Quality Attributes of Chitosan-Coated Cornelian Cherry (Cornus mas L.) Fruits under Different Storage Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Titratable Acidity, Total Soluble Solids, and pH
2.3. Total Phenolic Content and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Scavenging Capacity
2.4. Total Anthocyanin Content
2.5. Total Flavonoids (TF) Content
2.6. Antioxidant Capacity
2.7. PAL, PPO, and GPX Activity
2.8. Statistical Analysis
3. Results and Discussion
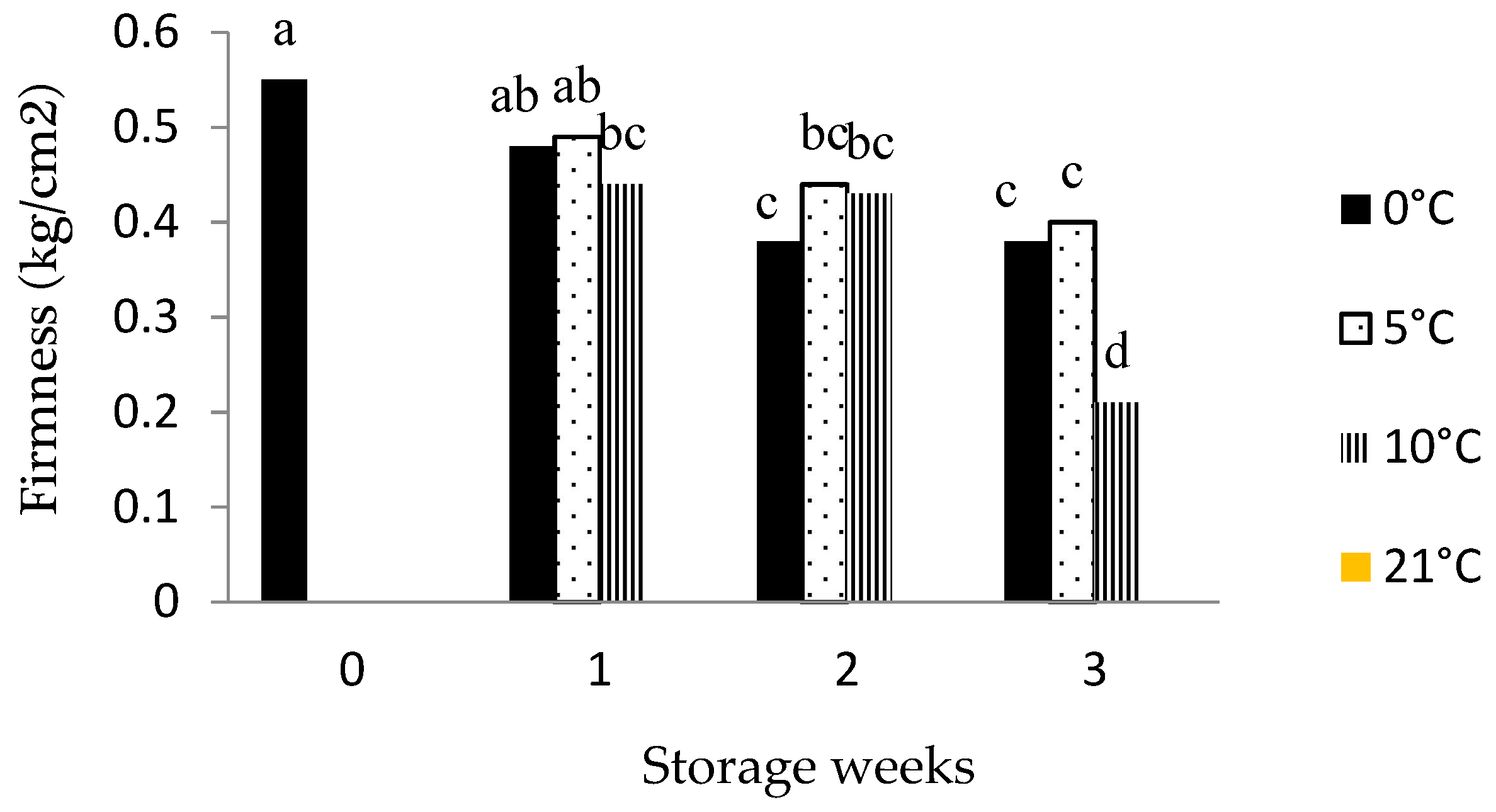
3.1. Fruit Firmness
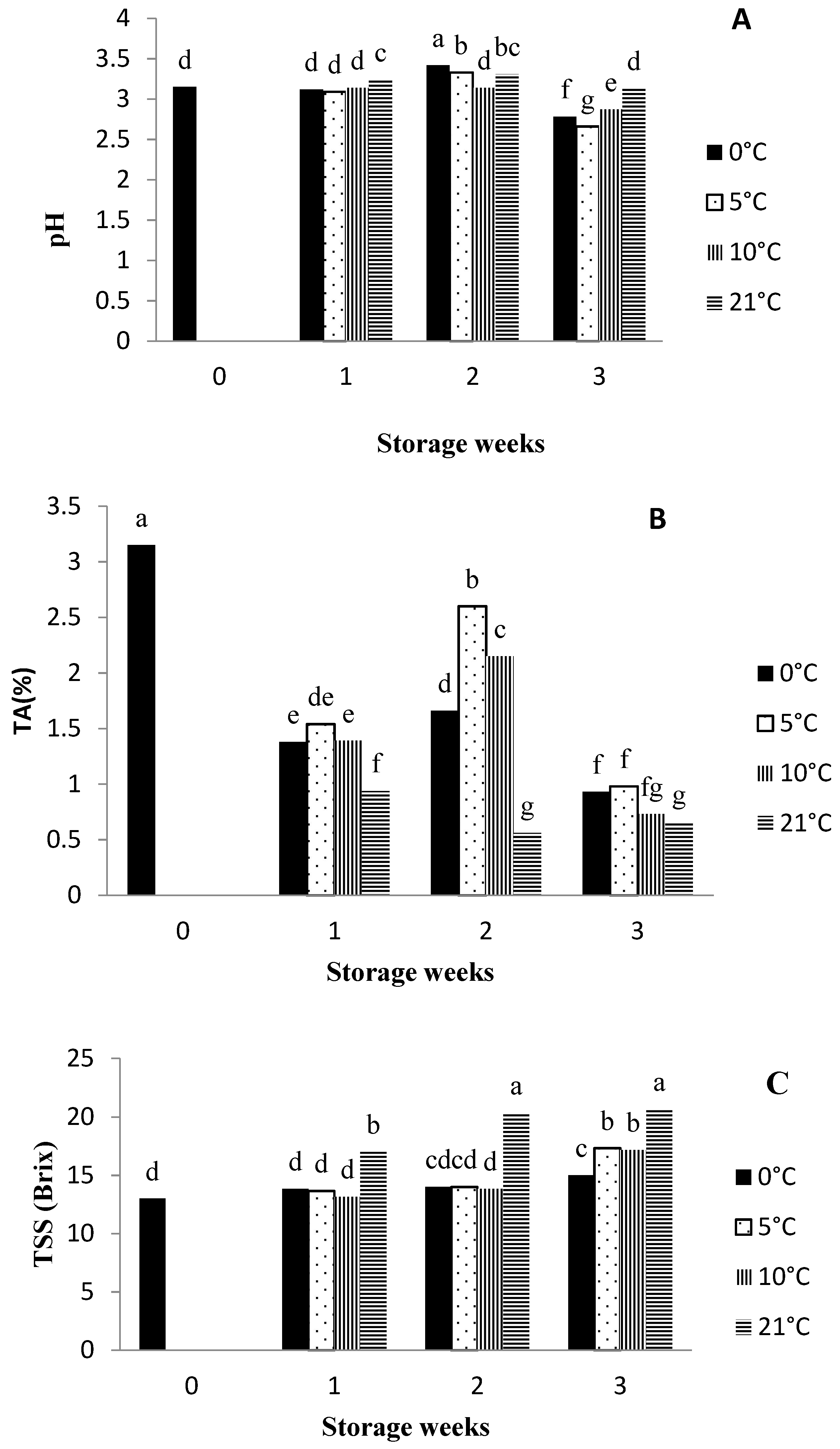
3.2. pH, Titratable Acidity (TA), and Total Soluble Solids (TSSs)
3.3. Fruit Color
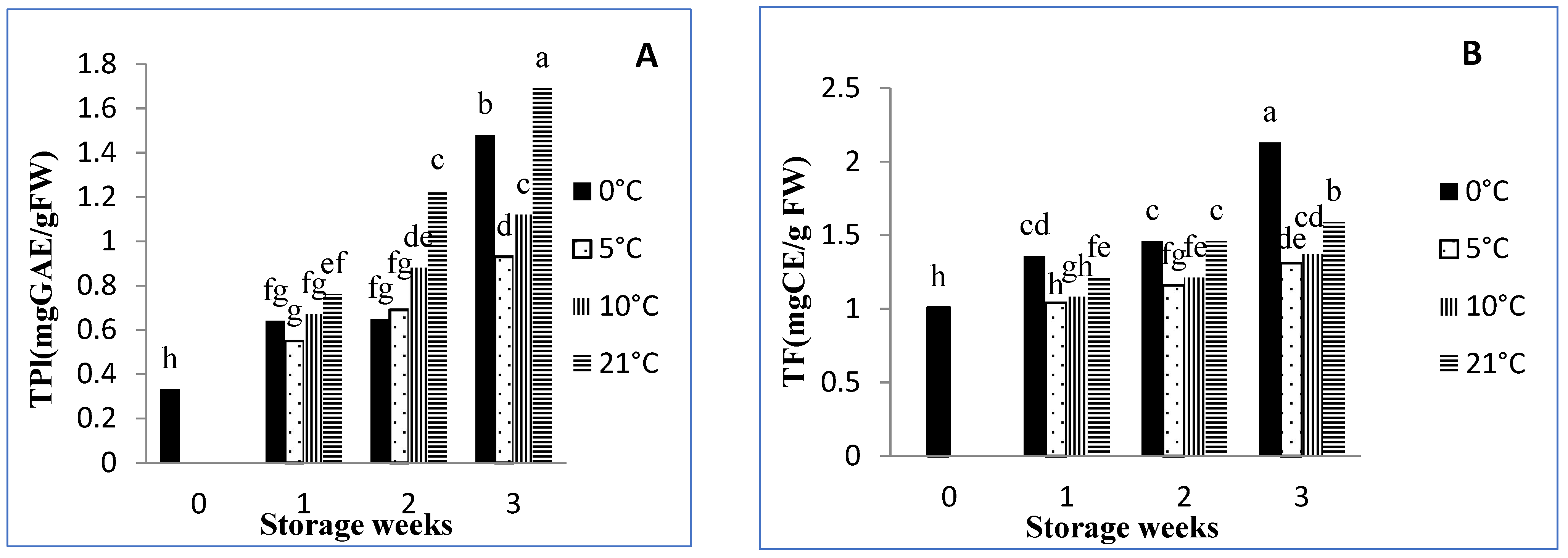
3.4. Total Phenolic Content
3.5. Total Flavonoids
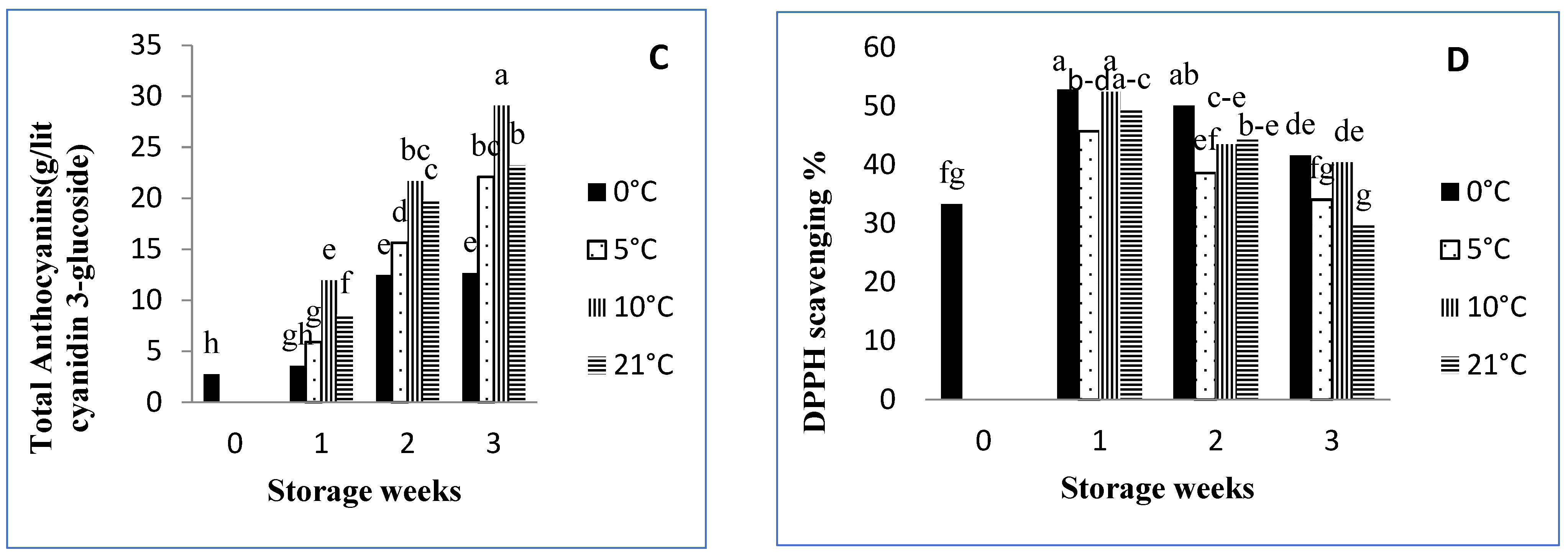
3.6. Total Anthocyanins
3.7. Total Antioxidant Activity
3.8. Antioxidant Enzyme Activity
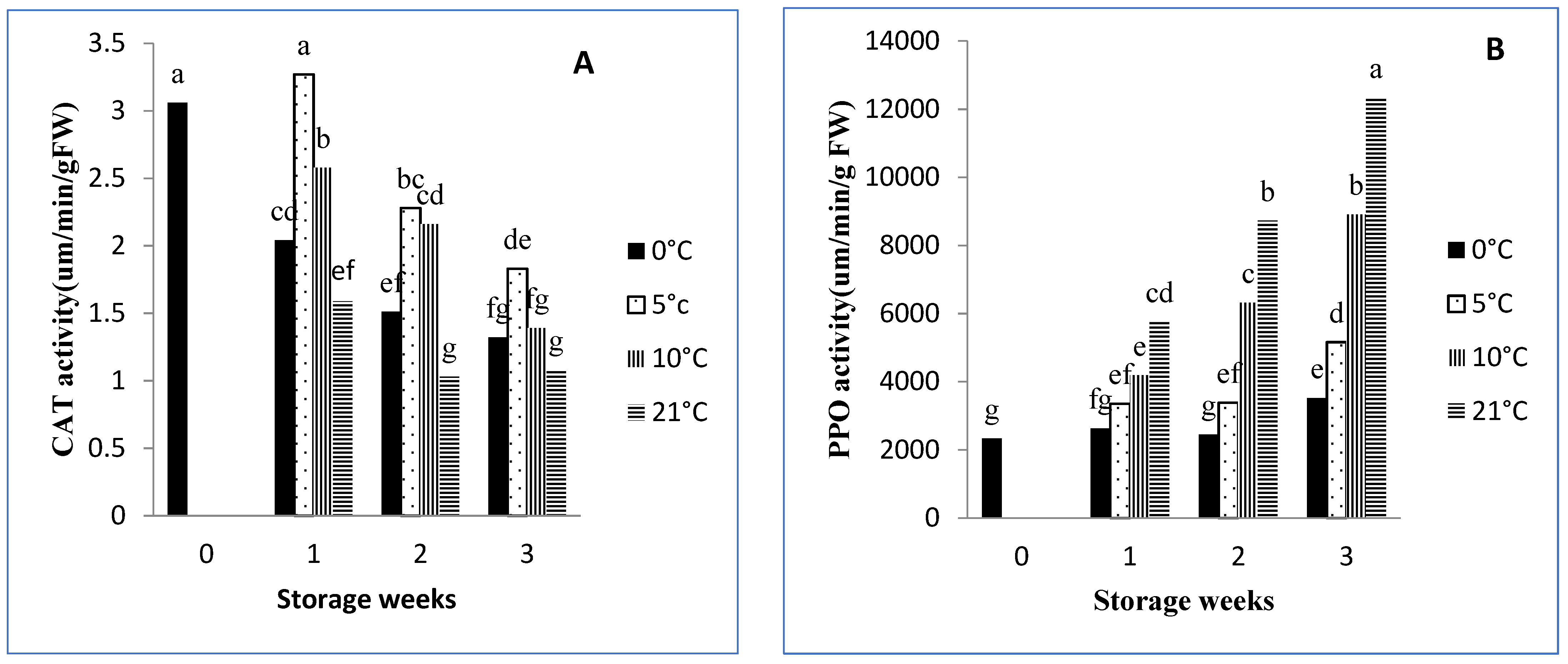
3.8.1. Catalase
3.8.2. PPO Activity
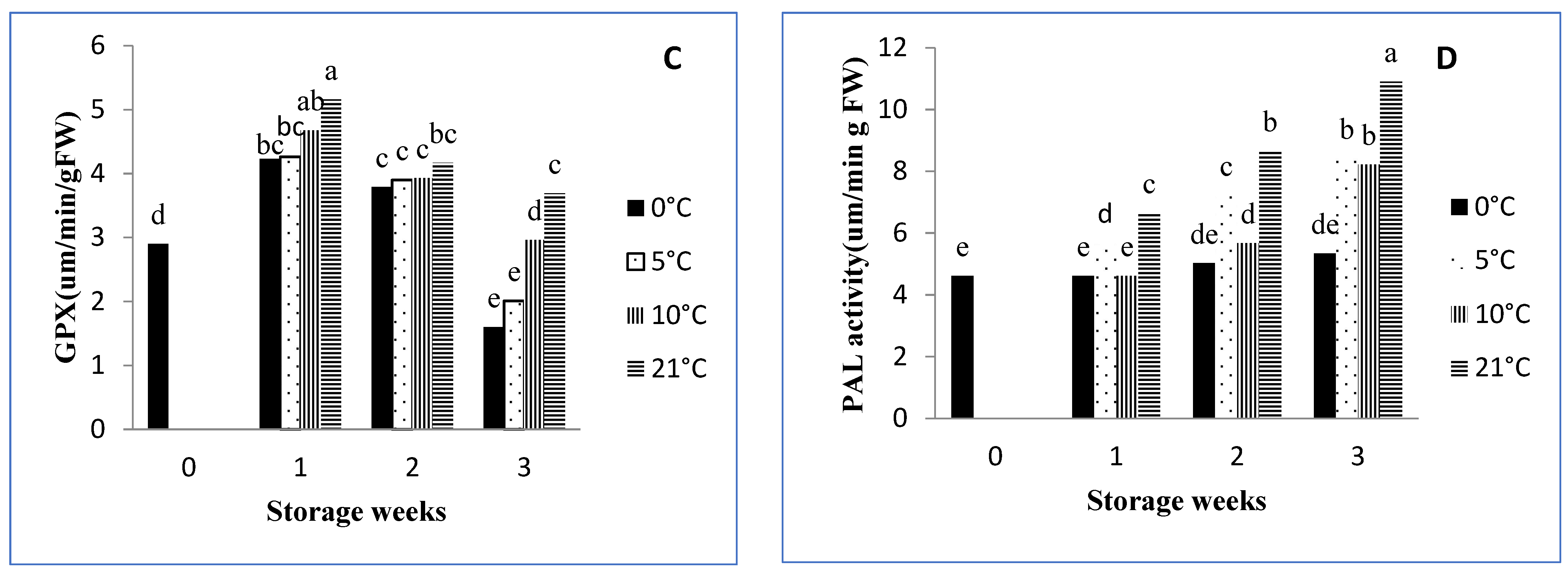
3.8.3. GPX Activity
3.8.4. PAL Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Cao, G.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolic on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Rimm, E.B.; Aschiero, A.; Giovannucci, E.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C. Vegetables, fruit, and cereal fiber intake and risk of coronary heart disease among men. J. Am. Med. Assoc. 1996, 275, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Schutzki, R.; Chandra, A.; Nair, M.G. Characterization, quantification, and bioactivities of anthocyanins in Cornus species. J. Agric. Food Chem. 2002, 50, 2519–2523. [Google Scholar] [CrossRef]
