Antioxidant and Anti-Obesity Potentials of Korean-Native Wild Vegetables (Allium species)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Characteristics
2.2. Cell Culture
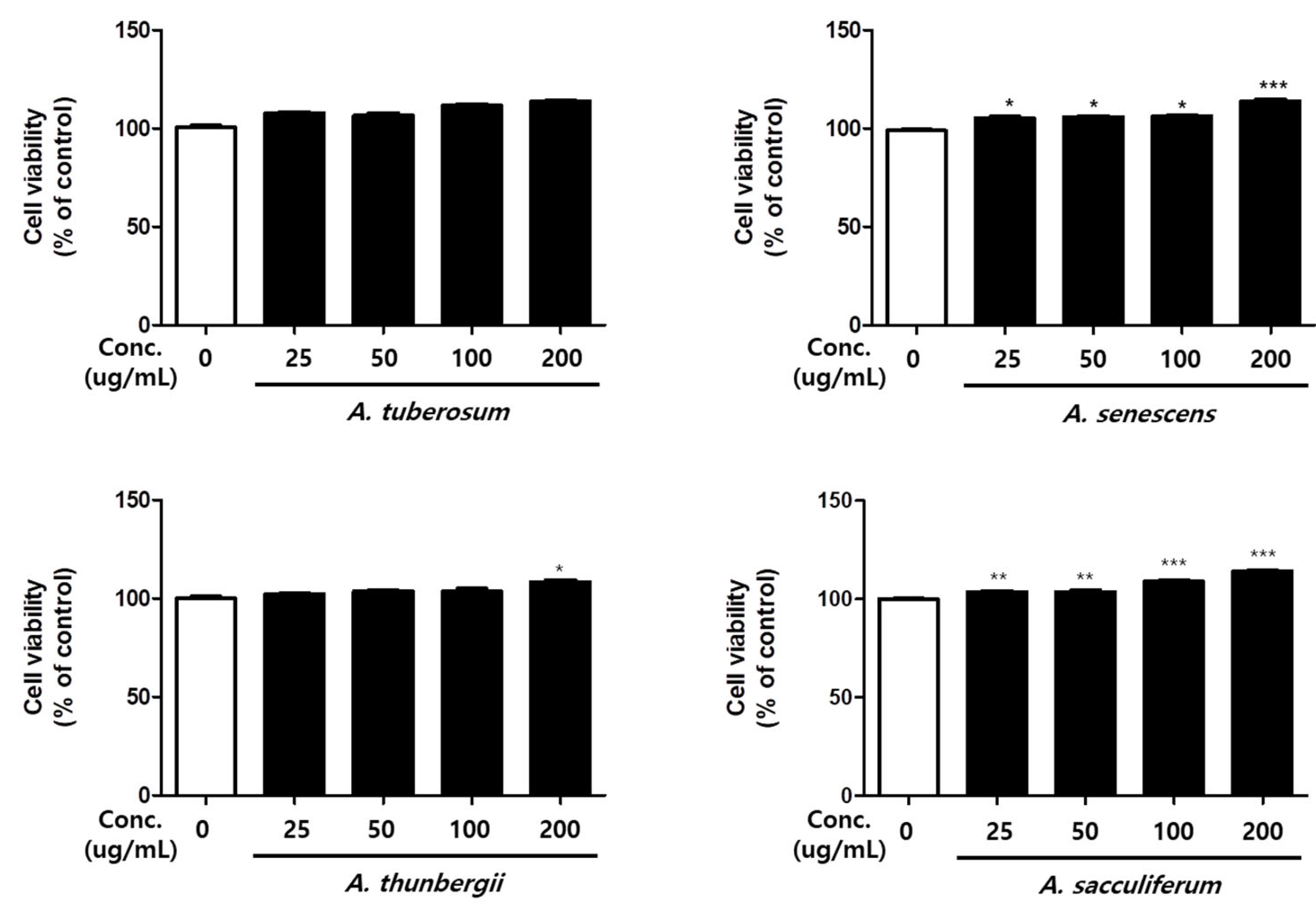
