scholar] [CrossRef]
- Kays, S.J.; Paull, R.E. Postharvest Biology, 2nd ed.; Exon Press: Athens, Greece, 2004. [Google Scholar]
- Dogan, A. Effects of Different Atmosphere Compositions under Palliflex System on Storage Life and Biochemical Composition of ‘Bursa Siyahi’ fig Cultivar. Ph.D. Thesis, Akdeniz University, Antalya, Turkey, 2019. [Google Scholar]
- Seymour, G.B.; Colquhoun, I.J.; Dupont, M.S.; Parsley, K.R.; Selvendran, R.R. Composition and structural features of cell wall polysaccharides from tomato fruits. Phytochemisty 1990, 29, 725–731. [Google Scholar] [CrossRef]
- Li, M.; Zhi, H.; Dong, Y. Textural property and cell wall metabolism of ‘Golden Bosc’ and ‘d’Anjou’ pears as influenced by oxygen regimes after long-term controlled atmosphere storage. Postharvest Biol. Technol. 2019, 151, 26–35. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, J. Differential responses of fruit quality and major targeted metabolites in three different cultivars of cold-stored figs (Ficus carica L.). Sci. Hortic. 2020, 260, 108877. [Google Scholar] [CrossRef]
- Cantín, C.M.; Giné-Bordonaba, J.; Echeverría, G. Optimal handling and postharvest strategies to reduce losses of ‘Cuello Dama Negro’ dark figs (Ficus carica L.). Int. J. Fruit Sci. 2020, 20 (Suppl. 2), 414–431. [Google Scholar] [CrossRef]
- Waghmare, R.B.; Annapure, U.S. Integrated effect of radiation processing and modified atmosphere packaging (MAP) on shelf-life of fresh fig. J. Food Sci. Technol. 2018, 55, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Damián, M.T.; Cruz-Arvizu, O.; Cruz-Alvarez, O. Effect of modified atmosphere packaging on nutraceutical quality and overall appearance of figs stored at 1 °C. Not. Bot. Horti. Agrobot. Cluj-Napoca 2020, 48, 2292–2305. [Google Scholar] [CrossRef]
- Crisosto, H.; Ferguson, L.; Bremer, V.; Stover, E.; Colelli, G. Fig (Ficus carica L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 134–158. [Google Scholar]
- Li, C.; Kader, A.A. Residual effects of controlled atmospheres on postharvest physiology and quality of strawberries. J. Am. Soc. Hortic. Sci. 1989, 114, 629–634. [Google Scholar]
- Wright, A.H.; Delong, J.M.; Arul, J.; Prange, R.K. The trend toward lower oxygen levels during apple (Malus × domestica Borkh) storage. J. Hortic. Sci. Biotechnol. 2015, 90, 1–13. [Google Scholar] [CrossRef]
- Freiman, Z.E.; Rodov, V.; Yablovitz, Z.; Horev, B.; Flaishman, M.A. Preharvest application of 1-methylcyclopropene inhibits ripening and improves keeping quality of ‘Brown Turkey’ figs (Ficus carica L.). Sci. Hortic. 2012, 138, 266–272. [Google Scholar] [CrossRef]
- Ercisli, S.; Tosun, M.; Karlidag, H.; Dzubur, A.; Hadziabulic, S.; Aliman, Y. Color and antioxidant characteristics of some fresh fig (Ficus carica L.) genotypes from Northeastern Turkey. Plant Foods Hum. Nutr. 2012, 67, 271–276. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z. Postharvest oxidative behaviour of 1-methylcyclopropene treated Japanese plums (Prunus salicina Lindell) during storage under controlled and modified atmospheres. Postharvest Biol. Technol. 2012, 74, 26–35. [Google Scholar] [CrossRef]
- Romero, I.; Sanchez-Ballesta, M.T.; Maldonado, R.; Escribano, M.I.; Merodio, C. Anthocyanin, antioxidant activity and stress-induced gene expression in high CO2-treated table grapes stored at low temperature. J. Plant Physiol. 2008, 165, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Gomes, M.H.; Almeida, D.P.F.; Pintado, M. Effect of modified atmosphere on polyphenols during storage of pasteurised strawberry purées. Food Sci. Technol. 2015, 60, 377–384. [Google Scholar] [CrossRef]
- Khorshidi, S.; Davarynejad, G.; Tehranifar, A.; Fallahi, E. Effect of modified atmosphere packaging on chemical composition, antioxidant activity, anthocyanin, and total phenolic content of cherry fruits. Hortic. Environ. Biotechnol. 2011, 52, 471–481. [Google Scholar] [CrossRef]
