Physiochemical Changes of Mung Bean [Vigna radiata (L.) R. Wilczek] in Responses to Varying Irrigation Regimes
Abstract
1. Introduction
2. Results and Discussion
2.1. Soil Moisture Content in Response to Irrigation Regimes in Mung Bean Genotypes
2.2. Physiological Properties in Response to Irrigation Regimes in Mung Bean Genotypes
2.2.1. Water Use Efficiency
2.2.2. Chlorophyll Content (mg g−1 FW)
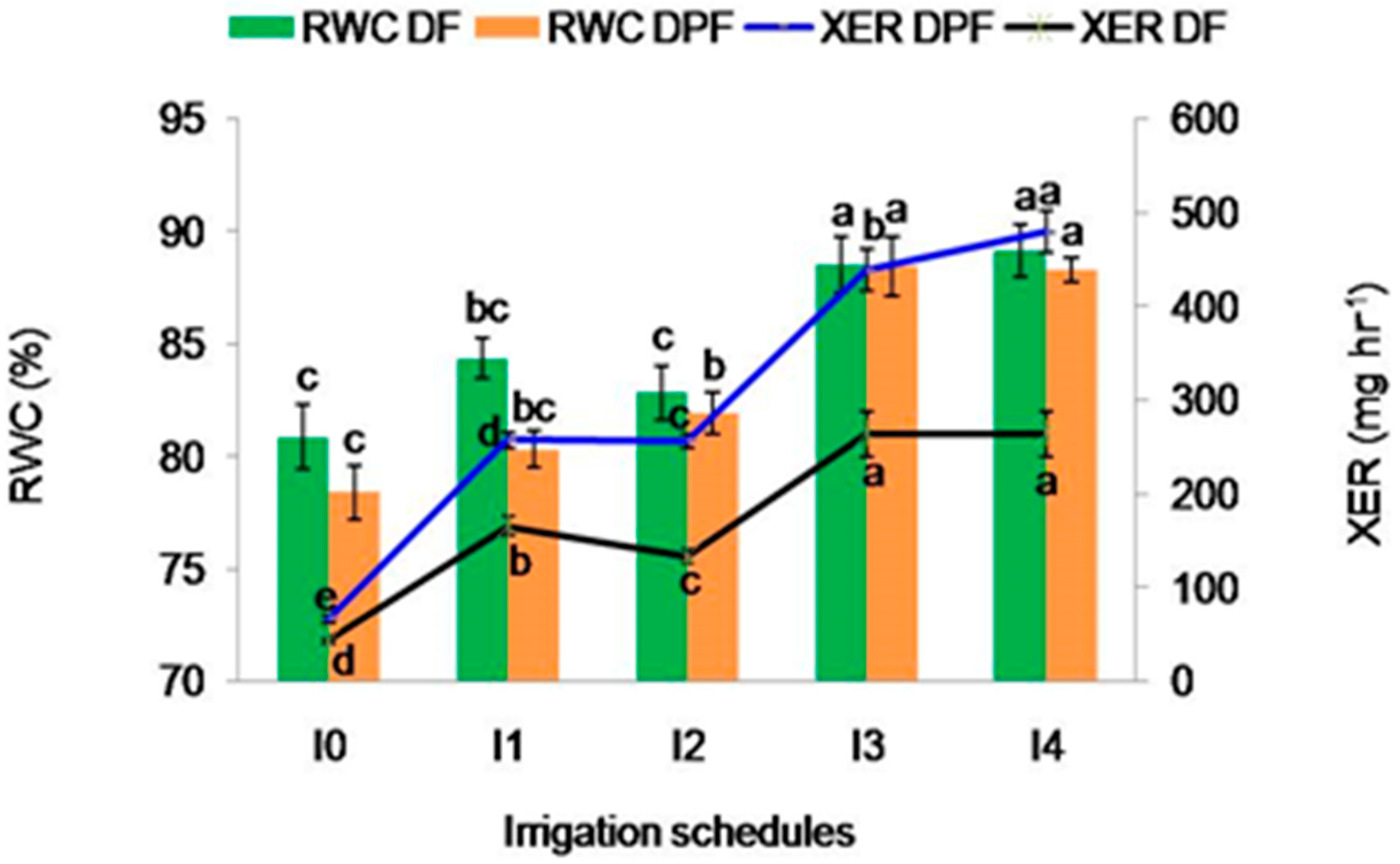
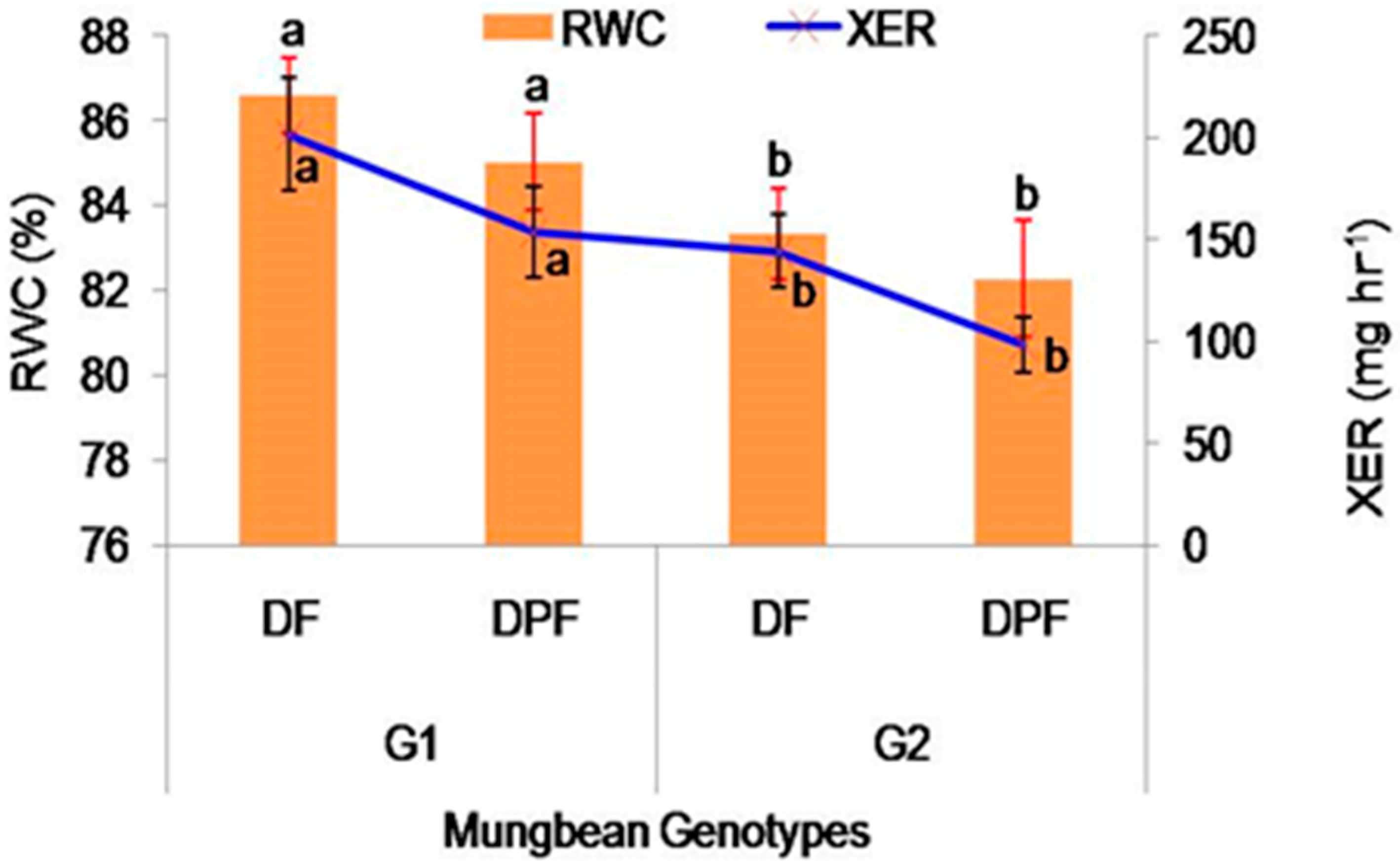
2.2.3. Relative Water Content
2.2.4. Xylem Exudation Rate (mg hr−1)
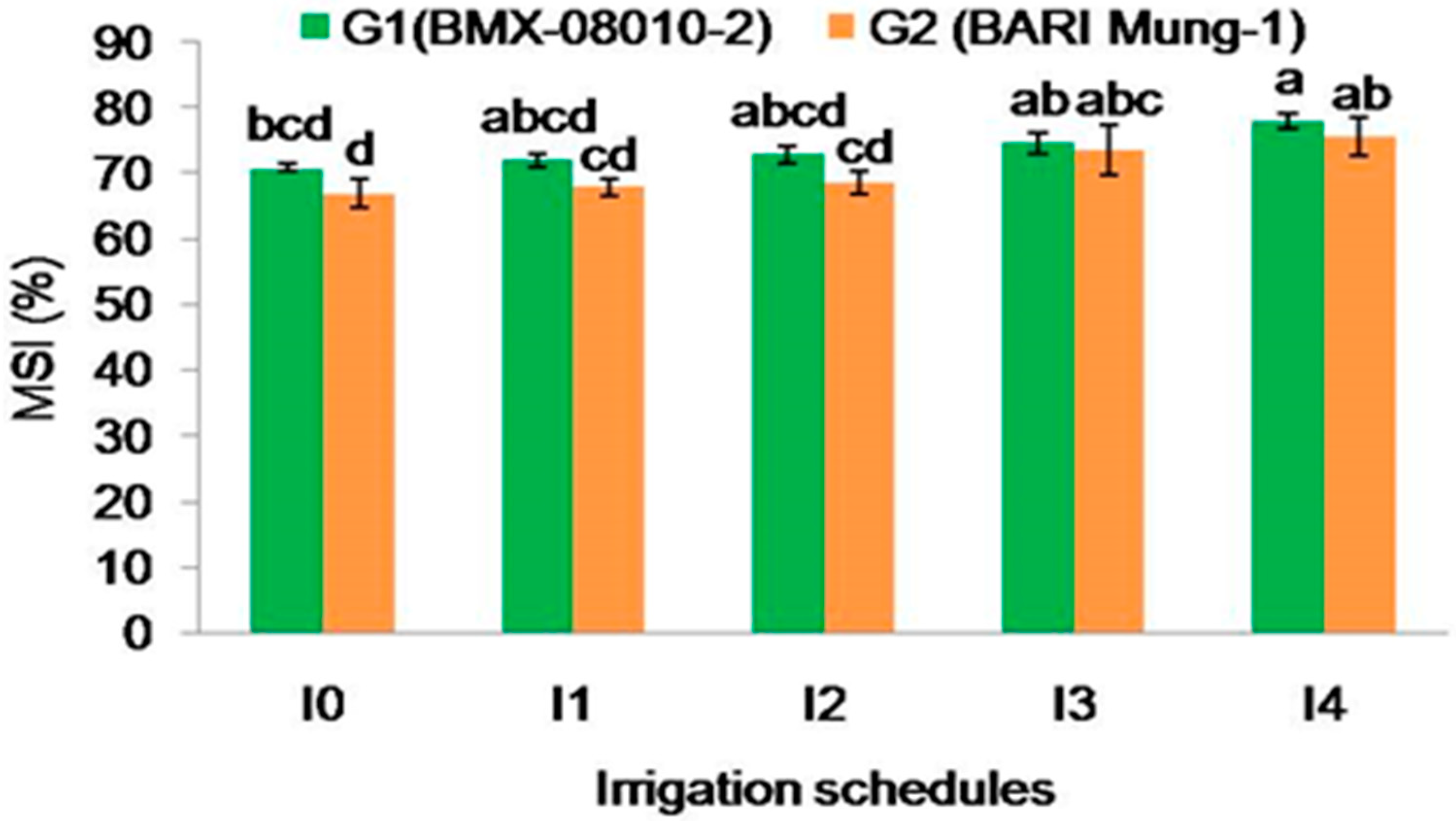
2.2.5. Membrane Stability Index (%)
2.3. Biochemical Properties in Response to Irrigation Regimes in Mung Bean Genotypes
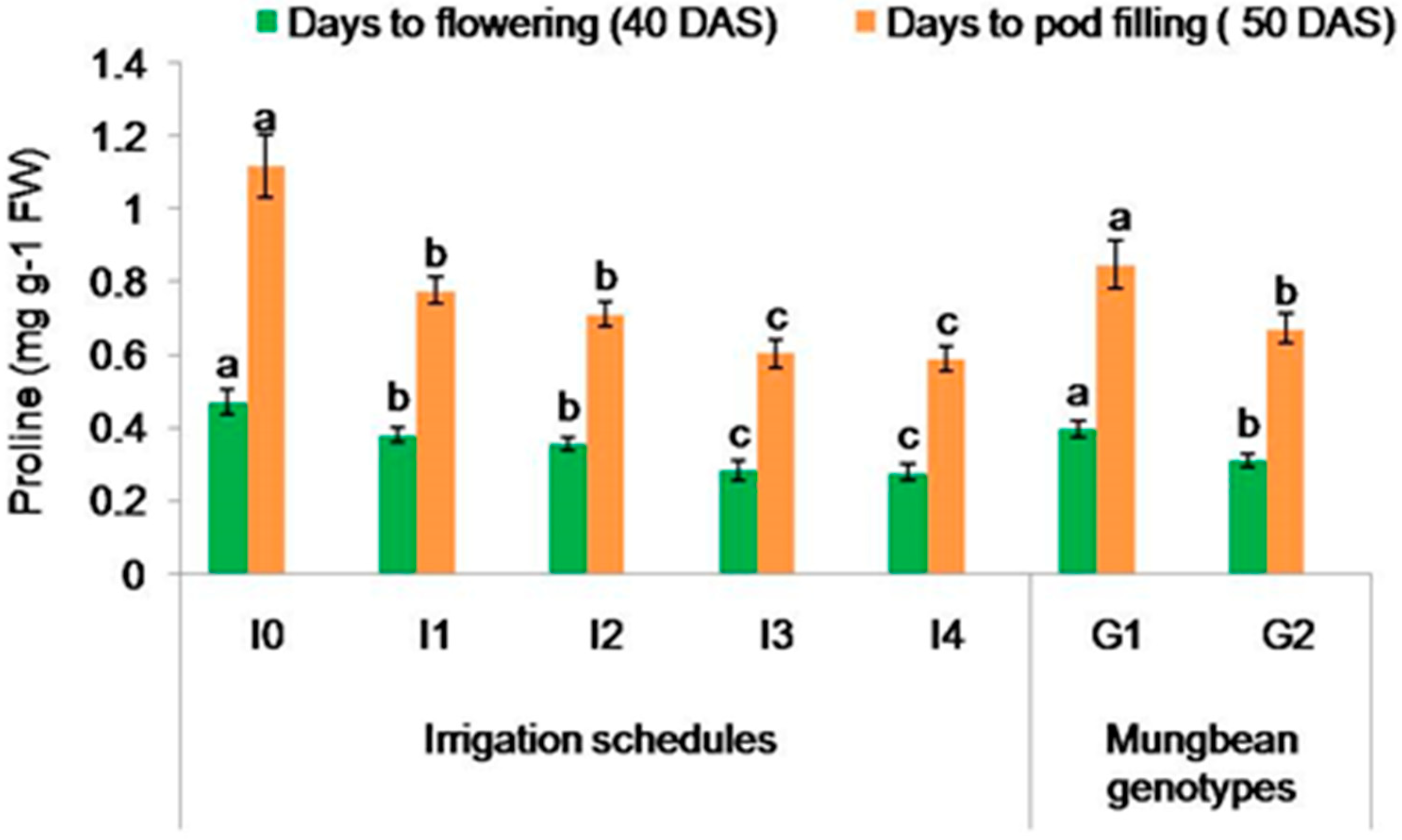
2.3.1. Proline Content (mg g−1 FW)
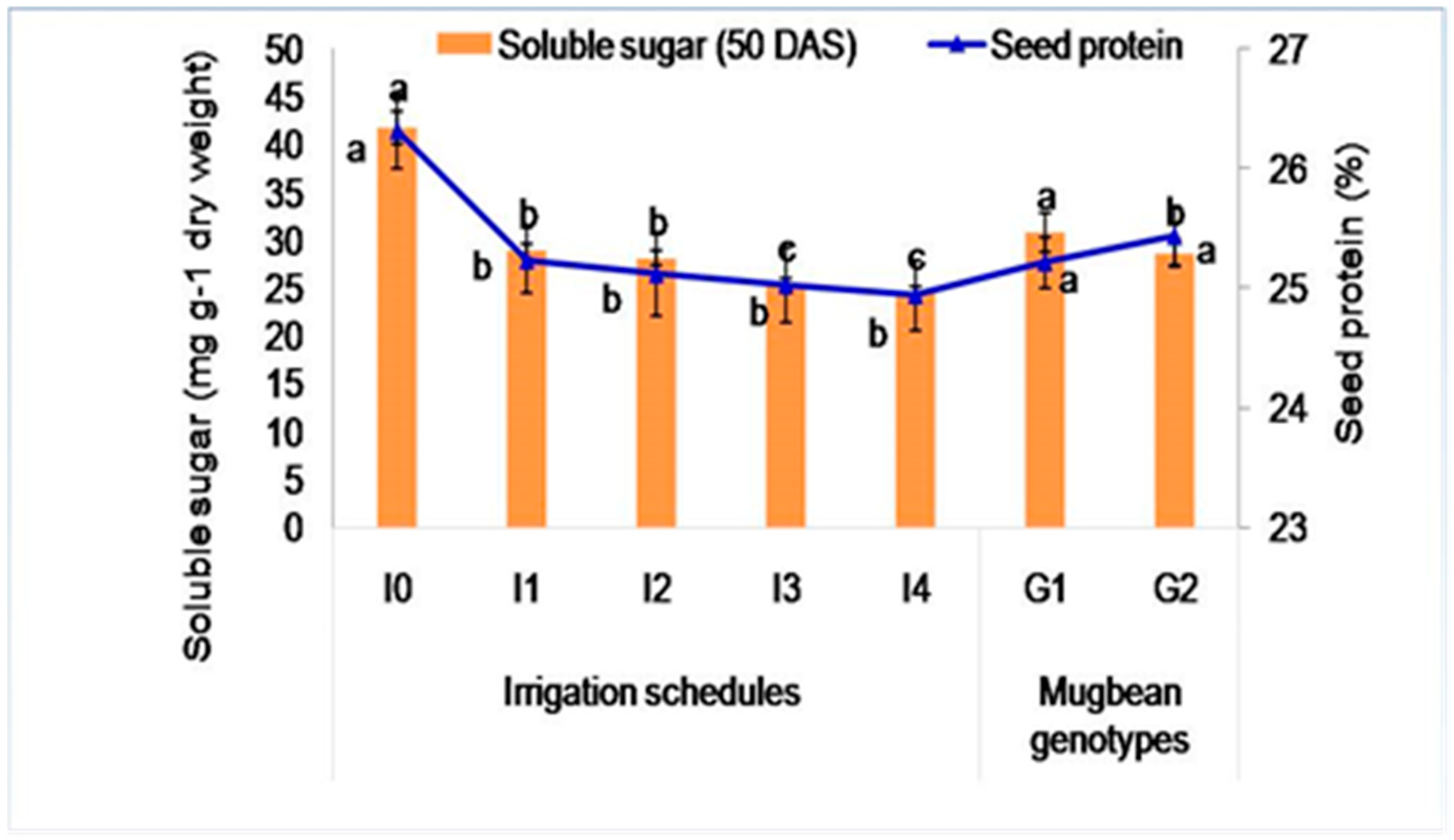
2.3.2. Soluble Sugar Content (mg g−1 Dry Weight)
2.3.3. Seed Protein Content (%)
2.4. Crop Harvests in Response to Irrigation Regimes in Mung Bean Genotypes
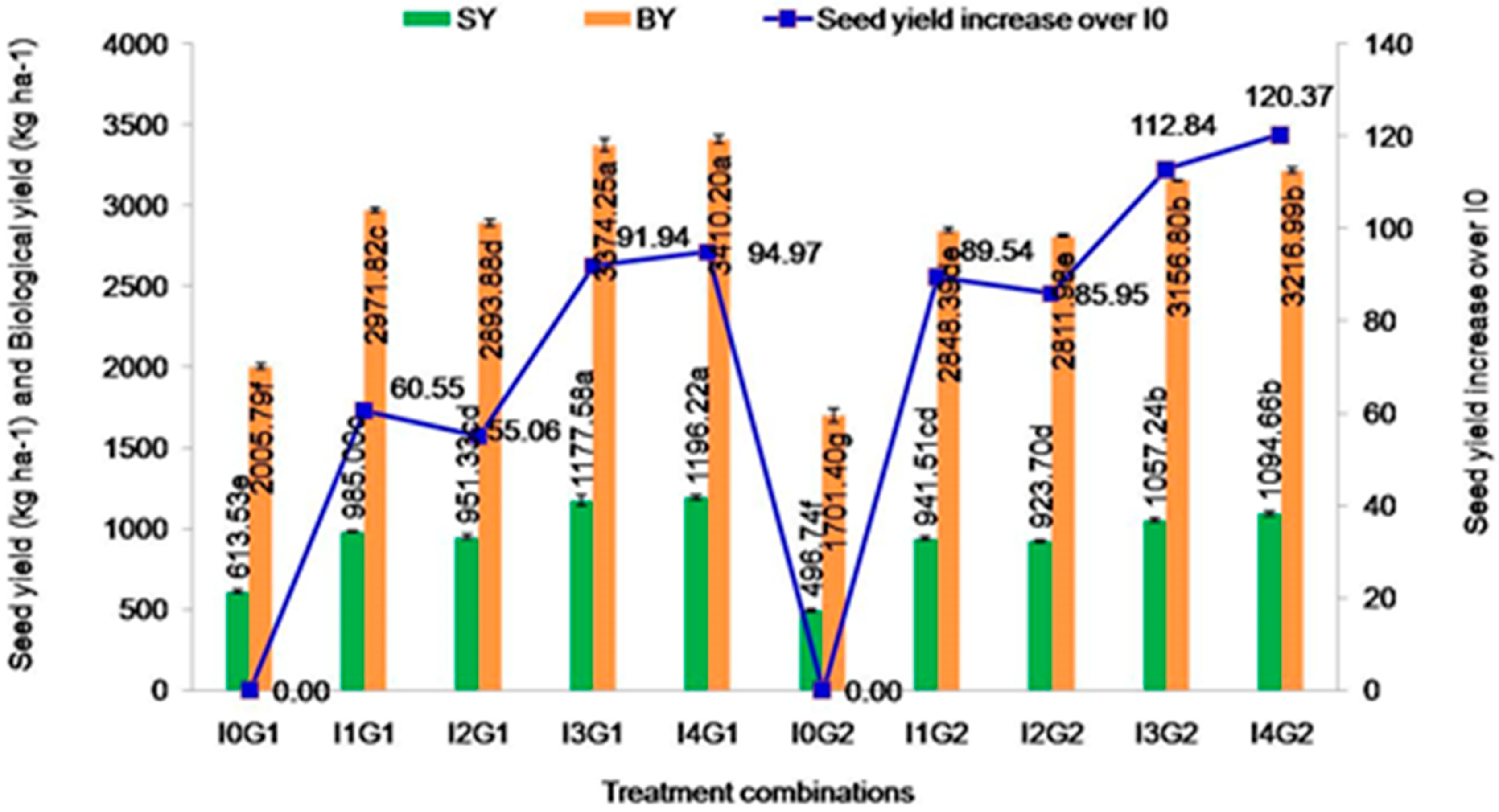
2.4.1. SeedYield
2.4.2. Biological Yield
3. Materials and Methods
3.1. Experimental Site andPlant Materials
3.2. Soil Status and Weather Information
3.3. Experimental Design and Treatments
3.4. Experimentation
3.4.1. Fertilization
3.4.2. Seed Sowing and Crop Management
3.5. Imposition of Treatments
3.6. Data Collection
3.6.1. Monitoring of Soil Moisture
3.6.2. Soil Water Depletion
3.6.3. Water Use Efficiency
3.6.4. Estimation of Chlorophyll Content
3.6.5. Relative Water Content (RWC)
3.6.6. Measurement of Xylem Exudation Rate
3.6.7. Estimation of the Membrane Stability Index
3.6.8. Estimation of Proline Content of the Third Trifoliate Leaf
3.6.9. Estimation of Soluble Sugars
3.6.10. Crop Harvests
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambrides, C.J.; Godwin, I.D.; Kole, C. (Eds.) Mungbean. In Genome Mapping and Molecular Breeding in Plants Pulses, Sugar and Tuber Crops; Springer: Berlin, Germany, 2007; pp. 69–90. [Google Scholar]
- Ashour, N.I.; Behairy, G.T.; Abd EL-Lateef, E.M.; Selim, M.M. A preliminary study on the potentiality of intercropping of mungbean (Vigna radiata Roxb.) with dwarf grain sorghum (Sorghum bicolor Moench) in Egypt. Bull. Natl. Res. Cent. 1991, 16, 53–60. [Google Scholar]
- FAO (Food and Agriculture Organization). Grassland Index. A Searchable Catalogue of Grass and Forage Legumes; FAO: Rome, Italy, 2012; pp. 37–43. [Google Scholar]
- Devendra, C.; Sevilla, C.; Pezo, D. Food-feed systems in Asia: Review. Asian-Aust. J. Anim. Sci. 2001, 14, 733–745. [Google Scholar]