2.3. Cell Viability
2.4. Differentiation of Pre-Adipocytes
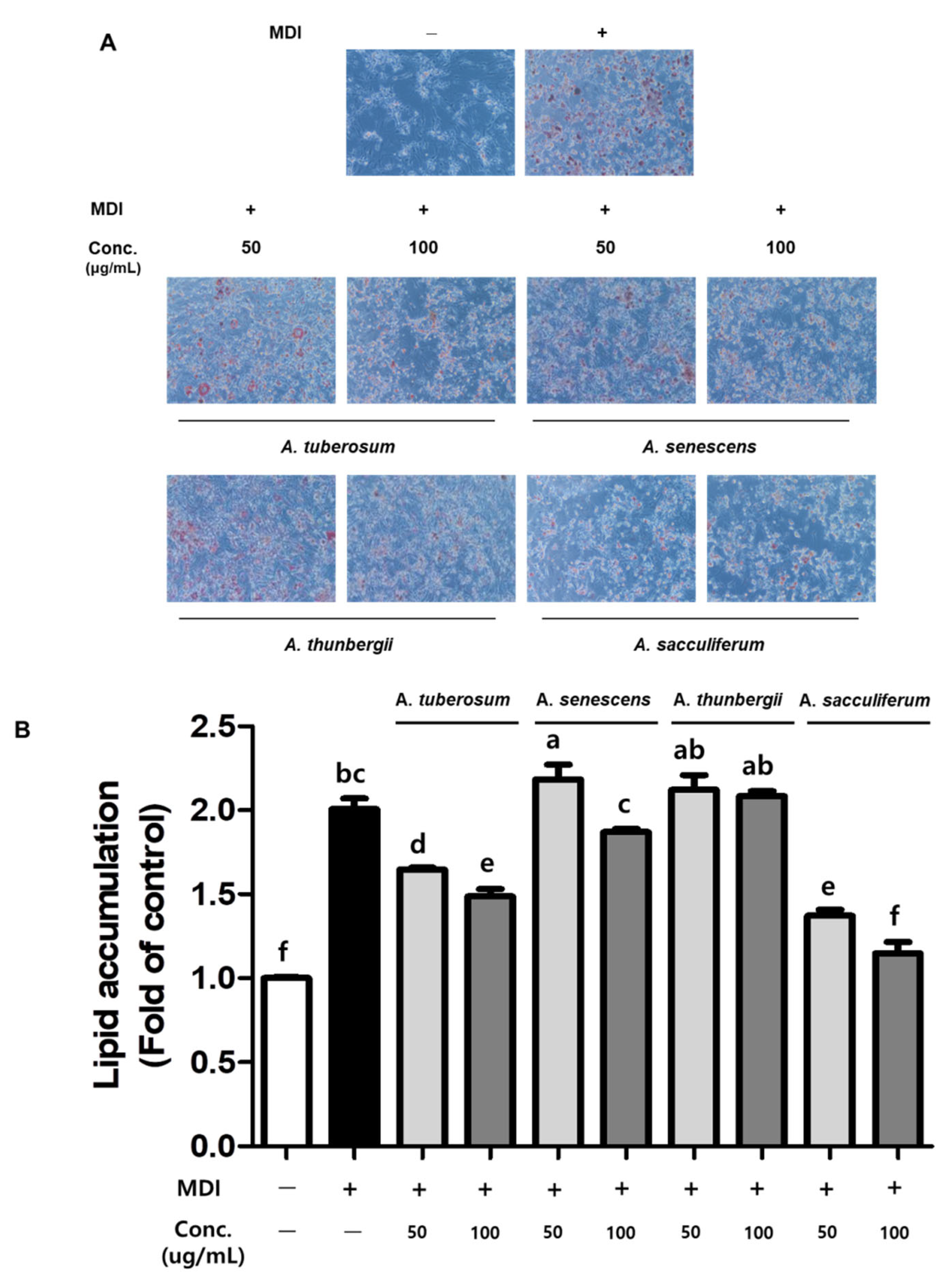
2.5. ORO Staining
2.6. Phenolic Analysis and Antioxidant Activity
2.6.1. DPPH Free Radical Scavenging Assay