- Hassanpour, H.; Hamidoghli, Y.; Samizadeh, H. Estimation of genetic diversity in some Iranian cornelian cherries (Cornus mas L.) accessions using ISSR markers. Biochem. Syst. Ecol. 2013, 48, 257–262. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G.R. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Camangi, F.; Braca, A. Quality-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010, 119, 1257–1261. [Google Scholar] [CrossRef] [Green Version]
- Redzic, S. Wild medicinal plants and their usage in traditional human therapy (Southern Bosnia and Herzegovina, W. Balkan). J. Med. Plants Res. 2010, 4, 1003–1027. [Google Scholar]
- Mamedov, N.; Craker, L.E. Cornelian cherry: A prospective source for phytomedicine. In Proceedings of the XXVI International Horticultural Congress: The Future for Medicinal and Aromatic Plants, Toronto, ON, Canada, 11–17 August 2002; Volume 629, pp. 83–86. [Google Scholar]
- Yousefpour Dokhanieh, A.; Solaeimani Aghdam, M.; Rezapour Fard, J.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hort. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Jianglian, D.; Shaoying, Z. Application of chitosan based coating in fruit and vegetable preservation: A review. J. Food Process. Technol. 2013, 4, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Muda Muhammad, M.T.; Sijam, K.; Siddiqui, Y. Potential of chitosan coating in delaying the postharvest anthracnose (Colletotrichum gloeosporioides Penz.) of Eksotika II papaya. Int. J. Food Sci. Technol. 2010, 45, 2134–2140. [Google Scholar] [CrossRef]
- Ali, A.; Mohamed, M.T.M.; Siddiqui, Y. Control of anthracnose by chitosan through stimulation of defence-related enzymes in Eksotika II papaya (Carica papaya L.) fruit. J. Biol. Life Sci. 2012, 3, 114–126. [Google Scholar] [CrossRef] [Green Version]
