Abstract
Background: Natural plant extracts and microbial antagonists have the potential for use in increasing the fungal resistance and productivity of horticulture plants. Methods: The purpose of this study was to evaluate the ability of both natural plant extracts and microbial antagonists as a biotical control of some fungal pathogens, i.e., Fusarium ssp., Exserohilum ssp. and Nigrospora ssp., along with improving the growth and productivity performance of zucchini under greenhouse conditions. Eucalyptus camaldulensis leaf extract (LE), Citrus sinensis LE, Ficus benghalensis fruit extract (FE), and two microbial antagonists Pseudomonas fluorescens (accession no. MW647093) and Trichoderma viride (accession no. MW647090) were tested under in vitro and in vivo conditions. Through morphological characteristics and the internal transcribed spacer (ITS) region, Fusarium solani (accession no. MW947256), F. oxysporum (accession no. MW947254), Exserohilum rostratum (accession no. MW947255), and Nigrospora lacticolonia (accession no. MW947253) were identified. HPLC analysis was used for the identification of phenolic compounds (PCs) and flavonoid compounds (FCs) in the extracts. Results: The highest inhibition percentage of fungal growth (IPFG) against F. oxysporum was obtained with P. fluorescens, T. viride, and E. camaldulensis LE (4000 mg/L); F. solani with P. fluorescens, T. viride, and C. sinensis LE (4000 mg/L); Exserohilum rostratum with P. fluorescens, Ficus benghalensis FE (4000 mg/L) and E. camaldulensis LE (4000 mg/L), and N. lacticolonia with P. fluorescens. Using HPLC analysis, the abundant PCs in E. camaldulensis LE were pyrogallol, and caffeic acid, those in C. sinensis LE were syringic acid and ferulic acid, and those in F. benghalensis FE were gallic acid and syringic acid. In addition, the abundant FCs in E. camaldulensis LE were kaempferol, and naringin, those in C. sinensis LE were hesperidin and quercetin, and those in F. benghalensis FE were kaempferol and quercetin. Under greenhouse experiments, T. viride and E. camaldulensis LE (4000 mg/L) followed by P. fluorescens + T. viride treatments gave the best results of zucchini plants in terms of leaf area, fruits number per plant, yield per plant, and total yield (marketable and non-marketable). Conclusions: Plant extracts and bioagents can be used to control some zucchini fungal pathogens and increase the productivity performance of zucchini plants.
1. Introduction
Zucchini (Cucurbita pepo L.) is a popular, seasonal vegetable crop that is cultivated in large areas of Egypt [1]. Zucchini is considered a low-calorie vegetable with health-promoting properties [2,3]. The fruits contain biologically active compounds including lutein, β-carotene, and folic acid, as well as vitamins and minerals [2,4,5]. Zucchini fruits have gained significant importance not only on the fresh food market but also as a raw material for various kinds of vegetable-based processed food items, especially in Mediterranean and European countries [6,7]. Zucchini is normally grown in soil extensively during the summer season and intensively under greenhouse conditions during the fall and winter seasons for national and international markets [8].
Zucchini plants grown in the field and under greenhouse conditions are usually infected by pathogens specific for Cucurbitaceae [9,10,11]. The most important among these pathogens are Exserohilum rostratum and Nigrospora lacticolonia [12,13,14,15], and fungal pathogens are considered as some of the most serious pathogens causing a significant reduction in date palm growth, production, and development [16,17,18]. Fusarium solani and F. oxysporum surviving in the soil environment as saprotrophic mycelium and chlamydospores are known to be pathogens of zucchini and other vegetables, causing plant decay due to the colonization of their underground organs [12,19]. The most important pathogens seem to be F. solani causing crown rot, F. oxysporum responsible for plant wilting, E. rostratum causing leaf spot and N. lacticolonia causing brown spot [13,15,20]. In recent years, researchers on biological control of fungal plant pathogens have placed much interest in increasing crop production by avoiding several problems linked to developing practices compatible with sustainable agriculture and chemical control. Microbial antagonists are being used to control fungal growth. Some of the most common antagonists—including Pseudomonas fluorescens and Trichoderma viride—have been utilized as the main mechanism against some fungal zucchini diseases [21]. Pseudomonas fluorescens is a harmless bacterial species that is found to protect the roots of plants from plant disease [22,23,24]. Trichoderma is used as a biological control agent against a wide range of commercially important plant pathogens [25,26,27]. They are known to produce a number of antibiotics, such as trichodermol A, harzianolide, and trichodermin [28,29,30,31].
Natural plant extracts modulate plant growth and are involved in plant defense responses, including limiting pathogen development [32]. They are used as antimicrobial agents against a broad spectrum of plant pathogenic fungi such as Alternaria solani, Aspergillus fumigatus, A. niger, Trichoderma longibrachiatum, A. flavus, A. fumigatus, Fusarium solani, F. oxysporum, Bipolaris orzyae, Botrytis cinerea, Curvularia lunata, F. verticilliodies, and F. graminearum [33,34,35,36,37,38,39]. Moreover, these can be used against plant bacterial pathogens such as Pectobacterium carotovorum, Pectobacterium atrosepticum, Dickeya solani, and Agrobacterium tumefaciens [40]. The action of natural compounds such as terpenoids, phenolics, and alkaloids are not specific, and their effects on pathogens are versatile [41]. However, some studies found that flavonoids were not associated with antifungal activity [42], while other works reported that the inhibition of fungal growth was mainly due to flavonoids [34,43]. Natural bioactive compounds used in plant protection kill pathogens (fungicidal effect) or limit their development (fungistatic effect), as well as induce plant defense reactions as elicitors [41].
This study was aimed at investigating the effects of natural plant extracts and microbial antagonists on the growth and total yield of zucchini plants under greenhouse conditions, to evaluate the diversity of the fungal isolates colonizing zucchini, isolation and by identification through morphological characteristics and the internal transcribed spacer (ITS) region. In addition, we planned to evaluate the plant extracts (Eucalyptus camaldulensis leaves, Citrus sinensis leaves, and Ficus benghalensis fruits) and microbial antagonists (Pseudomonas fluorescens and Trichoderma viride) to control selected Zucchini fungal pathogens in vitro.
2. Materials and Methods
2.1. Isolation of the Fungal Pathogens
Fungi were isolated from naturally infected zucchini plant variety AZIAD F1. (Figure 1). The fruit pieces were directly placed on a Potato Dextrose Agar (PDA) medium, into sterile Petri dishes. Plates were incubated at 25 °C for 7 days as described by Pitt and Hocking [44] and Abass et al. [17]. A standard tissue isolation technique was used to obtain fungal pathogen cultures as described by Naik et al. [45]. The fungus was identified in the laboratory using culturing, microscopic, and molecular methods.

Figure 1.
Symptoms of fungal diseases in naturally infected zucchini plants.
2.2. Morphological Identification of Fungal Isolates
The hyphae and conidia were examined in one-week-old colonies grown on PDA plates. The morphological identification was performed according to Matsushima [46]. Macroscopic characteristics included color, growth rate, and colony features, while microscopic characteristics including conidial size and shape were observed using a microscope (Reichert-jung, Dexter, MI, USA). Morphological characterizations were completed as described in previous works [15,47,48,49,50].
2.3. Isolation and Molecular Characterization of Genomic DNA Using ITS and Sequence Analysis
DNA extraction was performed from four tested fungal isolates grown on (PDA) medium. Fresh mycelia samples were collected from one-week-old cultures. The extraction of total genomic DNA of each fungal isolates was carried out according to previous recommendations [51,52,53].