2.6.2. ABTS Radical Scavenging Assay
2.6.3. Determination of TP Content
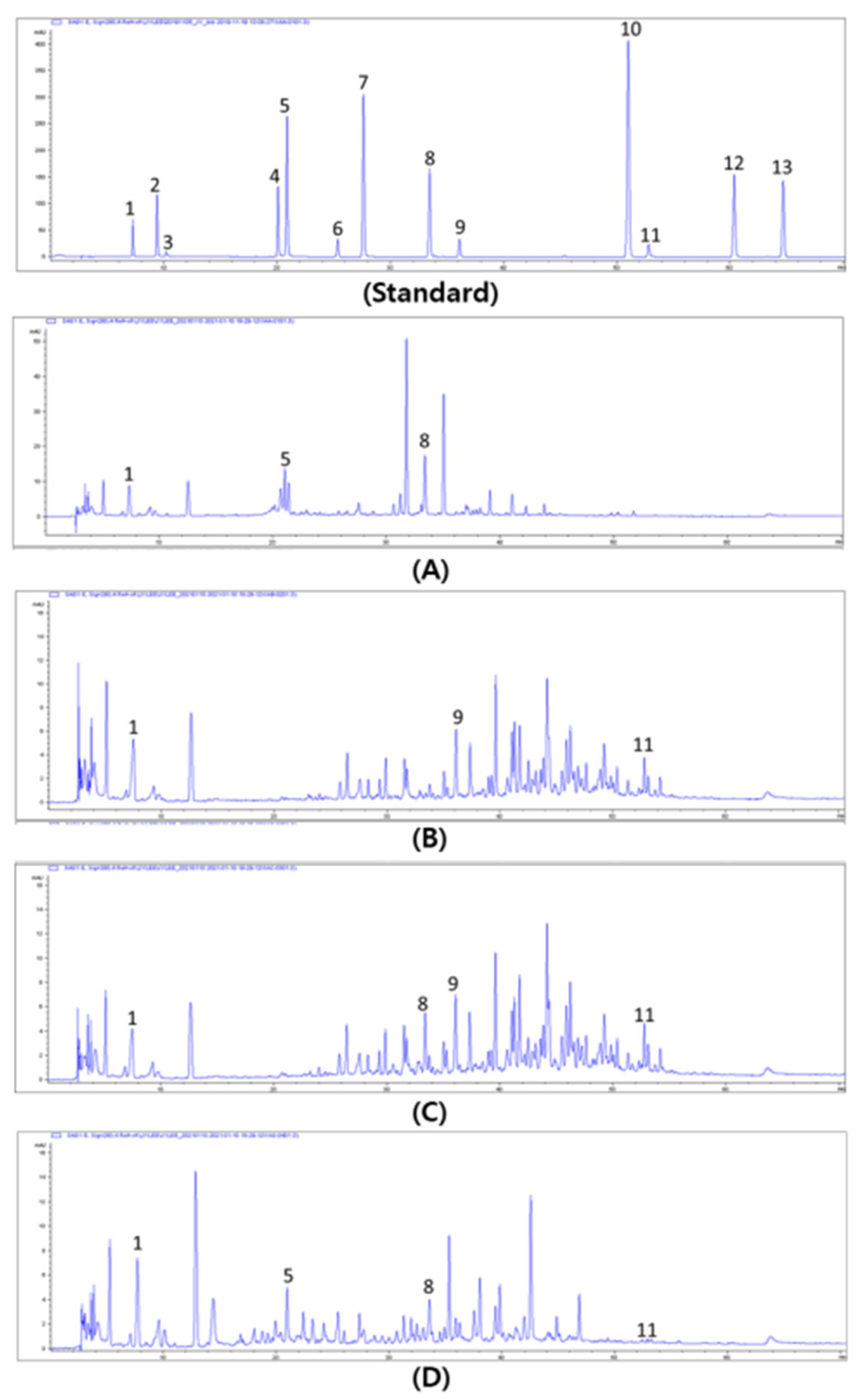
2.6.4. Phenolics Analysis Using HPLC
2.7. Statistical Analysis
3. Results
3.1. Growth Characteristic of Allium Spp.