- Doster, M.A.; Michailides, T.J. Fungal decay of first-crop and main crop figs. Plant Dis. 2007, 91, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Retamales, J.; Defilippi, B.G.; Arias, M.; Castillo, P.; Manriquez, D. High-CO2 controlled atmospheres reduce decay incidence in Thompson Seedless and Red Globe table grapes. Postharvest Biol. Technol. 2003, 29, 177–182. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Garner, D.; Crisosto, G. Carbon dioxide-enriched atmospheres during cold storage limit losses from Botrytis but accelerate rachis browning of ‘Redglobe’ table grapes. Postharvest Biol. Technol. 2002, 26, 181–189. [Google Scholar] [CrossRef]
- Tepeli, M.E.; İlhan, K.; Topuz, M.; Karabulut, Ö.A. Use of antimicrobial modified atmosphere packages against postharvest diseases in ‘Bursa Black’ figs. Ege Üniv. Ziraat Fak. Derg. 2020, 57, 1–10. [Google Scholar] [CrossRef]
- Saltveit, M. Biological basis for CA and MA. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Gil, M.I., Beaudry, R.M., Eds.; Academic Press: Oxford, UK, 2020; pp. 3–22. [Google Scholar]



| Atmosphere Composition | Storage Time (Days) | TSS (%) | TA (% Citric Acid) | Taste | Visual Appearance |
|---|---|---|---|---|---|
| Initial | 0 | 18.6 B 1 | 0.23 | 5.0 A | 5.0 A |
| PL1 | 14 | 18.8 b | 0.20 | 4.8 A | 4.9 A |
| PL2 | 18.7 b | 0.21 | 4.8 A | 4.9 A | |
| Control | 20.1 aA | 0.21 | 4.5 B | 4.6 A | |
| PL1 | 28 | 19.2 b | 0.20 | 4.4 B | 4.6 aB |
| PL2 | 19.5 b | 0.20 | 4.3 B | 4.5 aB | |
| Control | 20.5 aA | 0.21 | 4.1 C | 4.1 bB |
| Atmosphere Composition | Storage Time (Days) | TSS (%) | TA (% Citric Acid) | Taste | Visual Appearance |
|---|---|---|---|---|---|
| Initial | 0 | 18.6 C 1 | 0.23 | 5.0 A | 5.0 A |
| PL1 | 14 + 3 | 19.5 a | 0.20 | 4.6 aB | 3.9 aB |
| PL2 | 19.5 a | 0.20 | 4.5 aB | 3.9 aB | |
| Control | 21.2 aA | 0.21 | 4.0 bB | 2.7 bB | |
| PL1 | 28 + 3 | 19.2 b | 0.19 | 4.0 aC | 3.5 aB |
| PL2 | 18.9 b | 0.19 | 3.9 aC | 3.3 aC | |
| Control | 19.8 aB | 0.18 | 3.8 aB | 1.7 bC |
| Atmosphere Composition | Storage Time (Days) | Decay Incidence (%) | Decay Severity | Total Microorganisms (105 cfu) |
|---|---|---|---|---|
| Initial | 0 | - | - | 1.67 B, B, C * |
| PL1 | 14 | 0.0 bB 1 | 0.0 bB | 2.17 bA |
| PL2 | 0.0 bB | 0.0 bB | 2.27 bB | |
| Control | 5.6 aB | 3.2 aB | 3.03 aB | |
| PL1 | 28 | 25.9 cA | 13.4 cA | 2.37 cA |
| PL2 | 33.3 bA | 18.5 bA | 3.40 bA | |
| Control | 42.6 aA | 37.0 aA | 4.37 aA |
| Atmosphere Composition | Storage Time (Days) | Decay Incidence (%) | Decay Severity | Total Microorganisms (105 cfu) |
|---|---|---|---|---|
| Initial | 0 | - | - | 1.67 C |
| PL1 | 14 + 3 | 28.0 cB 1 | 14.4 bB | 2.37 bB |
| PL2 | 40.7 bB | 25.0 bB | 2.60 bB | |
| Control | 51.9 aB | 44.0 aB | 4.03 aB | |
| PL1 | 28 + 3 | 40.7 cA | 28.2 cA | 3.20 cA |
| PL2 | 53.7 bA | 44.4 bA | 4.10 bA | |
| Control | 87.0 aA | 69.5 aA | 5.90 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogan, A. Effects of Different Oxygen Levels with High-Carbon Dioxide Atmosphere on Postharvest Quality of Fresh Fig under Palliflex Storage Systems. Horticulturae 2022, 8, 353. https://doi.org/10.3390/horticulturae8050353
Dogan A. Effects of Different Oxygen Levels with High-Carbon Dioxide Atmosphere on Postharvest Quality of Fresh Fig under Palliflex Storage Systems. Horticulturae. 2022; 8(5):353. https://doi.org/10.3390/horticulturae8050353
Chicago/Turabian StyleDogan, Adem. 2022. "Effects of Different Oxygen Levels with High-Carbon Dioxide Atmosphere on Postharvest Quality of Fresh Fig under Palliflex Storage Systems" Horticulturae 8, no. 5: 353. https://doi.org/10.3390/horticulturae8050353
APA StyleDogan, A. (2022). Effects of Different Oxygen Levels with High-Carbon Dioxide Atmosphere on Postharvest Quality of Fresh Fig under Palliflex Storage Systems. Horticulturae, 8(5), 353. https://doi.org/10.3390/horticulturae8050353