- Hoorman, J.J.; Islam, R.; Sundermeier, A. Sustainable crop rotations with cover crops. Ohio State University Extension, Fact Sheet: Agriculture and Natural Resources, The Ohio State University, College of Food. Agric. Environ. Sci. 2009, SAG-9–09, 1–8. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Umer Chattha, M.; Arif, W.; Khan, I.; Soufan, W.; Bilal Chattha, M.; Hassan, M.U.; Ullah, N.; EL Sabagh, A.; Qari, S.H. Mitigation of Cadmium Induced Oxidative Stress by Using Organic Amendments to Improve the Growth and Yield of Mash Beans [Vigna mungo (L.)]. Agronomy 2021, 11, 2152. [Google Scholar] [CrossRef]
- Sarkar, M.N.I.; Ghosh, H.R. Techno-economic analysis and challenges of solar powered pumps dissemination in Bangladesh. Sustain. Energy Technol. Assess. 2017, 20, 33–46. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, M.S.; Sharker, K.K.; Nandi, S.K. Estimation of solar radiation on horizontal and tilted surface over Bangladesh. Comput. Water Energy Environ. Eng. 2016, 5, 54–69. [Google Scholar] [CrossRef]
- De Costa, W.A.; Shanmigathasan, K.N.; Joseph, K.D. Physiology of yield determination of mungbean (Vigna radiata L.) under various irrigation regimes in the dry and intermediate zones of Sri Lanka. Field Crops Res. 1999, 61, 1–12. [Google Scholar] [CrossRef]
- Siddique, M.R.B.; Hamid, A.; Islam, M.S. Drought stress effects on water relations of wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Ambachew, S.; Tena, A.; Assefa, M. Performance of mungbean under deficit irrigation application in the semi-arid highlands of Ethiopia. Agric. Water Manag. 2014, 136, 68–74. [Google Scholar] [CrossRef]
- Bibi, A.; Sadaqat, H.A.; Akram, H.M.; Mohammed, M.I. Physiological markers for screening sorghum (Sorghum bicolor) germplasm under water stress condition. Int. J. Agric. Biol. 2010, 12, 451–455. [Google Scholar]
- Hao, Z.F.; Li, X.H.; Su, Z.J.; Xie, C.X.; Li, M.S.; Liang, X.L.; Weng, J.F.; Zhang, D.X.; Li, L.; Zhang, S.H. A proposed selection criterion for drought resistance across multiple environments in maize. Breed. Sci. 2011, 61, 101–108. [Google Scholar] [CrossRef]
- Nandan, R.; Prasad, U.K. Effect of irrigation and nitrogen on growth, yield, nitrogen uptake and water use efficiency of French bean (Phaseolus vulgaris). Indian J. Agric. Sci. 1998, 67, 75–80. [Google Scholar]
- Rahim, S.F.; Gul, D.K.; Fazli, H.; Waheed, U. Effect of deficit irrigations and sowing methods on mung bean productivity. J. Biol. Agric. Healthc. 2014, 4, 74–83. [Google Scholar]
- Roy, S.; Barman, M.; Puste, A.M.; Gunri, S.K.; Jana, K. Growth, yield, water use efficiency and competitive functions of intercropping system of maize (Zea mays L.) and mungbean (Vigna radiata L.) as influenced by irrigation. SAARC J. Agric. 2015, 13, 94–107. [Google Scholar] [CrossRef]