Amplification of ITS regions of the rDNA was conducted using the universal primers, ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCC GCTTATTGATATGC-3′). The PCR amplification reactions were performed in a total volume of 25 µL, containing 3 µL of template DNA, 12.5 µL of PCR Green Master Mix (Thermo Scientific™, Gloucester, UK), 0.5 µL each of the universal forward primer (ITS1), reverse primer (ITS4) and 8.5 µL of molecular-grade water. The cycle initial denaturation step at 98 °C of 30 s, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 54 °C for 20 s; and a final extension for 10 min at 72 °C. Amplified products were separated on a 1.5% agarose gel in 0.5X TBE buffer (Tris-borate-EDTA), pre-stained with ethidium bromide (1 μg/mL) at 65 V for 15 min, GeneRuler 1 kb DNA ladder was used as a marker, and visualized under a UV transilluminator over ultraviolet light. The ITS region was sent for sequencing (Macrogen, Scientific Services Company, Seoul, Korea) [51,52,53,54,55], and thereafter identified by comparison with all available sequences in the National Centre for Biotechnology Information (NCBI) using the Basic Alignment Sequence Tool (BLAST).
2.4. Evaluation of Bioagents and Plant Extracts against Zucchini Fungal Pathogens In Vitro and In Vivo
2.4.1. Efficacy of Biological Control In Vitro
Two biological controls, namely, Pseudomonas fluorescens (accession number MW647093) and Trichoderma viride (accession number MW647090), were identified through DNA extraction as described in previous studies [43]. These biological controls were evaluated for their efficacy against some fungal isolated from zucchini plants using a dual-culture technique [35,43,53]. PDA medium (15 mL) was poured into 90 mm diameter Petri dishes and allowed to solidify for 15 min. Then, a 5 mm disc from each of the fungal pathogens was taken from growing margins of a one-week-old culture and placed at one end of the Petri dish that continued the PDA medium. The fungus was centered between two P. fluorescens lines in Petri dishes and incubated for one week at 28 °C. In the case of the fungal antagonist, the isolated T. viride strain (5 mm disc) was inoculated on the opposite side of the same Petri dish. The activity of the antagonistic organisms was recorded by measuring the colony diameter in each treatment and comparing it to the control value [43,53].
2.4.2. Preparation of Plant Extracts and Their HPLC Analysis
Eucalyptus camaldulensis leaves, Citrus sinensis leaves, and Ficus benghalensis fruits were collected from Alexandria, Egypt, during June 2019. The samples were air-dried under laboratory conditions and ground using a small laboratory Wiley mill. Approximately 50 g of each of the E. camaldulensis leaves, C. sinensis leaves, and F. benghalensis fruits was extracted with distilled water by the soaking method for 24 h under room temperature [35,56].
The phenolic compounds from the water extracts of each of the E. camaldulensis leaves, C. sinensis leaves, and F. benghalensis fruits were identified by HPLC (Agilent 1100) was composed of two LC pump, a UV/Vis detector, and C18 column (125 mm × 4.60 mm, 5 µm particle size). Chromatograms were obtained and analyzed using the Agilent ChemStation. Phenolic acids were separated by employing a gradient mobile phase of two solvents—Solvent A (Methanol) and Solvent B [Acetic acid in water (1:25)]. The gradient program was started with 100% B and was held at this concentration for the first 3 min. This was followed by 50% eluent A for the next 5 min after which the concentration of A was increased to 80% for the next 2 min and then reduced to 50% again for the following 5 min detection wavelength at 250 nm. Therefore, the order of phenolic compounds was according to authenticate standard compounds by using this mobile phase.
The identification of flavonoid compounds from those extracts was performed by HPLC (Agilent 1100), composed of two LC pumps, a UV/Vis detector, and C18 column (250 × 4.6 mm, 5 µm). The mobile phase was acetonitrile (A) and 0.2% (v/v) aqueous formic acid (B) with an isocratic elution (70:30) program. The detection wavelength was set at 360 nm.
2.4.3. Bioactivity In Vitro
The plant extracts were prepared at the concentrations 4000, 2000, 1000 and 500 mg/L by dissolving the extract in dimethyl sulfoxide (DMSO 0.01%) and were tested against the growth of the four fungal isolates. Fresh growth from each fungal isolate was harvested from 7-day-old culture grown on PDA media. The agar dilution method was through the integration of different coveted concentrations of the extracts into an agar medium, followed by a single 5 mm culture disk of the isolate taken from actively growing cultures and placed in the middle of the Petri dishes [57]. The Petri dishes were incubated at 28 °C for one week, the controls were contained only PDA medium and fungal discs in the middle. The inhibition percentage of fungal growth (IPFG)% of the tested fungi was calculated with the following formula [35,55,58]:
Inhibition percentage of fungal growth (IPFG)% = [(Growth in control − Growth in treatment)/Growth in control] × 100.
Growth values in the control and in the treatment are the average diameters (mm) of fungal colonies.
2.5. Experiments under Greenhouse Conditions
Two successive experiments were carried out during 2019 and 2020. The first experiment began in late September and ended in mid-December. The second experiment began in late December and ended in early February under greenhouse conditions at Abis Experimental Farm Station (31°13′ N latitude, 29°59′ E longitude), Faculty of Agriculture, Alexandria University, Egypt. During the period of Zucchini (Cucurbita pepo L.) growth, the average temperature (°C) condition under the greenhouse experimental is shown in Figure 2.
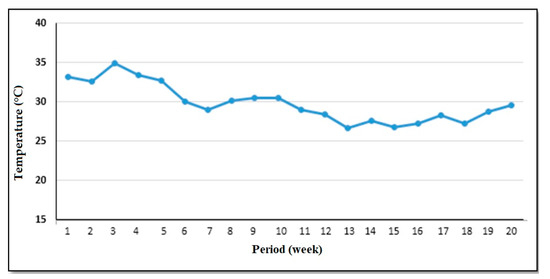
Figure 2.
Average temperature (°C) under greenhouse conditions during the 2019 and 2020 experimental periods for zucchini growth.
AZIAD F1 cultivar was used in this study, and this cultivar was imported from the Sakata Tackey company Japan vegetable seed. It is a desirable variety in the Egyptian market and bears low temperatures and high production [59].
Seeds for Zucchini variety AZIAD F1 were sown in late September and also in late December, in the first and second experiments, respectively, in clay soil. The row spacing was 30 cm between the plants and 1 m between the lines in a row. The experimental layout was a randomized complete block design (RCBD). Each treatment had three replicates, and each replicate had seven plots, where each plot had 35 plants. Nitrogen, phosphorus, and potassium fertilizers were fertigated at rates of 60, 70 and 100 kg N, P2O5 and K2O fed−1, respectively. Ammonium sulfate (NH4)2SO4, (20.5% N), phosphoric acid (58%), and potassium sulfate (48% K2O) were the sources of N, P2O5, and K2O, respectively. The drip irrigation system consisted of laterals GR of 16 mm in diameter with drippers at 0.3 m distance. The drippers had a discharge rate of 4 L h−1. Water irrigation was applied through the drip irrigation system.
Seven treatments (Table 1) were used in the present work. A drench of 50 mL suspension from each treatment was prepared. Inoculation with T. viride was mixed thoroughly with the soil, then watered and left to insure establishment and distribution of the inoculum in soil. Inoculation with P. fluorescens was sprayed on the plant. Inoculation with T. viride + P. fluorescens and the application of plant extracts (E. camaldulensis LE, C. sinensis LE, and F. benghalensis FE) were sprayed on the plant, respectively. In the case of plant extracts, the concentration used was selected based on the in vitro results to investigate its ability to reduce the incidence of fungal diseases in zucchini plants under greenhouse conditions [34,43].

Table 1.
Treatments used in the greenhouse experiment.
All treatments were added to plants four times during the entire growing season of zucchini plants. The first addition was after two weeks from the sowing date then additions were added weekly.
After 45 days from sowing, the leaf number per plant and the leaf area were measured using the following equation:
Leaf area = −5.25 + 0.67(MLTD) + 1.48(ML) + 0.74(TD); where (ML) midrib length and (ML) distance between tertiary lobes according to Fargo et al. [60].
Harvesting was initiated after 50 days of the sowing date and lasted for 6 weeks. Fruits were harvested twice a week. Fruits number per plant, yield per plant (kg), and total yield (marketable and non-marketable kilograms per square meter) were recorded after each harvesting accumulatively.