- EL Ghaouth, A.; Arul, J.; Ponnampalam, R.; Boulet, M. Use of chitosan coating to reduce water loss and maintain quality of cucumber and bell pepper fruits. J. Food Process. Preserv. 1991, 15, 359–368. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G. Double Layer Coatings: A new technique for maintaining physico-chemical characteristics and antioxidant properties of dragon fruit during storage. Food Bioprocess Technol. 2014, 7, 2366–2374. [Google Scholar] [CrossRef]
- Esmaili, M.; Ebrahimzadeh, A.; Hassanpour, H.; Hassanpour Aghdam, M.B. The effects of different chitosan concentrations on storage life and postharvest quality of cornelian cherry (Cornus mass L.). J. Food Res. 2020, 29, 139–152. (In Persian) [Google Scholar]
- Selcuk, N.; Erkan, M. The effects of 1-MCP treatment on fruit quality of medlar fruit (Mespilus germanica L. cv. Istanbul) during long term storage in the palliflex storage system. Postharvest Biol. Technol. 2015, 100, 81–90. [Google Scholar] [CrossRef]
- Chiou, M.J.; Wang, Y.D.; Kuo, C.M.; Chen, J.C.; Chen, J.Y. Functional Analysis of Mitogen-Activated Protein Kinase-3 (MAPK3) and Its Regulation of the Promoter Region in Zebrafish. DNA Cell Biol. 2007, 26, 781–790. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1–13. [Google Scholar] [CrossRef]
- Shin, Y.; Liu, R.H.; Nock, J.F.; Holliday, D.; Watkins, C.B. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol. Technol. 2007, 45, 349–357. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nguyen, T.B.T.; Ketsa, S.; van Doorn, W.G. Relationship between browning and the activities of polyphenol oxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol. Technol. 2003, 30, 187–193. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B.N. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; DiPatre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Shin, Y.; Ryu, J.A.; Liu, R.H.; Nock, J.F.; Watkins, C.B. Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol. Technol. 2008, 49, 201–209. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. (Eds.) Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 7–47. [Google Scholar]
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Tembo, L.; Chiteka, Z.A.; Kadzere, I.; Akinnifesi, F.K.; Tagwira, F. Storage temperature affects fruit quality attributes of Ber (Ziziphus mauritiana Lamk.) in Zimbabwe. Afr. J. Biotechnol. 2008, 7, 3092–3099. [Google Scholar]
- Pailly, O.; Tison, G.; Amouroux, A. Harvest time and storage conditions of ‘Star Ruby’grapefruit (Citrus paradisi Macf.) for short distance summer consumption. Postharvest Biol. Technol. 2004, 34, 65–73. [Google Scholar] [CrossRef]
- Demir, F.; Kalyoncu, I.H. Some nutritional, pomological and physical properties of cornelian cherry (Cornus mas L.). J. Food Eng. 2003, 60, 335–341. [Google Scholar] [CrossRef]
- Thumula, P. (Ed.) Studies on Storage Behavior of Tomatoes Coated with Chitosan-Lysozyme Films; McGill University: Montréal, QC, USA, 2006. [Google Scholar]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT-Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Bianca, M.; Luminita, D. Influence of temperature and preserving agents on the stability of Cornelian cherries anthocyanins. Molecules 2014, 19, 8177–8188. [Google Scholar]