- Singh, R.J.; Idnani, L.K.; Rai, R.K. Grain yield, water use efficiency, economics and soil moisture extraction pattern of summer greengram (Vigna radiata L.) as influenced by planting and irrigation methods, irrigation schedules and VAM inoculation. Ann. Agric. Res. 2006, 27, 306–310. [Google Scholar]
- Idnani, L.K.; Gautam, H.K. Water economization in summer greengram (Vigna radiata var radiata) as influenced by irrigation regimes and configurations. Indian J. Agric. Sci. 2008, 78, 214–219. [Google Scholar]
- Robertson, M.J.; Fukai, S.; Peoples, M.B. The effect of timing and severity of water deficit on growth development, yield accumulation and nitrogen fixation of mung bean. Field Crops Res. 2004, 86, 67–80. [Google Scholar]
- Simsek, M.; Comlekcioglu, N.; Ozturk, I. The effects of the regulated deficit irrigation on yield and some yield components of common bean (Phaseolus vulgaris L.) under semi-arid conditions. Afr. J. Biotechnol. 2011, 10, 4057–4064. [Google Scholar]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Pospíšilová, J.; Dodd, I.C. Role of plant growth regulators in stomatal limitation to photosynthesis during water stress. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; Taylor and Francis: New York, NY, USA, 2005; pp. 811–825. [Google Scholar]
- Nazran, A.; Ahmed, J.U.; Karim, A.J.M.S.; Ghosh, T.K. Physiological responses of mungbean (Vignaradiata) varieties to drought stress. Bangladesh J. Agric. Res. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Gaff, D.F.; Oliver, M. The evolution of desiccation tolerance in angiosperm plants: A rare yet common phenomenon. Func. Plant Biol. 2013, 40, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Vance, N.C.; Zaerr, J.B. Influence of drought stress and low irradiance on plant water relations and structural constituents in needles of Pinus ponderosa seedlings. Tree Physiol. 1991, 8, 175–184. [Google Scholar] [CrossRef]
- Islam, M.S.; Hasan, M.K.; Islam, B.; Renu, N.A.; Hakim, M.A.; Islam, M.R.; Chowdhury, M.K.; Ueda, A.; Saneoka, H.; Raza, M.A.; et al. Responses of water and pigments status, dry matter partitioning, seed production, and traits of yield and quality to foliar application of GA3 in Mungbean (Vigna radiata L.). Front. Agron. 2021, 2, 596850. [Google Scholar] [CrossRef]
- Choudhury, A.K. Water Stress Tolerance of French Bean (Phaseolus vulgaris L.). Ph.D. Thesis, Department of Agronomy, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh, 2009. [Google Scholar]
- Bajji, M.; Kinet, J.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Sibel, T.; Birol, T. Some physiological responses of drought stress in wheat genotypes with different ploidity in turkey. World J. Agric. Sci. 2007, 3, 178–183. [Google Scholar]