2.6. Statistical Analysis
The reduction in linear growth of F. oxysporum, F. solani, E. rostratum, and N. lacticolonia as affected by the different concentrations of E. camaldulensis leaves, C. sinensis leaves, F. benghalensis fruits extracts, and the biocontrol agents P. fluorescens and T. viride was analyzed using analysis of variance in a completely randomized design using SAS (Statistical Analysis System), and compared with the values for of the control. Data obtained from the field experiment were statistically analyzed using one-way ANOVA. The means among the treatments were compared using minimum significant difference measured by LSD test at alpha 0.05 [61,62].
3. Results
3.1. Isolation of the Fungal Pathogen
Four pure cultures of fungal isolates were obtained from infected zucchini plants cultivar as shown in Figure 3. Based on the morphological and molecular identification, Fusarium solani, F. oxysporum, Exserohilum rostratum, and Nigrospora lacticolonia were identified.
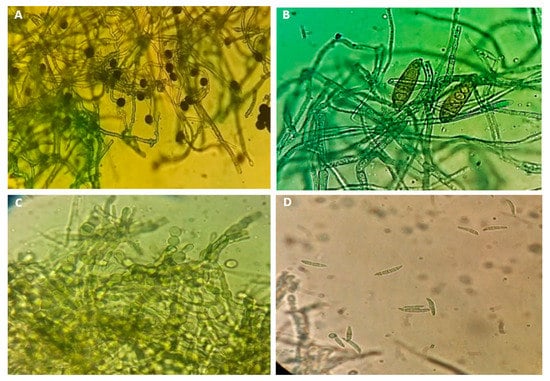
Figure 3.
Morphological characteristics of the conidia, N. lacticolonia (A), E. rostratum (B), chlamydospores and conidia of F. oxysporum ((C,D), respectively) and conidia of F. solani at 40× magnification.
3.2. Morphological Characteristics of Fungal Isolates
Based on morphological characteristics (Figure 3), all the isolates can be divided into three different morphotypes. The colony color of F. solani on PDA plates was white-creamy to white-greyish, the pigmentation was colorless and white-creamy with dark brown zonation; microscopic characteristics included conidiogenous cell was long and branched monophialides and the macroconidia septation was 3–7. In the case of F. oxysporum, the colony color and pigmentation were pale to dark peach; microscopic characteristics, macroconidia morphology was straight and relatively slender, the apical cell morphology was tapered and curved, the basal morphology was foot-shaped, the macroconidia septation was the three most common. The characteristics of E. rostratum colonies on PDA plates were deep-brown, circular with abundant aerial mycelia that appeared woolly or cottony. The conidiophores were slightly curved, erected, and septate. The basal cell in a conidiophore was swollen. N. lacticolonia in the color of the culture was initially orange, with dark brown patches in the reverse, N. lacticolonia in the smaller ellipsoidal conidia (Figure 3).
3.3. Molecular Identification Based on rDNA-ITS Sequences
The amplified DNA fragments were purified and both strands were sequenced through the ITS region. The sequence data alongside BLAST search proved the identity to be F. solani (accession no. MW947256), F. oxysporum (accession no. MW947254), E. rostratum (accession no. MW947255), and N. lacticolonia (accession no. MW947253).
3.4. Evaluation of Bioagents and Plant Extracts against Zucchini Fungal Pathogens In Vitro
The data presented in Table 2 show the highly significant effects of the tested biocontrol agents T. viride (Accession no. MW647090) and P. fluorescens (accession no. MW647093) against the four isolates of F. oxysporum, F. solani, E. rostratum, and N. lacticolonia.

Table 2.
Antifungal activity of P. fluorescens and T. viride bioagents and E. camaldulensis LE, C. sinensis LE, and F. benghalensis FE on F. oxysporum, F. solani, N. lacticolonia, and E. rostratum isolates under in vitro condition.
Moreover, the visual observation of the activity of plant extracts (Eucalyptus camaldulensis LE, Citrus sinensis LE, and Ficus benghalensis FE showed promising antifungal activity against the four isolates. Any increase in extract concentration led to an increase in the mycelial inhibition percentage of fungi. P. fluorescens, T. viride and E. camaldulensis LE (4000 mg/L) showed the highest activity against Fusarium oxysporum growth with inhibition percentages of fungal growth (IPFG) of 94.8%, 80.03% and 77.8%, respectively. P. fluorescens, T. viride, and C. sinensis LE (4000 mg/L) were observed as the highest IPFG against Fusarium solani with values of 92.2%, 81.10%, and 80%, respectively, followed by E. camaldulensis LE (4000 mg/L) with 78.53%. The highest IPFGs against E. rostratum were observed as P. fluorescens, F. benghalensis FE (4000 mg/L), and E. camaldulensis LE (4000 mg/L) with percentages of 96.7%, 81.46% and 78.53%, respectively. The highest inhibition percentage of fungal growth (IPFG) by P. fluorescens was 97.8% against N. lacticolonia, followed by antagonistic properties of T. viride (78.9%) and E. camaldulensis LE (4000 mg/L) with 74.83%.
T. viride inhibited the mycelial growth of all fungal isolates but could not overgrow the pathogen until 3 to 4 days. Furthermore, conidia production decreased compared to the control plates. T. viride hyphae coiled around hyphae of fungal isolates causing vacuolization and disintegration of isolates hyphae indicating strong antagonistic activity of the T. viride isolate, followed by the activity found against the fungal isolates when the E. camaldulensis LE, C. sinensis LE, and F. benghalensis FE were applied at a concentration of 4000 mg/L. Figure 4 shows the visual observations of the antifungal activity of P. fluorescens, T. viride and plant extract for example (C. sinensis LE) against F. solani, F. oxysporum, E. rostratum, and N. lacticolonia.
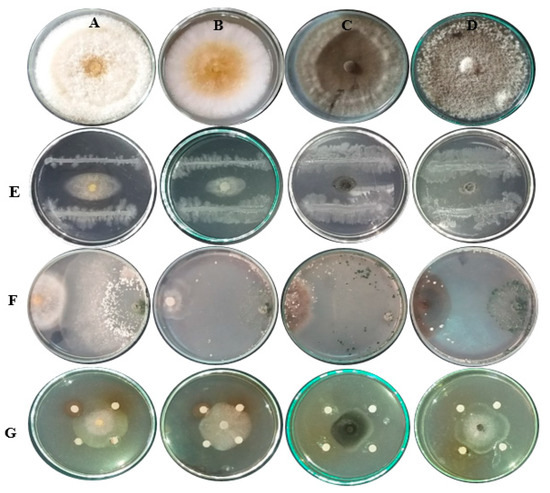
Figure 4.
Visual observations of the antifungal activity of P. fluorescens (E), T. viride (F) and C. sinensis LE (G), against F. solani (A), F. oxysporum (B), E. rostratum (C) and N. lacticolonia (D).
3.5. HPLC Analysis of Extracts
The phenolic compounds (PCs) and flavonoid compounds (FCs) identified in the studied extracts are presented in Table 3 and Figure 5. The most abundant PCs in E. camaldulensis LE were pyrogallol (13.63 µg/mL), caffeic acid (7.41 µg/mL), and p-coumaric acid (6.33 µg/mL), and the abundant FCs were kaempferol (15.03 µg/mL), naringin (14.16 µg/mL), 7-OH flavone (12.09 µg/mL), and quercetin (11.14 µg/mL). C. sinensis LE showed the presence of abundant PCs of syringic acid (8.42 µg/mL) and ferulic acid 7.56 µg/mL), while the abundant FCs were hesperidin (14.19 µg/mL), quercetin (9.52 µg/mL) and kaempferol (6.14 µg/mL). In F. benghalensis FE, the abundant PCs were gallic acid (10.42 µg/mL), p-coumaric acid (5.14 µg/mL), and syringic acid (5.22 µg/mL), while the abundant FCs were kaempferol (12.06 µg/mL), quercetin (8.14 µg/mL), and hesperidin (6.44 µg/mL).

Table 3.