- Sánchez-González, L.; Pastor, C.; Vargas, M.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of hydroxy propylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol. Technol. 2011, 60, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Shewfelt, R.L.; Thai, C.N.; Davis, J.W. Prediction of changes in color of tomatoes during ripening at different constant temperatures. J. Food Sci. 1988, 53, 1433–1437. [Google Scholar] [CrossRef]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of color and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Rodriguez-Saonm, L.E.; Giusti, M.M.; Wrolstad, R.E. Color and pigment stability of red radish and red-fleshed potato anthocyanins in juice model systems. J. Food Sci. 1999, 64, 451–456. [Google Scholar] [CrossRef]
- Rezaee Kivi, A.; Sartipnia, N.; Babai Khalkhali, M. Comparative phenolic content and antioxidant activities of four wild raspberries in Iran. J. Appl. Sci. Agric. 2014, 9, 2419–2424. [Google Scholar]
- Soleimani-Aghdam, M.; Yousefpour-Dokhanieh, A.; Hassanpour, H.; Pezapour-Fard, J. Enhancement of antioxidant capacity of cornelian cherry (Cornus mas L.) fruit by postharvest calcium treatment. Sci. Hortic. 2013, 161, 160–164. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Genovese, M.I.; doNascimento, J.R.O.; Hassimotto, N.M.A.; dosSantos, R.J.; Lajolo, F.M. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 2005, 91, 113–121. [Google Scholar] [CrossRef]
- Celik, S.; Bakirci, I.; Sat, I.G. Physico-chemical and organoleptic properties of yogurt with cornelian cherry paste. Int. J. Food Prop. 2006, 9, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Piljac-Žegarac, J.; Šamec, D. Antioxidant stability of small fruits in postharvest storage at room and refrigerator temperatures. Food Res. Int. 2011, 44, 345–350. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Ferreyra, R.M.; Viña, S.Z.; Mugridge, A.; Chaves, A.R. Growth and ripening season effects on antioxidant capacity of strawberry cultivar Selva. Sci. Hortic. 2007, 112, 27–32. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Sala, J.M.; Lafuente, M.T. Catalase enzyme activity is related to tolerance of mandarin fruits to chilling. Postharvest Biol. Technol. 2000, 20, 81–89. [Google Scholar] [CrossRef]
- Hong, K.; Xu, H.; Wang, J.; Zhang, L.; Hu, H.; Jia, Z.; Gu, H.; He, Q. Quality changes and internal browning developments of summer pineapple fruit during storage at different temperatures. Sci. Hortic. 2013, 151, 68–74. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants–recent progress. Phytochemistry 1987, 26, 11–20. [Google Scholar] [CrossRef]
- Ruoyi, K.; Zhifang, Y.; Zhaoxin, L. Effect of coating and intermittent warming on enzymes, soluble pectin substances and ascorbic acid of Prunus persica (cv. Zhonghuashoutao) during refrigerated storage. Food Res. Int. 2005, 38, 331–336. [Google Scholar] [CrossRef]
- Goupy, P.; Amiot, M.J.; Forget, F.; Duprat, F.; Aubert, S.; Nicolas, J. Enzymatic browning of model solutions and apple phenolic extracts by apple polyphenol oxidase. J. Food Sci. 1995, 60, 497–501. [Google Scholar] [CrossRef]
- Ritter, H.; Schulz, G.E. Structural basis for the entrance into the phenylpropanoid metabolism catalyzed by phenylalanine ammonia-lyase. Plant Cell 2004, 16, 3426–3436. [Google Scholar] [CrossRef] [PubMed]
- Elyatem, S.M.; Kader, A. Post-harvest physiology and storage behavior of pomegranate fruits. Sci. Hortic. 1984, 24, 287–298. [Google Scholar] [CrossRef]
- Hassanpour, H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT-Food Sci. Technol. 2015, 60, 495–501. [Google Scholar] [CrossRef]