3.2. Antioxidant Activities of Allium Spp.
3.3. Cell Viability of Allium Spp.
3.4. Effect of The Differentiation Inhibition of Allium Spp. by ORO Staining
3.5. Profiling of Phenolics in Allium Spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dandona, P.; Ghanim, H.; Chaudhuri, A.; Dhindsa, S.; Kim, S.S. Macronutrient intake induces oxidative and inflammatory stress: Potential relevance to atherosclerosis and insulin resistance. Exp. Mol. Med. 2010, 42, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, D.; Potgieter, M.; Ambele, M.A.; Adam, L.; Durandt, C.; Pepper, M.S. The role of reactive oxygen species in adipogenic differentiation. In Genome Editing; Springer: Cham, Switzerland, 2017; Volume 1083, pp. 125–144. [Google Scholar]
- Haida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef]
- Wang, Y.W.; Jones, P.J. Conjugated linoleic acid and obesity control: Efficacy and mechanisms. Int. J. Obes. 2004, 28, 941–955. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–498. [Google Scholar]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant phenolics—Secondary metabolites with diverse functions. Recent Adv. Polyphen. Res. 2008, 1, 1–35. [Google Scholar]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Chrząszcz, M.; Krzemińska, B.; Celiński, R.; Szewczyk, K. Phenolic Composition and Antioxidant Activity of Plants Belonging to the Cephalaria (Caprifoliaceae) Genus. Plants 2021, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Oh, B.U. A partial revision of Allium (Amaryllidaceae) in Korea and north-eastern China. Bot. J. Linn. Soc. 2011, 167, 153–211. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, H.S.; Park, K.W.; Kim, J.Y.; Lee, M.K.; Jeong, I.Y.; Seo, K.I. Mechanisms of thiosulfinates from Allium tuberosum L.-induced apoptosis in HT-29 human colon cancer cells. Toxicol. Lett. 2009, 188, 142–147. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Gu, Y.; Wang, P.; Song, W.; Ma, J.; Yang, X. The variability of bacterial communities in both the endosphere and ectosphere of different niches in Chinese chives (Allium tuberosum). PLoS ONE 2020, 15, e0227671. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Olatunji, O.J.; Zhou, Y.; Hou, X. Allium tuberosum: Antidiabetic and hepatoprotective activities. Food Res. Int. 2017, 102, 681–689. [Google Scholar] [CrossRef]
- Park, H.S.; Choi, E.J.; Lee, J.H.; Kim, G.H. Evaluation of Allium vegetables for anti-adipogenic, anti-cancer, and anti-inflammatory activities in vitro. J. Life Sci. 2013, 5, 127–132. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, G.H. Inhibitory effects of Allium senescens L. methanol extracts on reactive oxygen species production and lipid accumulation during differentiation in 3T3-L1 cells. Korean J. Food Sci. Technol. 2014, 46, 498–504. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.H.; Badamtsetseg, B.; Lee, S.; Kim, S. Anti-Proliferative Effect of Allium senescens L. Extr. Hum. T-Cell Acute Lymphocytic Leuk. Cells. Mol. 2021, 26, 35. [Google Scholar]
- Choi, H.Y.; Kim, G.H. Inhibitory effects of Allium sacculiferum Max. methanol extracts on ROS production and lipid accumulation during differentiation of 3T3-L1 cells. J. Korean Soc. Food Sci. Nutr. 2014, 43, 822–828. [Google Scholar] [CrossRef][Green Version]
- Choi, H.J.; Jang, C.G.; Ko, S.C.; Oh, B.U. A taxonomic review of Korean Allium (Alliaceae). Korean J. Plant Taxon. 2004, 34, 119–152. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. Assay Guidanced Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016; pp. 1–25. [Google Scholar]