Phenolic and flavonoid compounds identified in E. camaldulensis LE, C. sinensis LE, and F. benghalensis FE.
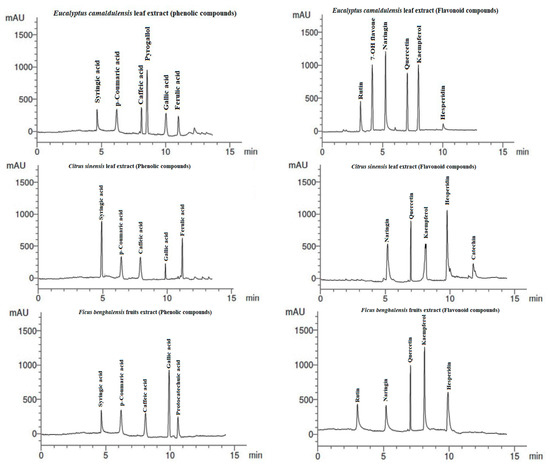
Figure 5.
HPLC of phenolic and flavonoid compounds identified in water extracts from E. camaldulensis leaves, C. sinensis leaves, and F. benghalensis fruits.
3.6. Characterization of Zucchini Vegetative Growth
The vegetative growth parameters, e.g., number of leaves per plant and leaf area (cm2), were significant, depending on the treatments with natural plant extract and bio-agent antagonists in the two experiments in (Figure 6a,b and Figure 7a,b). Regardless of the biological antagonists, there were significantly more leaves of the plants treated by inoculation T. viride, and E. camaldulensis LE (400 mg/L), followed by T. viride + P. fluorescens in the two experiments. However, T. viride and E. camaldulensis LE gave the highest leaf area (cm2), in the two growing experiments. Plants in the control treatment showed the smallest leaf size with the lowest number of leaves per plant compared with the plants treated with plant extracts and microbial agents.
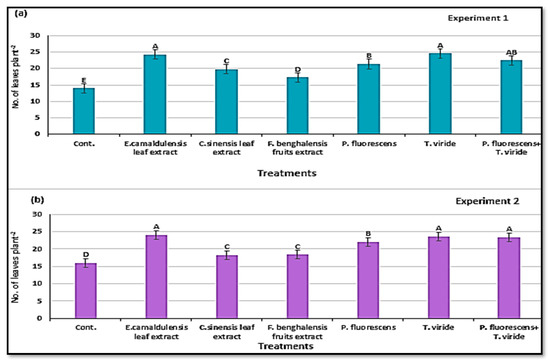
Figure 6.
Number of leaves per plant (means ± S.E) of zucchini as affected by the natural plant extracts and microbial antagonists. Letters in figure indicate the means ± S.E of treatments with the same letter/s were not significantly different according to LSD at 0.05 level of probability. (a) Experiment 1; (b) experiment 2.
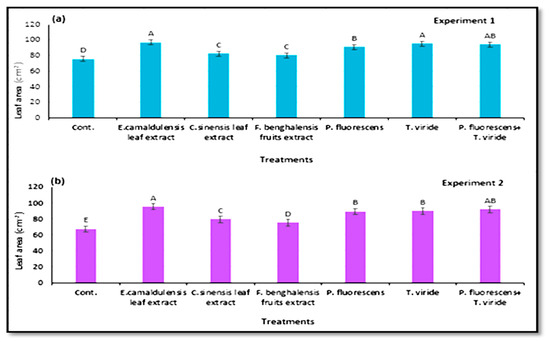
Figure 7.
Leaf area (means ± S.E) of zucchini as affected by the natural plant extracts and microbial antagonists. Letters in Figure indicate the means ± S.E of treatments with the same letter/s were not significantly different according to LSD at 0.05 level of probability. (a) Experiment 1; (b) experiment 2.
3.7. Total Yield
Regarding the influences of the microbial antagonist factors on the number of fruits per plant, this varied from 27.8 to 28.6 in the case of E. camaldulensis LE to 25.7–26.4 in the case of T. viride, P. fluorescens + T. viride (25.1–25.9/plant), and P. fluorescens (24.3–25.2/plant) treatments. Moreover, it showed statistically positive results in both growing experiments (Table 4). Similarly, yield per plant was 1.950–1.847 kg and 1.830–1.787 kg for the T. viride and E. camaldulensis LE treatments, respectively, in both experiments.

Table 4.
Number of fruit per plant and total yield per plant (kg) of zucchini as affected by natural plant extracts and microbial antagonists.
Data in Table 5 and Figure 8a,b show that the total yield per square meter and marketable yield were affected by the natural plant extracts and microbial antagonists studied. T. viride and E. camaldulensis LE showed the highest marketable yield (5.83 and 5.52 kg m−2) and (5.84 and 5.50 kg m−2) in the first and second experiment, respectively, without significant differences between them, followed by the treatment P. fluorescens + T. viride. The control treatment reduced the marketable yield between 41–39.9% and 41.1–41.3% with respect to T. viride and E. camaldulensis LE in the first and second experiment, respectively.

Table 5.
Marketable yield and non-marketable yield (kg.m−2) of zucchini as affected by natural plant extracts and microbial antagonists.
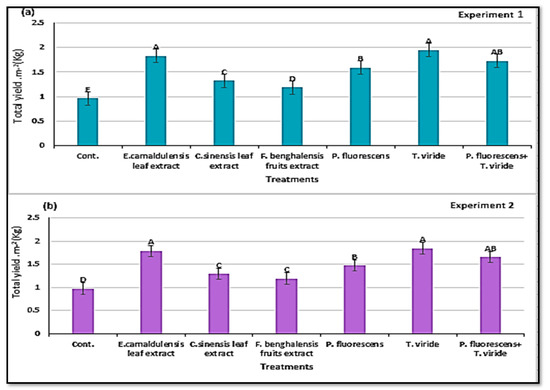
Figure 8.
Total yield per square meter (means ± S.E) of zucchini as affected by the natural plant extracts and microbial antagonists. Letters in figure indicate the means ± S.E of treatments with the same letter/s were not significantly different according to LSD at 0.05 level of probability. (a) Experiment 1; (b) experiment 2.
Non-marketable yield was also significantly affected by the natural plant extracts and microbial antagonists. Microbial and E. camaldulensis LE resulted in the lowest non-marketable yield of zucchini fruits. Non-marketable yield represented small percentages of 5.75%, 10.15%, 11.13% and 4.09% as affected by the treatments P. fluorescens +T. viride, T. viride, P. fluorescens, and E. camaldulensis LE, respectively, in the first experiment, while it was 5.61%, 10.23 %, 10.16% and 7.55%, respectively, in the second experiment with respect to total fruit yield (Table 5).
4. Discussion
The uses of plant extracts and microbial bioagents as eco-friendly treatments for the management of plant diseases have recorded high significance in recent years [43,63,64,65,66,67,68]. The literature supports the implementation of biostimulant and biocontrol tools due to nature, antimicrobial activity, easy biodegradability, non-phytotoxicity, and resistance in the host. In agroecological practices, there are clear demonstrations of their potential to reduce chemical inputs, save energy and provide farmers with new opportunities for sustainable fertilization and disease control due to their being readily available [43,69,70].
The present work was mainly conducted to study the effect of natural plant extracts and microbial antagonists to control some zucchini fungal pathogens for this purpose. The tested hypothesis assumed that natural plant extracts and microbial antagonists would result in an increase in the growth and yield parameters of zucchini plants. The results indicated that the growth parameters were positively and significantly influenced by a very promising magnitude. With regard to some measurements such as leaves number per plant and leaf area in both experiments of cultivation, they have improved positively by the application of plant extracts and microbial bioagents. The results also showed that all the microbial bioagent treatments resulted in a significant increase in the growth criteria of zucchini plants, and significant superiority was found in the integration treatment on all biological control agents for T. viride in increasing the number of leaves, leaf area (cm2), and marketable yield (kg).