| MS | DF | SS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chroma | Hue | b* | a* | L* | TSSs | TA | pH | Firmness | ||
| ** 121.038 | 3.58 ns | ** 23.87 | ** 97.48 | ** 44.54 | ** 37.34 | ** 1.16 | ** 0.05 | ** 0.28 | 3 | Temperature |
| ** 1094.68 | ** 476.01 | ** 717.20 | ** 510.58 | ** 1089.19 | ** 44.12 | ** 11.98 | ** 0.40 | ** 0.18 | 3 | Day |
| ** 46.32 | ** 23.84 | ** 13.60 | ** 37.24 | ** 19.61 | ** 6.27 | ** 0.47 | ** 0.03 | ** 0.044 | 9 | Day × Temperature |
| 3.02 | 5.39 | 1.05 | 2.9 | 1.76 | 0.46 | 0.02 | 0.0016 | 0.001 | 32 | Error |
| 6.17 | 8.31 | 7.44 | 6.99 | 3.81 | 4.50 | 8.64 | 1.28 | 8.31 | - | C.V. |
| Chroma | Hue | b* | a* | L* | Weeks | Temperature (°C) |
|---|---|---|---|---|---|---|
| 41.81 a | 36.72 a | 24.99 a | 33.51 a | 48.79 a | 0 | 0 |
| 29.24 b | 27.97 bcd | 13.67 bc | 25.84 b | 34.92 bc | 1 | |
| 27.26 bcd | 25.85 de | 11.86 cd | 24.53 b | 32.76 cde | 2 | |
| 26.28 b–e | 23.59 ef | 10.47 de | 24.085 b | 30.56 efg | 3 | |
| 41.81 a | 36.72 a | 24.99 a | 33.51 a | 48.79 a | 0 | 5 |
| 28.91 bc | 30.57 bc | 14.72 b | 24.88 b | 35.4 b | 1 | |
| 25.9 c–e | 21.89 f | 9.59 ef | 24.03 b | 31.15 def | 2 | |
| 25.39 de | 21.26 f | 9.26 ef | 23.62 b | 29.085 fgh | 3 | |
| 41.81 a | 36.72 a | 24.99 a | 33.51 a | 48.79 a | 0 | 10 |
| 23.97 ef | 31.31 b | 12.44 c | 20.49 cd | 28.23 gh | 1 | |
| 21.58 f | 22.22 ef | 8.15 fg | 19.98 d | 27.45 h | 2 | |
| 17.99 g | 22.6 ef | 6.92 g | 16.6 e | 27.24 h | 3 | |
| 41.81 a | 36.72 a | 24.99 a | 33.51 a | 48.79 a | 0 | 21 |
| 26.01 c–e | 27.58 cd | 12.06 cd | 22.97 bc | 33.52 bcd | 1 | |
| 22.16 f | 22.38 ef | 8.44 fg | 20.49 cd | 28.23 gh | 2 | |
| 8.52 h | 22.67 ef | 3.13 h | 7.9 f | 22.87 i | 3 |
| MS | DF | SS | |||