- Ratnasekera, D.; Subhashi, A.P.T. Morpho-physiological response of selected mungbean (Vigna radiata L.) Sri Lankan genotypes to drought stress. J. AgriSearch 2015, 2, 162–166. [Google Scholar]
- Ahmadizadeh, M.; Nori, A.; Shahbazi, H.; Habibpour, M. Effects of drought stress on some agronomic and morphological traits of durum wheat (Triticum durum Desf.) landraces under greenhouse condition. Afr. J. Biotechnol. 2011, 10, 14097–14107. [Google Scholar]
- Parvin, S.; Javadi, T.; Ghaderi, N. Proline, protein, RWC and MSI contents affected by paclobutrazol and water deficit treatments in strawberry cv. Paros. Cercet. Agron. Mold. 2015, 48, 107–114. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Assaha, D.V.M.; Islam, M.S.; Hassan, M.M.; EL Sabagh, A.; Saneoka, H. NaCl enhance the growth of swiss chard (Beta vulgaris L.) leaves under potassium-deficient conditions. J. Soil Sci. Plant Nutr. 2021, 21, 1949–1956. [Google Scholar] [CrossRef]
- Bajji, M.; Lutts, S.; Kinet, J.M. Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Sci. 2001, 160, 669–681. [Google Scholar] [CrossRef]
- Zadehbagheri, M.; Kamelmanesh, M.M.; Javanmardi, S.; Sharafzadeh, S. Effect of drought stress on yield and yield components, relative leaf water content, proline and potassium ion accumulation in different white bean (Phaseolus vulgaris L.) genotype. Afr. J. Agric. Res. 2012, 7, 5661–5670. [Google Scholar]
- Sanchez, F.J.; Manzanarcs, M.; Andres, R.F.; Tcrnorio, J.L.; De Ayerbe, L.; De Andres, F.F. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crop. Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Stoyanov, Z.Z. Effect of water stress on leaf water relations of young bean plants. J. Cent. Eur. Agric. 2005, 6, 5–14. [Google Scholar]
- Kabbadj, A.; Makoudi, B.; Mouradi, M.; Pauly, N.; Frendo, P.; Ghoulam, C. Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS ONE 2017, 12, e0190284. [Google Scholar] [CrossRef] [PubMed]
- Yasir, T.A.; Khan, A.; Skalicky, M.; Wasaya, A.; Rehmani, M.I.A.; Sarwar, N.; Mubeen, K.; Aziz, M.; Hassan, M.M.; Hassan, F.A.; et al. Exogenous Sodium Nitroprusside Mitigates Salt Stress in Lentil (Lens culinaris Medik.) by Affecting the Growth, Yield, and Biochemical Properties. Molecules 2021, 26, 2576. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W. Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.). Genet. Mole. Res. 2015, 14, 9943–9950. [Google Scholar] [CrossRef]
- Islam, M.S.; Fahad, S.; Hossain, A.; Chowdhury, M.K.; Iqbal, M.A.; Dubey, A.; Kumar, A.; Rajendran, K.; Danish, S.; Habib Ur Rahman, M.; et al. Legumes under Drought Stress: Plant Responses, Adaptive Mechanisms and Management Strategies in Relation to Nitrogen Fixation. In Engineering Tolerance in Crop Plants against Abiotic Stress; Fahad, S., Sönmez, O., Saud, S., Wang, D., Wu, C., Adnan, M., Arif, M., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2021; pp. 179–208. [Google Scholar] [CrossRef]