- Lee, J.Y.; Park, J.Y.; Kim, H.D.; Lee, S.E.; Lee, J.H.; Lee, Y.; Seo, K.H. Anti-oxidant and anti-adipocyte differentiation of Aster glehni and Aster yomena. J. Nutr. Health 2019, 52, 250–257. [Google Scholar] [CrossRef]
- Kinkel, A.D.; Fernyhough, M.E.; Helterline, D.L.; Vierck, J.L.; Oberg, K.S.; Vance, T.J.; Dodson, M.V. Oil red-O stains non-adipogenic cells: A precautionary note. Cytotechnology 2004, 46, 49–56. [Google Scholar] [CrossRef]
- Nazlić, M.; Kremer, D.; Grubešić, R.J.; Soldo, B.; Vuko, E.; Stabentheiner, E.; Dunkić, V. Endemic Veronica saturejoides Vis. ssp. saturejoides–Chemical composition and antioxidant activity of free volatile compounds. Plants 2020, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Tanruean, K.; Poolprasert, P.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phytochemical Analysis and Evaluation of Antioxidant and Biological Activities of Extracts from Three Clauseneae Plants in Northern Thailand. Plants 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Bärlocher, F.; Graça, M.A. Total Phenolics. Methods to Study Litter Decomposition; Springer: Berlin/Heidelberg, Germany, 2020; pp. 157–161. [Google Scholar]
- Lee, J.Y.; Park, J.Y.; Seo, H.T.; Seong, H.A.; Ji, Y.J.; Lee, S.E.; Kim, H.D. Samnamul (Shoots of Aruncus dioicus) Inhibit Adipogenesis by Downregulating Adipocyte-Specific Transcription Factors in 3T3-L1 Adipocytes. Processes 2020, 8, 1576. [Google Scholar] [CrossRef]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Chen, X.; Na, J.; Sun, W. 3D printing of HEK 293FT cell-laden hydrogel into macroporous constructs with high cell viability and normal biological functions. Biofabrication 2015, 7, 015010. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M. Adipocyte differentiation: From fibroblast to endocrine cell. Exp. Biol. Med. 2001, 226, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Ilavenil, S.; Arasu, M.V.; Lee, J.C.; Kim, D.H.; Roh, S.G.; Park, H.S.; Choi, K.C. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Phytomedicine 2014, 21, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Finglas, P. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Choi, H.J.; Oh, B.U. Taxonomy of the Allium sect. Sacculiferum in Korea: With a special reference to the morphology. Korean J. Plant Taxon. 2003, 33, 339–357. [Google Scholar] [CrossRef]
- Achakzai, A.K.K.; Achakzai, P.; Masood, A.; Kayani, S.A.; Tareen, R.B. Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pak. J. Bot 2009, 41, 2129–2135. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Kwak, C.S.; Kim, M.J.; Kim, S.G.; Park, S.; Kim, I.G.; Kang, H.S. Antioxidant and antiobesity activities of oral treatment with ethanol extract from sprout of evening primrose (Oenothera laciniata) in high fat diet-induced obese mice. J. Nutr. Health 2019, 52, 529–539. [Google Scholar] [CrossRef]
- Gülçin, İ.; Berashvili, D.; Gepdiremen, A. Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J. Ethnopharmacol. 2005, 101, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Lim, S.W.; Ki, H.H.; Nepali, S.; Lee, Y.M.; Kim, D.K. Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. Int. J. Mol. Med. 2014, 34, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. [Google Scholar] [CrossRef] [PubMed]
- Juman, S.; Yasui, N.; Okuda, H.; Ueda, A.; Negishi, H.; Miki, T.; Ikeda, K. Caffeic acid phenethyl ester inhibits differentiation to adipocytes in 3T3-L1 mouse fibroblasts. Biol. Pharm. Bull. 2010, 33, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