|---|---|---|---|---|---|
| DPPH | Anthocyanin | TF | TP | ||
| ** 107/12 | ** 153/77 | ** 0/23 | ** 0/29 | 3 | temperature |
| ** 691/89 | ** 922/52 | ** 0/75 | ** 1/98 | 3 | storage time |
| * 28/73 | ** 25/24 | ** 0/07 | ** 0/097 | 9 | storage time × temperature |
| 11/78 | 1/88 | 0/0039 | 0/008 | 32 | error |
| 8/36 | 11/10 | 4/92 | 11/47 | - | C.V. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimzadeh, A.; Esmaeili, M.; Hassanpour, H.; Hassanpouraghdam, M.B.; Ercisli, S.; Bozhuyuk, M.R.; Dokoupil, L.; Mlcek, J. Quality Attributes of Chitosan-Coated Cornelian Cherry (Cornus mas L.) Fruits under Different Storage Temperatures. Horticulturae 2021, 7, 540. https://doi.org/10.3390/horticulturae7120540
Ebrahimzadeh A, Esmaeili M, Hassanpour H, Hassanpouraghdam MB, Ercisli S, Bozhuyuk MR, Dokoupil L, Mlcek J. Quality Attributes of Chitosan-Coated Cornelian Cherry (Cornus mas L.) Fruits under Different Storage Temperatures. Horticulturae. 2021; 7(12):540. https://doi.org/10.3390/horticulturae7120540
Chicago/Turabian StyleEbrahimzadeh, Asghar, Maryam Esmaeili, Hamid Hassanpour, Mohammad Bagher Hassanpouraghdam, Sezai Ercisli, Mehmet Ramazan Bozhuyuk, Libor Dokoupil, and Jiri Mlcek. 2021. "Quality Attributes of Chitosan-Coated Cornelian Cherry (Cornus mas L.) Fruits under Different Storage Temperatures" Horticulturae 7, no. 12: 540. https://doi.org/10.3390/horticulturae7120540
APA StyleEbrahimzadeh, A., Esmaeili, M., Hassanpour, H., Hassanpouraghdam, M. B., Ercisli, S., Bozhuyuk, M. R., Dokoupil, L., & Mlcek, J. (2021). Quality Attributes of Chitosan-Coated Cornelian Cherry (Cornus mas L.) Fruits under Different Storage Temperatures. Horticulturae, 7(12), 540. https://doi.org/10.3390/horticulturae7120540