- Turner, N.C.; Jones, M.M. Turgor maintenance by osmotic adjustment: A review and evaluation. In Adaptation of Plants to Water and High Temperature Stress; Turner, N.C., Kramer, P.J., Eds.; Wiley: New York, NY, USA, 1980; pp. 87–103. [Google Scholar]
- Kundu, P.B.; Paul, N.K. Effect of water stress on chlorophyll, proline and sugar accumulation in rap (Brassica campestries L). Bangladesh J. Bot. 1997, 26, 83–85. [Google Scholar]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptation in environmental stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Ingram, J.; Bartels, D. The molecular basis of dehydration tolerance in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1996, 17, 377–403. [Google Scholar] [CrossRef]
- Trouverie, J.; Thévenot, C.; Rocher, J.P.; Sotta, B.; Prioul, J.L. The role of abscisic acid in the response of a specific vacular invertase to water stress in the adult maize leaf. J. Exp. Bot. 2003, 54, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, M.; Moradi, F.; Ahmadi, A.; Poostini, K.; Najafian, G. Effect of exogenous application of ABA and CK at different stages of grain development on some physiological aspects of source and sink relationship in two bread wheat cultivars. Iran. J. Crop Sci. 2006, 8, 268–282. [Google Scholar]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Koch, K. Carbohydrate-modulated gene expression in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S. Sugar-induced signal transduction in plants. Ann. Rev. Plant Biol. 2001, 51, 49–81. [Google Scholar] [CrossRef]
- Mujtaba, S.M.; Alam, S.M. Drought Phenomenon and Crop Growth. Industry and Economy Megazine, 2002. [Google Scholar]
- Bayat, A.; Ahmadvand, G.; Dorri, H. The effect of water stress on the yield and yield components of spotted beans genotypes. J. Agron. Sci. Iran 2010, 45, 42–45. [Google Scholar]
- Mohammadzadeh, A.; Majnoonhoseini, N.; Moghaddam, H.; Akbari, M. The effect of various water stress and nitrogen levels on the yield and yield components in red beans genotype. J. Agric. Sci. 2011, 43, 29–38. [Google Scholar]
- Yagoob, H.; Yagoob, M. The effects of water deficit stress on protein yield of mung bean genotypes. Peak J. Agric. Sci. 2014, 2, 30–35. [Google Scholar]
- Ahmad, A.; Selim, M.M.; Alderfasi, A.A.; Afzal, M. Effect of drought stress on mungbean (Vigna radiata L.) under arid climatic conditions of Saudi Arabia. Ecosys. Sustain. Dev. 2015, 192, 185–193. [Google Scholar]