The in vitro treatments using P. fluorescens showed high efficiency in reducing the pathogen growth, where the biocontrol effect was better due to its rapid growth and sporulation, resulting in higher competition success for space and available resources compared to other species [71,72,73]. However, few studies have conducted in vivo experiments on fungi disease control by P. fluorescens, T. viride, P. fluorescens with T. viride, and some plant extracts [74,75].
According to this study, biological control agents could be the best alternative and showed significant results against soil-borne pathogens on zucchini plants. Previously, agro-industrial waste mixed with biological control agents (Lactobacillus plantarum, L. casei, Rhodobacter sphaeroides, Rhodopseudomonas palustris, Saccharomyces sp., Streptococcus lactis, and Streptomyces sp.) was shown as a promising strategy against verticillium wilt of olive [76,77,78,79,80,81]. In addition, potential biocontrol agents against F. oxysporum f.sp. cubense (Foc) races STR4 and TR4 on banana plants was achieved [82].
However, Trichoderma produce phytohormones, vitamins, and solubilizing minerals in addition to their role in direct inhibition of pathogen growth, ultimately, zucchini plants can be easily grown up in field conditions [83,84]. Many strains of P. fluorescens are known to enhance plant growth promotion and reduce the severity of various diseases. The efficacy of the bacterial antagonists in controlling fungal diseases was often better alone, and sometimes in combination with fungicides [85]. The highest reduction in disease severity under greenhouse conditions was detected in tomato seedlings plants treated with formulated Bacillus amyloliquefaciens (74.4%), P. aeruginosa (66.7%), P. fluorescens (40%) and B. subtilis (53.3%) [68]. The antagonistic effect of Trichoderma towards F. oxysporum f.sp. vanillae, P. meadii, and Colletotrichum vanillae in vanilla, where the coculture of the phytopathogens and Trichodema clearly showed dominance of the Trichoderma species [86].
The biological activities of E. camaldulensis LE, C. sinensis LE, and F. benghalensis FE against the isolated fungi could be rested to the presence of PCs and FCs. According to the HPLC chromatograms the abundant PCs compound identified in E. camaldulensis LE was pyrogallol, caffeic acid and p-coumaric acid, which belongs to the class of cinnamate and is widely distributed in nature [87]. Flavonoids protect plants from different biotic and abiotic stresses and act as unique UV-filter, functioning as signal molecules, allelopathic compounds, phytoalexins, detoxifying agents, and antimicrobial defensive compounds. Moreover, FCs have roles against frost hardiness, drought resistance, and may play a functional role in plant heat acclimation, freezing tolerance, and photo protection [88,89,90,91]. Resistance mechanisms refer to traits that inhibit or limit attack, while tolerance strategies do not limit attack but reduce or offset consequences on the plant fitness by adjusting its physiology to buffer the effects of herbivory or diseases [92]. In earlier, several research workers have demonstrated that some plants extract possess antifungal activity against several plant diseases [33,34,38,93]. Similar to the present findings, E. camaldulensis LE has potential as an antifungal agent, where it is able to act as a moderate antifungal agent against household molds, and wood rot fungi [94], and phytopathogenic fungi [95]. Recently, leaf extracts of several aromatic plants were found to have a strong inhibitory effect against fungi in vitro and in vivo [96,97].
The application of extracts even in small amounts and after a single administration in certain stages of crop development proved that PCs alter the growth, nutrition, or resistance of organisms or organs, leading to an improvement in crop quality and quantity with an increase in production [35,98,99,100]. Previously, benzoic acid, quinol, salicylic acid, myricetin, and rutin were identified as abundant polyphenolic compounds found in E. camaldulensis bark extract with promising antifungal activity against the growth of F. culmorum, Rhizoctonia solani, and Botrytis cinerea [101]. n-Hexane oily extracts from E. camaldulensis aerial parts when applied showed promising antifungal activity against F. culmorum, R. solani, and P. chrysogenum [93]. In the present study, pyrogallol was found in high concentration in leaf extract but it was not found in bark extract of E. camaldulensis [101].
The polyphenolic composition of flavonoids and phenolic acids in the soluble fractions of the methanolic extracts of E. camaldulensis showed the presence of gallic, protocatechuic, vanillic, and ellagic acids, and protocatechic aldehyde was identified, along with eriodictyol, quercetin, naringenin, vanillin, naringin, quercitrin, luteolin, and kaempferol [102]. Extract from the leaves of E. camaldulensis showed four major components HHDP-glucopyranose, chlorogenic acid, and phloroglucinol derivatives [103].
E. camaldulensis LE showed good antifungal activity against Penicillium funiculosum, Penicillium ochrochloron, Aspergillus niger, A. flavus, Rhizoctonia solani, and F. oxisporum, and these activity could be related to ellagic acid, quercetin 3-O-rhamnoside, quercetin 3-O-b-D-glucuronide, caffeic acid, chlorogenic acid, ferulic acid, and p-coumaric acid [104]. Eucalyptus LE in water, methanol, and n-hexane proved more effective than stem and bark for controlling the growth of F. solani [105]. Early blight of tomato caused by Alternaria solani was inhibited by 49.31% when E. camaldulensis extract was applied [106]. Aqueous LEs from E. camaldulensis exhibited highly pronounced antifungal potential against Alternaria alternata, Drechslera hawaiiensis, and D. tetramera [107].
C. sinensis LE showed moderate activity against the isolated and identified fungi F. oxysporum, F. solani, N. lacticolonia and E. rostratum isolates compared to other bio-assayed extracts. C. sinensis LE showed an inhibition percentage of 26.12% against the growth of F. oxysporum at 10% concertation [108]. Leaves are rich in flavonoids, flavonols, polymerized phenols and hydrolyzable tannins content [109]. PCs present in Citrus indicates their role as an antimicrobial agent since they are extensively used in preventing the diseases caused by bacteria or fungi compared to bactericides or fungicides [109,110,111,112]. Moreover, other FCs such as hesperetin, naringenin, hesperidin, cyanidin 3-glucoside, limocitrin, quercetagetin, quercitrin, and kaempferol derivatives were isolated from different parts of C. sinensis [113,114,115].
The bioactivity of F. benghalensis FE could be related to the presence of PCs and FCs [116,117,118]; several PCs and FCs were also identified in different parts from F. benghalensis, such as gallic acid, rhein, anthraquinone, gallocatechin, theaflavin-3,3′-digallate, and flavone in leaves [119]. Chlorogenic acid, caffeic acid, naringenin, quercetin, kaempferol, malondialdehyde, and morin from root extract and cyanidin 3-glucoside, chlorogenic acid, caffeic acid and quercetin from FE were identified [120]. Fruit extracts had significant antibacterial activity but no antifungal activity [117].
Ellagic acid, gallic acid, rutin, myricetin, and naringenin were the major compounds identified in methanolic extract of Musa paradisiaca L. peels, with good antifungal activity against the growth of F. culmorum, and Rhizoctonia solani [121]. PCs and FCs from plant extracts are considered to be bacteriostatic and fungistatic [33], where they cause the swelling of hyphal tips, leaking of plasma, cell wall distortion, plasma seeping around hyphae abnormal branching or fusion of hyphae, and consequent wrinkling of the hypha surface [122].
Phenolic and flavonoid molecules are of interest in industry sectors such as in nutraceutical product formulation as therapeutic agents for diabetes and cancer [123,124,125], in food as additives and preservatives [124,126], in cosmetics as UV-protection and antioxidant agents [127,128], and in the textile industry and packaging as antimicrobial agents [129,130]. However, most prior studies were carried out at the in vitro scale, and there is a lack of information about their in vivo action resulting from their bioavailability and absorption [124,131].
p-Hydroxybenzoic, p-coumaric, and protocatechuic acids as commercial compounds with an encapsulation technique such as atomization/coagulation were used for protection [128], and the commercial compound catechin is used in encapsulation techniques as an inclusion complex to increase solubility and resistance to heat, light, and oxygen [132]. Phenolic extract from Punica granatum L. was used commercially for spray drying to improve storage stability (4 °C per 90 days) and for the protection of bioactivities [133]. Catechin from Grape juice was used in spray drying to improve thermal resistance [134]. In addition, quercetin was used as a commercial compound using the superparamagnetic iron oxide nanoparticles technique to increase bioavailability [135].