| Plant | Collection Site | Voucher No. | Local Names | Parts Used |
|---|---|---|---|---|
| A. tuberosum | Mt.Obong, (Yangyang, Gangwon) | MPS001706 | Buchu | Aerial part |
| A. senescens | Peak. Hyangro, (Yangyang, Gangwon) | MPS005298 | Dume-buchu | Aerial part |
| A. thunbergii | Mt.Sobaek, (Danyang, Chungbuk) | MPS004662 | San-buchu | Aerial part |
| A. sacculiferum | NIHHS (Eumseong, Chungbuk) | MPS005491 | Chamsan-buchu | Aerial part |
| Plant | Plant Height (Mm) | Stem Diameter (Mm) | Length of Longest Root (Mm) | Number of Leaves |
|---|---|---|---|---|
| A. tuberosum | 212.5 ± 4.3 b | 0.4 ± 0.0 c | 78.7 ± 3.5 b | 4.0 ± 1.4 a |
| A. senescens | 242.0 ± 9.5 a | 46.7 ± 2.9 a | 114.8 ± 10.9 a | 3.7 ± 0.6 ab |
| A. thunbergii | 244.5 ± 11.0 a | 35.0 ± 10.6 b | 124.0 ± 17.7 a | 2.3 ± 0.0 b |
| A. sacculiferum | 110.8 ± 18.7 c | 2.0 ± 0.7 c | 40.0 ± 4.2 c | 3.3 ± 0.0 ab |
| Plants | Antioxidant Activity 1 | TP 2 | Yields 3 | |
|---|---|---|---|---|
| Dpph | Abts+ | |||
| A. tuberosum | 694.2 ± 10.7 b | 100.2 ± 1.7 b | 17.6 ± 0.3 a | 29.7 |
| A. senescens | 880.0 ± 51.4 a | 101.1 ± 3.7 b | 16.0 ± 0.2 c | 55.3 |
| A. thunbergii | 980.0 ± 5.1 a | 396.2 ± 2.6 a | 14.5 ± 0.7 d | 55 |
| A. sacculiferum | 723.1 ± 12.3 c | 44.0 ± 0.7 c | 17.3 ± 0.1 b | 53.1 |
| Ascorbic acid | 55.34 ± 2.08 d | 31.29 ± 1.47 d | - | - |
| Phenolics | A. tuberosum | A. senescens | A. thunbergii | A. sacculiferum |
|---|---|---|---|---|
| Homogentic acid | 50.8 ± 0.7 c | 75.7 ± 1.6 a | 47.8 ± 0.5 d | 63.0 ± 0.5 b |
| Caffeic acid | 3.9 ± 0.1 a | N.D.1 | N.D. | 3.6 ± 0.1 b |
| Ferulic acid | 15.9 ± 0.1 a | N.D. | 0.6 ± 0.2 b | 0.3 ± 0.1 c |
| Veratric acid | N.D. | 58.9 ± 1.3 a | 54.4 ± 1.0 b | N.D. |
| Quercetin | N.D. | 53.8 ± 0.6 a | 44.0 ± 1.0 b | N.D. |
| Factors | TP 1 | Homogentic Acid | Caffeic Acid | Ferulic Acid | DPPH 2 | ABTS 3 | Lipid Accumulation |
|---|---|---|---|---|---|---|---|
| TP | −0.373 | 0.932 | 0.464 | −0.796 ** | −0.439 | −0.826 *** | |
| Homogentic acid | −0.238 | −0.474 | −0.024 | −0.587 * | −0.120 | ||
| Caffeic acid | 0.618 * | −0.931 | −0.633 * | −0.889 *** | |||
| Ferulic acid | −0.595 * | −0.231 | −0.248 | ||||
| DPPH | 0.811 ** | 0.867 *** | |||||
| ABTS | 0.784 ** | ||||||
| Lipid accumulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Seo, K.H.; Lee, E.Y.; Ji, Y.-J.; Lee, Y.J.; Kang, M.H.; Seong, H.-A.; Kim, H.D. Antioxidant and Anti-Obesity Potentials of Korean-Native Wild Vegetables (Allium species). Horticulturae 2021, 7, 541. https://doi.org/10.3390/horticulturae7120541
Lee JY, Seo KH, Lee EY, Ji Y-J, Lee YJ, Kang MH, Seong H-A, Kim HD. Antioxidant and Anti-Obesity Potentials of Korean-Native Wild Vegetables (Allium species). Horticulturae. 2021; 7(12):541. https://doi.org/10.3390/horticulturae7120541
Chicago/Turabian StyleLee, Ji Yeon, Kyung Hye Seo, Eun Young Lee, Yun-Jeong Ji, Yun Ji Lee, Min Hye Kang, Hyun-A Seong, and Hyung Don Kim. 2021. "Antioxidant and Anti-Obesity Potentials of Korean-Native Wild Vegetables (Allium species)" Horticulturae 7, no. 12: 541. https://doi.org/10.3390/horticulturae7120541
APA StyleLee, J. Y., Seo, K. H., Lee, E. Y., Ji, Y.-J., Lee, Y. J., Kang, M. H., Seong, H.-A., & Kim, H. D. (2021). Antioxidant and Anti-Obesity Potentials of Korean-Native Wild Vegetables (Allium species). Horticulturae, 7(12), 541. https://doi.org/10.3390/horticulturae7120541