- Sadasivan, R.; Natrajaratnam, N.; Dabu, R.; Muralidharan, V.; Rangasmay, S.R.S. Response of mungbean cultivars to soil moisture stress at different growth phases. In Proceedings of the International Symposium on Mungbean, Bankok, Thailand, 16–20 November 1987; AVRDC Taiwan: Shenhua, Taiwan, 1988; pp. 260–262. [Google Scholar]
- Pannu, R.K.; Singh, D.P. Influence of water deficits on morpho-physiological and yield behaviour of mung bean (Vigna radiata (L.) Wilczek). In Proceedings of the International Symposium on Mungbean, Bankok, Thailand, 16–20 November 1987; Shanmugasundaram, S., Mclean, B.T., Eds.; AVRDC Taiwan: Shenhua, Taiwan, 1988; pp. 252–259. [Google Scholar]
- Nielson, D.C.; Nelson, N.O. Black bean sensitivity to water stress at varius growth stages. Crop Sci. 1998, 38, 422–427. [Google Scholar] [CrossRef]
- Thaltooth, A.T.; Tawfik, M.M.; Mohamed, H.M. A comparative study on the effect of foliar application of Zinc, Potassium and Magnesium on growth, yield and some chemical constituents of mungbean plants grown under water stress conditions. World J. Agric. Sci. 2006, 2, 37–46. [Google Scholar]
- Ricciardi, L.; Polignano, G.B.; De Giovanni, C. Genotypic response of faba bean to water stress. Euphytica 2001, 118, 39–46. [Google Scholar] [CrossRef]
- Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.A.; Muhammad Sabir Tariq, R.; Ayub, M.A.; Hossain, A.; Hassan, M.M.; Brestic, M.; Sohidul Islam, M.; et al. Selenium Alleviates the Adverse Effect of Drought in Oilseed Crops Camelina (Camelina sativa L.) and Canola (Brassica napus L.). Molecules 2021, 26, 1699. [Google Scholar] [CrossRef]
- Khan, I.A. The growth and yield responses of mungbean to variable seeding densities and irrigation levels. Crop Husb. 2001, 16, 115–120. [Google Scholar]
- Sangakara, U.R. Yield and seed quality of mungbean as affected by irrigation in a dry season. J. Agron. Crop Sci. 1994, 172, 327–332. [Google Scholar] [CrossRef]
- Islam, M.R. Improving mungbean productivity under drought stress through screening of genotypes and potassium application. Ph.D. Thesis, Department of Agronomy, Hajee Mohammad Danesh Science and Technology University (HSTU), Basherhat, Bangladesh, 2020. [Google Scholar]
- BARC (Bangladesh Agricultural Research Council). Fertilizer Recommendation Guide; BARC: Dacca, Bangladesh, 2012; pp. 251–254. [Google Scholar]
- Azad, A.K.; Gosshami, B.K.; Rahman, M.L.; Malaker, P.K.; Hasan, M.S.; Rahman, M.H.H. Krishi Projukti Hatboi. In Handbook on Agro-Technology, 7th ed.; Bangladesh Agricultural Research Institute: Dacca, Bangladesh, 2017; p. 52. [Google Scholar]
- Michael, A.M. Irrigation Theory and Practice; Vikas Publishing House Pvt. Ltd.: New Delhi, India, 1996; p. 512. [Google Scholar]