Finally, according to the in vitro results, future evaluation using naturally or artificially infected plants should be carried out using those bioagents and plant extracts, especially Eucalyptus camaldulensis leaf extract.
5. Conclusions
Plant extracts and microbial antagonists for the control of fungal pathogens such as Fusarium oxysporum, Fusarium solani, Exserohilum rostratum and Nigrospora lacticolonia are associated with beneficial effects in zucchini plants. Improved productivity of zucchini plants was obtained in terms of leaf area, fruit number per plant, yield per plant (kg), and total yield (marketable and non-marketable kilograms per square meter). The results for the three studied plant extracts and bioagents against zucchini fungal pathogens in vivo suggest that Trichoderma viride and Eucalyptus camaldulensis leaf extract have the superior effect against zucchini fungal pathogens, followed by Pseudomonas fluorescens with Trichoderma viride. In the future, we suggest evaluating plant extracts and bioagents on naturally infected plants; this could possibly reduce the amount of agricultural chemicals used in the control, which may leak into other ecosystems, and thus help to reduce their environmental burdens on agriculture.
Author Contributions
Conceptualization, H.S.H., A.A.M., M.Z.M.S. and D.Y.A.-E.; methodology, H.S.H., A.A.M., M.Z.M.S. and D.Y.A.-E.; software, H.S.H., A.A.M., M.N.F., M.Z.M.S., H.M.A., M.A. and D.Y.A.-E.; validation, H.S.H., A.A.M., M.N.F. and D.Y.A.-E.; formal analysis, H.S.H., A.A.M., M.Z.M.S. and D.Y.A.-E.; investigation, H.S.H., A.A.M., M.N.F., M.Z.M.S., H.M.A., M.A. and D.Y.A.-E.; resources, H.S.H., A.A.M., M.N.F., M.Z.M.S., H.M.A., M.A. and D.Y.A.-E.; data curation, H.S.H., A.A.M., M.Z.M.S. and D.Y.A.-E.; writing—original draft preparation, H.S.H., A.A.M., M.N.F., M.Z.M.S., H.M.A., M.A. and D.Y.A.-E.; writing—review and editing, H.S.H., A.A.M., M.N.F., M.Z.M.S., H.M.A., M.A. and D.Y.A.-E.; visualization, H.S.H., A.A.M., M.N.F., M.Z.M.S., H.M.A., M.A. and D.Y.A.-E.; supervision, M.N.F. and M.Z.M.S.; project administration, D.Y.A.-E.; funding acquisition, H.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project (RSP-2021/123) King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahmoud, A. Occurrence of Fusarium wilt on summer squash caused by Fusarium oxysporum in Assiut, Egypt. J. Phytopathol. Pest Manag. 2016, 3, 34–45. [Google Scholar]
- Kopczyńska, K.; Kazimierczak, R.; Średnicka-Tober, D.; Barański, M.; Wyszyński, Z.; Kucińska, K.; Perzanowska, A.; Szacki, P.; Rembiałkowska, E.; Hallmann, E. The profile of selected antioxidants in two courgette varieties from organic and conventional production. Antioxidants 2020, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Piasecka-Jóźwiak, K.; Rozmierska, J.; Chabłowska, B.; Stecka, K.; Skąpska, S.; Kliszcz, M.; Szkudzińska-Rzeszowiak, E. Starter cultures for lactic acid fermentation of sweet pepper, pattypan squash and tomatoes. Pol. J. Food Nutr. Sci. 2013, 63, 95–102. [Google Scholar] [CrossRef][Green Version]
- Muntean, E.; Lazăr, V.; Muntean, N. HPLC-PDA analysis of carotenoids and chlorophylls from Cucurbita pepo L. convar Giromontiina fruits. Bul. USAMV CN 2006, 62, 94–99. [Google Scholar]
- Kręcisz, M.; Stępień, B.; Pasławska, M.; Popłoński, J.; Dulak, K. Physicochemical and quality properties of dried courgette slices: Impact of vacuum impregnation and drying methods. Molecules 2021, 26, 4597. [Google Scholar] [CrossRef]
- Verdone, M.; Rao, R.; Coppola, M.; Corrado, G. Identification of zucchini varieties in commercial food products by DNA typing. Food Control 2018, 84, 197–204. [Google Scholar] [CrossRef]
- Lust, T.A.; Paris, H.S. Italian horticultural and culinary records of summer squash (Cucurbita pepo, Cucurbitaceae) and emergence of the zucchini in 19th-century Milan. Ann. Bot. 2016, 118, 53–69. [Google Scholar] [CrossRef]
- Contreras, J.I.; Baeza, R.; Alonso, F.; Cánovas, G.; Gavilán, P.; Lozano, D. Effect of distribution uniformity and fertigation volume on the bio-productivity of the greenhouse zucchini crop. Water 2020, 12, 2183. [Google Scholar] [CrossRef]
- Sumner, D.; Dowler, C.; Johnson, A.; Glaze, N.; Phatak, S.; Chalfont, R.; Epperson, J. Rot diseases of cucumber in irrigated multiple-cropping system with pest management. Plant Dis. 1983, 67, 1071–1075. [Google Scholar] [CrossRef]
- Sumner, D.R.; Phatak, S.C.; Gay, J.D.; Chalfant, R.B.; Brunson, K.E.; Bugg, R.L. Soilborne pathogens in a vegetable double-crop with conservation tillage following winter cover crops. Crop. Prot. 1995, 14, 495–500. [Google Scholar] [CrossRef]
- Kimati, H.; Gimenes-Fernandes, N.; Soave, J.; Kurozawa, C.; Brignani Neto, F.; Bettiol, W. Guia de Fungicidas Agrícolas: Recomendações por Cultura; Grupo Paulista de Fitopatologia: Jaboticabal, Brazil, 1997; p. 225. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W. Fusarium Species: An Illustrated Manual for Identification; The Pennsylvania State University Press: University Park, PA, USA, 1983; p. 193. [Google Scholar]
- Hao, Y.; Aluthmuhandiram, J.V.S.; Chethana, K.W.T.; Manawasinghe, I.S.; Li, X.; Liu, M.; Hyde, K.D.; Phillips, A.; Zhang, W. Nigrospora species associated with various hosts from Shandong Peninsula, China. Mycobiology 2020, 48, 169–183. [Google Scholar] [CrossRef]
- Khaekhum, S.; Ekprasert, J.; Suebrasri, T.; Mongkolthanaruk, W.; Riddech, N.; Jogloy, S.; Boonlue, S. The first member of Exserohilum rostratum beneficial for promoting growth and yield of sunchoke (Helianthus tuberosus L.). Rhizosphere 2021, 19, 100379. [Google Scholar] [CrossRef]
- Farag, M.F. A first record of Exserohilum rostratum as a new pathogen causing bean blight in Egypt. J. Plant. Pathol. Microbiol. 2020, 11, 496. [Google Scholar] [CrossRef]
- El Hassni, M.; El Hadrami, A.; Daayf, F.; Chérif, M.; Barka, E.A.; El Hadrami, I. Biological control of bayoud disease in date palm: Selection of microorganisms inhibiting the causal agent and inducing defense reactions. Environ. Exp. Bot. 2007, 59, 224–234. [Google Scholar] [CrossRef]
- Abass, M.H.; Hameed, M.A.; Ahmed, A.N. First report of Nigrospora sphaerica (Sacc.) Mason as a potential pathogen on date palm (Phoenix dactylifera L.). Can. J. Plant Pathol. 2013, 35, 75–80. [Google Scholar] [CrossRef]
- Abass, M.H.; Mohammed, N.H. Morphological, molecular and pathological study on Nigrospora oryzae and Nigrospora sphaerica, the leaf spot fungi of date palm. Basra J. Date Palm Res. 2014, 13, 26–38. [Google Scholar]
- Jamiołkowska, A. Fungi isolated from underground part of hot pepper (Capsicum annuum) plants cultivated in the field. Phytopathol. Pol。 2009, 51, 37–44. [Google Scholar]
- Wagner, A. Fungal communities colonising stems of hot pepper (Capsicum annuum). Phytopathol. Pol 2004, 33, 23–29. [Google Scholar]
- Baker, R.; Paulitz, T. Theoretical basis for microbial interactions leading to biological control of soilborne plant pathogens. In Principles and Practice of Managing Soilborne Plant Pathogens; Hall, R., Ed.; American Phytopathological Society: St. Paul, MN, USA, 1996; pp. 50–79. [Google Scholar]
- Haas, D.; Keel, C. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 2003, 41, 117–153. [Google Scholar] [CrossRef]
- Cabanãs, C.G.-L.; Schilirã, E.; Valverde-Corredor, A.; Mercado-Blanco, J. The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces systemic defense responses in aerial tissues upon colonization of olive roots. Front. Microbiol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Gomaa, E.Z. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica 2012, 50, 263–272. [Google Scholar] [CrossRef]
- Sivan, A.; Chet, I. Integrated control of media on growth and interactions between a range of soil borne glasshouse pathogens and antagonistic fungi. Phytopathology 1993, 10, 127–142. [Google Scholar]
- Whipps, J.M.; Lumsden, R.D. Commercial use of fungi as plant disease biological control agents: Status and prospects. Fungal Biocontrol Agents Prog. Probl. Potential 2001, 9–22. [Google Scholar] [CrossRef]
- McLean, K.; Dodd, S.; Sleight, B.; Hill, R.; Stewart, A. Comparison of the behaviour of a transformed hygromycin resistant strain of Trichoderma atroviride (M1057hygR) with the wildtype strain (M1057). N. Z. Plant Prot. 2004, 57, 72–76. [Google Scholar] [CrossRef]
- Simon, A.; Sivasithamparam, K. Interactions among Gaeumannomyces graminis var. tritici, Trichoderma koningii, and soil bacteria. Can. J. Microbiol. 1988, 34, 871–876. [Google Scholar] [CrossRef]
- Schirmböck, M.; Lorito, M.; Wang, Y.L.; Hayes, C.K.; Arisan-Atac, I.; Scala, F.; Harman, E.G.; Kubicek, C.P. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 1994, 60, 4364–4370. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma: III. Hyphal interaction. Trans. Br. Mycol. Soc. 1971, 57, 363–IN2. [Google Scholar] [CrossRef]
- Claydon, N.; Hanson, J.R.; Truneh, A.; Avent, A.G. Harzianolide, a butenolide metabolite from cultures of Trichoderma harzianum. Phytochemistry 1991, 30, 3802–3803. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; El-Hefny, M.; Ali, H.M.; Abdel-Megeed, A.; El-Settawy, A.A.; Böhm, M.; Mansour, M.M.; Salem, A.Z.M. Plants-derived bioactives: Novel utilization as antimicrobial, antioxidant and phytoreducing agents for the biosynthesis of metallic nanoparticles. Microb. Pathog. 2021, 158, 105107. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mohamed, A.; Ali, H.; Al Farraj, D. Characterization of phytoconstituents from alcoholic extracts of four woody species and their potential uses for management of six Fusarium oxysporum isolates identified from some plant hosts. Plants 2021, 10, 1325. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Hefny, M.; El-Shanhorey, N.A.; Ali, H.M. Foliar application of bio-stimulants enhancing the production and the toxicity of Origanum majorana essential oils against four rice seed-borne fungi. Molecules 2020, 25, 2363. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.A.; Abdel-Megeed, A.; Nasser, R.A.; Salem, M.Z.M. comparative evaluation of some woody tree methanolic extracts and paraloid B-72 against Phytopathogenic mold fungi Alternaria tenuissima and Fusarium culmorum. Bioresources 2015, 10, 2570–2584. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Alotaibi, S.; Abo Elgat, W.A.; Taha, A.; Fares, Y.; El-Shehawi, A.; Ghareeb, R. Antifungal activities of wood and non-wood kraft handsheets treated with Melia azedarach extract using SEM and HPLC analyses. Polymers 2021, 13, 2012. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elkader, D.; Salem, M.Z.M.; Komeil, D.; Al-Huqail, A.; Ali, H.; Salah, A.; Akrami, M.; Hassan, H. Post-harvest enhancing and Botrytis cinerea control of strawberry fruits using low cost and eco-friendly natural oils. Agronomy 2021, 11, 1246. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Olivares-Pérez, J.; Salem, A. Studies on biological activities and phytochemicals composition of Hibiscus species-A review. Life Sci. J. 2014, 11, 1–8. [Google Scholar]
- Ashmawy, N.; Salem, M.Z.M.; El-Hefny, M.; El-Kareem, M.S.A.; El-Shanhorey, N.A.; Mohamed, A.A.; Salem, A.Z.M. Antibacterial activity of the bioactive compounds identified in three woody plants against some pathogenic bacteria. Microb. Pathog. 2018, 121, 331–340. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Di Foggia, M.; Quintana, A.M.; Roberti, R. Assessing the potential of the Terrestrial Cyanobacterium Anabaena minutissima for controlling Botrytis cinerea on tomato fruits. Horticulturae 2021, 7, 210. [Google Scholar] [CrossRef]
- Stankovic, M.; Stefanovic, O.; Čomić, L.; Topuzović, M.; Radojević, I.; Solujić, S. Antimicrobial activity, total phenolic content and flavonoid concentrations of Teucrium species. Open Life Sci. 2012, 7, 664–671. [Google Scholar] [CrossRef]
- Mohamed, A.; Salah, M.; El-Dein, M.; El-Hefny, M.; Ali, H.; Farraj, D.; Hatamleh, A.; Salem, M.Z.M.; Ashmawy, N. Ecofriendly bioagents, Parthenocissus quinquefolia, and Plectranthus neochilus extracts to control the early blight pathogen (Alternaria solani) in tomato. Agronomy 2021, 11, 911. [Google Scholar] [CrossRef]
- Pitt, J.; Hocking, A. Fungi and Food Spoilage; Blackie Academic & Professional: New South Wales, Australia, 1997. [Google Scholar]
- Naik, M.; Prasad, Y.; Bhat, K.; Rani, G.D. Morphological, physiological, pathogenic and molecular variability among isolates of Alternaria solani from tomato. Indian Phytopathol. 2010, 63, 168–173. [Google Scholar]
- Matsushima, T. Icones Microfungorum a Matsushima Lectorum; Taylor & Francis Group: Oxfordshire, UK, 1976; p. 955. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi; Academic Press: London, UK, 1980. [Google Scholar]
- Hafizi, R.; Salleh, B.; Latiffah, Z. Morphological and molecular characterization of Fusarium solani and F. oxysporum associated with crown disease of oil palm. Braz. J. Microbiol. 2013, 44, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.P.; Roy, S. Morphological and molecular characterization of Fusarium sp. causing wilt disease of isabgol (Plantago ovata Forsk.) and its management strategies. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100244. [Google Scholar] [CrossRef]
- Choi, I.-Y.; Kim, J.-H.; Lee, W.-H.; Park, J.-H.; Shin, H.-D. First report on Fusarium wilt of zucchini caused by Fusarium oxysporum, in Korea. Mycobiology 2015, 43, 174–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Edel, V.; Steinberg, C.; Gautheron, N.; Recorbet, G.; Alabouvette, C. Genetic diversity of Fusarium oxysporum populations isolated from different soils in France. FEMS Microbiol. Ecol. 2001, 36, 61–71. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Gomaa, F.H. Molecular characterization and biological control of some rice seed-borne fungal pathogens. J. Phytopathol. Pest Manag. 2019, 6, 40–53. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press Inc.: New York, NY, USA, 1990; p. 315. [Google Scholar]