- Martin, D.L.; Stegman, E.C.; Freres, E. Irrigation scheduling principals. In Management of Farm Irrigation Systems; Hoffman, G.L., Howell, T.A., Solomon, K.H., Eds.; ASAE: St. Joseph, MI, USA, 1990; pp. 155–372. [Google Scholar]
- Molden, D. Accounting for Water Use and Productivity. SWIM Paper 1; International Irrigation Management Institute: Colombo, Sri Lanka, 1997. [Google Scholar]
- Witham, H.; Blades, D.F.; Devin, R.H. Exercise in Plant Physiology, 2nd ed.; PWS Publishers: Boston, MA, USA, 1986; pp. 128–131. [Google Scholar]
- Barr, H.D.; Weatherley, P.E. A reexamination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Islam, M.S. Morpho-Physiology of Black Gram and Mungbean as Influenced by Salinity. Master’s Thesis, Department of Agronomy, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh, 2001. [Google Scholar]
- Sairam, R.K. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 1994, 32, 594–597. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; IRRI: Los Baños, Laguna, Philippines, 1976; p. 83. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: Singapore, 1984; pp. 302–307. [Google Scholar]
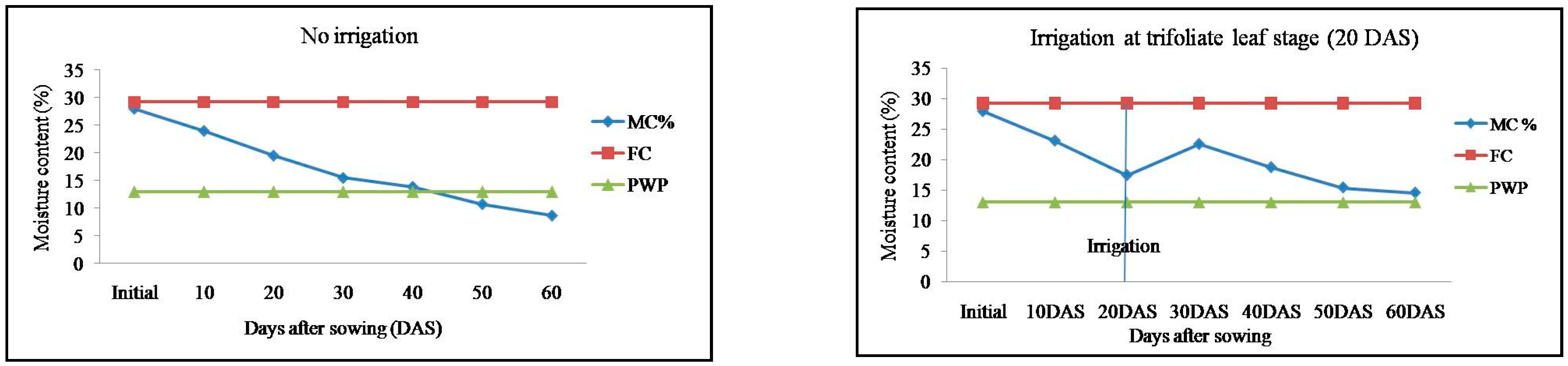
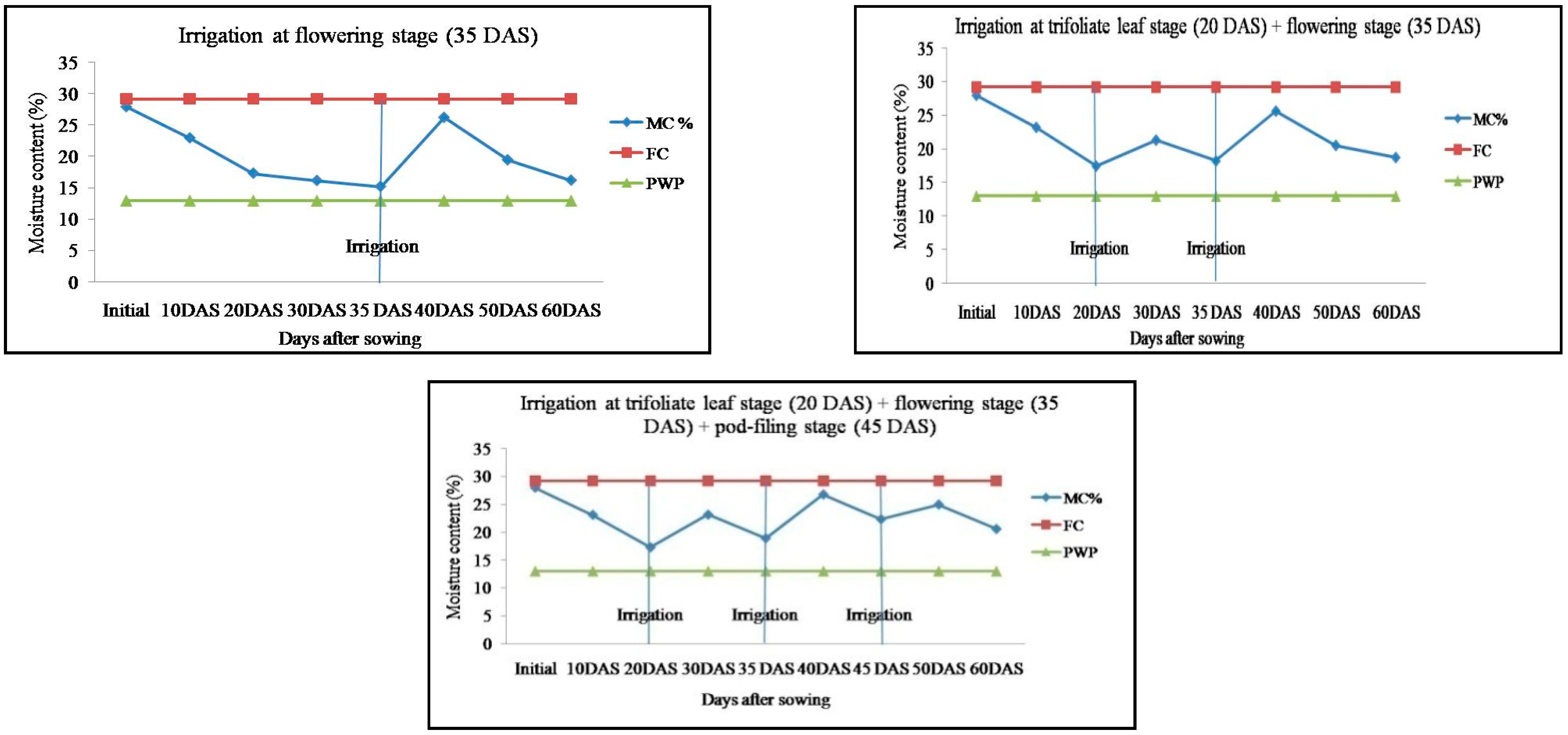
| Treatments | No. of Irrigation | Post-Sowing Irrigation (mm) | Irrigation (mm) | Effective Rainfall (mm) | Soil Water Depletion (mm) | Total Water Use (mm) | Water Use Efficiency (kg ha−1 mm−1) |
|---|---|---|---|---|---|---|---|
| I0 | 0 | 48 | 0 | 0 * | 121 | 169 | 3.79 |
| I1 | 1 | 48 | 74 | 0 | 84 | 206 | 4.68 |
| I2 | 1 | 48 | 88 | 0 | 74 | 210 | 4.46 |
| I3 | 2 | 48 | 143 | 0 | 58 | 249 | 4.49 |
| I4 | 3 | 48 | 183 | 0 | 46 | 277 | 4.14 |
| Treatment | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Chlorophyll a/b Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| DF (40 DAS) | DPF (50 DAS) | DF (40 DAS) | DPF (50 DAS) | DF (40 DAS) | DPF (50 DAS) | DF (40 DAS) | DPF (50 DAS) | |
| Irrigation schedules | ||||||||
| I0 | 1.74b (0) # | 1.57c (0) # | 0.75b (0) # | 0.65b (0) # | 2.48b (0) # | 2.22c (0) # | 2.38a | 2.43a |
| I1 | 1.87a (7.47) | 1.65bc (5.10) | 0.84a (12) | 0.73ab (12.31) | 2.71a (9.27) | 2.37bc (6.76) | 2.23bc | 2.26b |
| I2 | 1.79b (2.87) | 1.68abc (7.01) | 0.75b (0) | 0.75a (15.38) | 2.54b (2.42) | 2.43ab (9.46) | 2.34ab | 2.23b |
| I3 | 1.88a (8.05) | 1.77ab (12.74) | 0.85a (13.33) | 0.81a (24.62) | 2.73a (10.08) | 2.57ab (15.77) | 2.22c | 2.20b |
| I4 | 1.89a (8.62) | 1.79a (14.01) | 0.84a (12) | 0.81a (24.62) | 2.71a (9.27) | 2.61a (17.57) | 2.25bc | 2.21b |
| LSD (0.05) | 0.06 | 0.13 | 0.06 | 0.09 | 0.10 | 0.20 | 0.11 | 0.14 |
| CV (%) | 2.31 | 5.67 | 5.54 | 8.77 | 2.95 | 6.30 | 3.78 | 4.68 |
| LS | *** | * | ** | * | ** | * | * | * |
| Genotypes | ||||||||
| G1 | 1.88a | 1.74a | 0.84a | 0.78a | 2.72a | 2.53a | 2.25b | 2.23a |
| G2 | 1.78b | 1.64b | 0.77b | 0.72b | 2.54b | 2.35b | 2.31a | 2.31b |
| LS | ** | ** | *** | ** | *** | ** | * | * |
| Interactions | ||||||||
| I × G | ns | ns | ns | ns | ns | ns | ns | ns |
| Items | Soil Texture (Clay Loamy) | pH | OM (%) | N (%) | p (µg mL−1) | K (meq 100 g−1 soil) | S (µg mL−1) | B (µg mL−1) | Zn (µg mL−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial soil | Sand (18.6%) | Silt (32.0%) | Clay (49.4%) | 7.36 | 1.10 | 0.06 | 31.12 | 0.31 | 10.75 | 0.35 | 1.43 |
| Critical level | - | - | - | - | - | 0.12 | 10.00 | 0.12 | 10.00 | 0.20 | 0.60 |
| Month | Decades | Air Temperature | Mean Relative Humidity (%) | Rainfall (mm) | Sunshine Hour (day−1) | |
|---|---|---|---|---|---|---|
| Max. (°C) | Min. (°C) | |||||
| March | 1 | 31.0 | 17.7 | 72.6 | 22.0 | 5.5 |
| 2 | 29.8 | 15.9 | 71.9 | 12.0 | 7.4 | |
| 3 | 32.8 | 22.2 | 79.6 | 4.0 | 6.2 | |
| April | 1 | 35.3 | 24.6 | 75.5 | 0.1 | 6.9 |
| 2 | 35.3 | 21.6 | 71.2 | 2.8 | 8.7 | |
| 3 | 33.7 | 23.6 | 82.5 | 11.0 | 5.5 | |
| May | 1 | 34.4 | 24.4 | 81.6 | 1.5 | 6.9 |
| 2 | 34.9 | 24.5 | 80.9 | 5.3 | 7.7 | |
| 3 | 35.9 | 26.4 | 80.5 | 0.5 | 7.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.R.; Kamal, M.M.; Alam, M.A.; Hossain, J.; Soufan, W.; Skalicky, M.; Brestic, M.; Habib-ur-Rahman, M.; EL Sabagh, A.; Islam, M.S. Physiochemical Changes of Mung Bean [Vigna radiata (L.) R. Wilczek] in Responses to Varying Irrigation Regimes. Horticulturae 2021, 7, 565. https://doi.org/10.3390/horticulturae7120565
Islam MR, Kamal MM, Alam MA, Hossain J, Soufan W, Skalicky M, Brestic M, Habib-ur-Rahman M, EL Sabagh A, Islam MS. Physiochemical Changes of Mung Bean [Vigna radiata (L.) R. Wilczek] in Responses to Varying Irrigation Regimes. Horticulturae. 2021; 7(12):565. https://doi.org/10.3390/horticulturae7120565
Chicago/Turabian StyleIslam, Mohammad Rafiqul, Mohd. Mostofa Kamal, Mohammad Ashraful Alam, Jamil Hossain, Walid Soufan, Milan Skalicky, Marian Brestic, Muhammad Habib-ur-Rahman, Ayman EL Sabagh, and Mohammad Sohidul Islam. 2021. "Physiochemical Changes of Mung Bean [Vigna radiata (L.) R. Wilczek] in Responses to Varying Irrigation Regimes" Horticulturae 7, no. 12: 565. https://doi.org/10.3390/horticulturae7120565
APA StyleIslam, M. R., Kamal, M. M., Alam, M. A., Hossain, J., Soufan, W., Skalicky, M., Brestic, M., Habib-ur-Rahman, M., EL Sabagh, A., & Islam, M. S. (2021). Physiochemical Changes of Mung Bean [Vigna radiata (L.) R. Wilczek] in Responses to Varying Irrigation Regimes. Horticulturae, 7(12), 565. https://doi.org/10.3390/horticulturae7120565