- Mohamed, A.A.; Behiry, S.I.; Ali, H.M.; El-Hefny, M.; Salem, M.Z.; Ashmawy, N.A. Phytochemical compounds of branches from P. halepensis oily liquid extract and S. terebinthifolius essential oil and their potential antifungal activity. Processes 2020, 8, 330. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Salem, M.Z.M.; Al-Huqail, A.A.; Ali, H.M. Application of glycine, folic acid, and moringa extract as bio-stimulants for enhancing the production of ‘Flame Seedless’ grape cultivar. Bioresources 2021, 16, 3391–3410. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Rahman, M.A.; Begum, M.F.; Alam, M.F. Screening of Trichoderma isolates as a biological control agent against Ceratocystis paradoxa causing pineapple disease of sugarcane. Mycobiology 2009, 37, 277–285. [Google Scholar] [CrossRef]
- Elmohsen, Y.A.; Salman, S.R.; Helmy, Y.I.; El-Shinawy, M.Z.; Abou-Hadid, A.F. The effect of grafting on squash plants grown under low plastic tunnel in winter season. Egypt J. Hortic. 2021, 48, 181–192. [Google Scholar] [CrossRef]
- Fargo, W.S.; Bonjour, E.L.; Wagner, T.L. An estimation equation for squash leaf area using leaf measurements. Can. J. Plant. Sci. 1986, 66, 677–682. [Google Scholar] [CrossRef]
- SAS. User Guide: Statistics (Release 8.02); SAS Institute: Cary, NC, USA, 2001. [Google Scholar]
- Steel, R.; Torrie, J. Principles and Procedures of Statistics, 2nd ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Ashmawy, N.A.; Salem, M.Z.M.; El Shanhorey, N.; Al-Huqail, A.A.; Ali, H.M.; Behiry, S.I. Eco-friendly wood-biofungicidal and antibacterial activities of various Coccoloba uvifera L. leaf extracts: HPLC analysis of phenolic and flavonoid compounds. Bioresources 2020, 15, 4165–4187. [Google Scholar] [CrossRef]
- Savitha, A.; Naik, M.; Ajithkumar, K. Eco-friendly management of Alternaria Sesami incitant blight of sesame. J. Plant Dis. Sci. 2011, 6, 150–152. [Google Scholar]
- Hari, C.; Surender, S. Control of chickpea wilt (Fusarium oxysporum f sp. ciceri) using bioagents and plant extracts. Indian J. Agric. Sci. 2005, 75, 115–116. [Google Scholar]
- Joshi, S.; Gupta, K. Eco-friendly management of leaf spot of chickpea caused by Alternaria spp. Ann. Plant Prot. Sci. 2009, 17, 504–505. [Google Scholar]
- Tapwal, A.; Garg, S.; Gautam, N.; Kumar, R. In vitro antifungal potency of plant extracts against five phytopathogens. Braz. Arch. Biol. Technol. 2011, 54, 1093–1098. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Bagy, H.M.M.K.; Hashem, M.; Alamri, S.A.M.; Mostafa, Y.S. Biological control of the tomato wilt caused by Clavibacter michiganensis subsp. michiganensis using formulated plant growth-promoting bacteria. Egypt J. Biol. Pest. Control 2019, 29, 54. [Google Scholar] [CrossRef]
- Jardin, P.D.; Verheggen, F.; Fassotte, B. Implementing plant biostimulants and biocontrol strategies in the agroecological management of cultivated ecosystems. Biotechnol. Agron. Soc. Environ. 2016, 20, 299–313. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Santos, M.; Moreno-Gavíra, A.; Marín-Rodulfo, T.; Gea, F.; Diánez, F. Biological control of fungal diseases by Trichoderma aggressivum f. europaeum and its compatibility with fungicides. J. Fungi 2021, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Timms-Wilson, T.M.; Beringer, J.E.; Rhodes, D.; Renwick, A.; Stevenson, L.; Bailey, M.J. Ecological basis for biocontrol of damping-off disease by Pseudomonas fluorescens 54/96. J. Appl. Microbiol. 1999, 87, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhong, T.; Chen, K.; Du, M.; Chen, G.; Chen, X.; Wang, K.; Zalán, Z.; Takács, K.; Kan, J. Antifungal activity of volatile organic compounds produced by Pseudomonas fluorescens ZX and potential biocontrol of blue mold decay on postharvest citrus. Food Control 2021, 120, 107499. [Google Scholar] [CrossRef]
- Ganeshan, G.; Kumar, A.M. Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J. Plant Interact. 2005, 1, 123–134. [Google Scholar] [CrossRef]
- Blakeman, J.P.; Fokkema, N.J. Potential for biological control of plant diseases on the Phylloplane. Annu. Rev. Phytopathol. 1982, 20, 167–190. [Google Scholar] [CrossRef]
- Oloo, J. Evaluation of Local Trichoderma Isolates for Their Efficiency in Biological Control of Fusarium Oxysporum F. Sp Phaseoli in Common Bean. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2013. [Google Scholar]
- Varo-Suárez, A.; Raya-Ortega, M.; Agustí-Brisach, C.; García-Ortiz-Civantos, C.; Fernández-Hernández, A.; Mulero-Aparicio, A.; Trapero, A. Evaluation of organic amendments from agro-industry waste for the control of verticillium wilt of olive. Plant Pathol. 2018, 67, 860–870. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.; Trapero, A. Enhanced production of microsclerotia in recalcitrant Verticillium dahliae isolates and its use for inoculation of olive plants. J. Appl. Microbiol. 2016, 121, 473–484. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.; Trapero, A. Selection and evaluation of micro-organisms for biocontrol of Verticillium dahliaein olive. J. Appl. Microbiol. 2016, 121, 767–777. [Google Scholar] [CrossRef]
- Montes-Osuna, N.; Mercado-Blanco, J. Verticillium wilt of olive and its control: What did we learn during the last decade? Plants 2020, 9, 735. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Cardoni, M.; Cabanás, C.G.-L.; Valverde-Corredor, A.; Villadas, P.J.; Fernández-López, M.; Mercado-Blanco, J. Linking belowground microbial network changes to different tolerance level towards Verticillium wilt of olive. Microbiome 2020, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Pérez, M.D.L.O.; Jiménez-Ruiz, J.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Barroso, J.B.; Luque, F.; Mercado-Blanco, J. Tolerance of olive (Olea europaea) cv Frantoio to Verticillium dahliae relies on both basal and pathogen-induced differential transcriptomic responses. New Phytol. 2017, 217, 671–686. [Google Scholar] [CrossRef]
- Cabanás, C.G.-L.; Fernández-González, A.; Cardoni, M.; Valverde-Corredor, A.; López-Cepero, J.; Fernández-López, M.; Mercado-Blanco, J. The banana root Endophytome: Differences between mother plants and suckers and evaluation of selected bacteria to control Fusarium oxysporum f.sp. cubense. J. Fungi 2021, 7, 194. [Google Scholar] [CrossRef]
- Uddin, A.; Hussain, M.; Rahman, S.; Ahmad, H.; Roni, M. Effect of Trichoderma concentration on the growth and yield of tomato. Bangladesh Res. Publ. J. 2015, 11, 228–232. [Google Scholar]
- Pavithra, G.; Sandeep, B.; Yadav, S.; Choudhary, S. Role of Trichoderma viride and GA 3 in the growth of summer squash. Plant Arch. 2019, 19, 1173–1176. [Google Scholar]
- Sharma, M.; Saini, I.; Kaushik, P.; Aldawsari, M.M.; Al Balawi, T.; Alam, P. Mycorrhizal fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in eggplant. Saudi J. Biol. Sci. 2021, 28, 3685–3691. [Google Scholar] [CrossRef]
- Radjacommare, R.; Venkatesan, S.; Samiyappan, R. Biological control of phytopathogenic fungi of vanilla through lytic action of Trichoderma species and Pseudomonas fluorescens. Arch. Phytopathol. Plant Prot. 2010, 43, 1–17. [Google Scholar] [CrossRef]
- Paz, J.E.W.; Contreras, C.R.; Munguía, A.R.; Aguilar, C.N.; Inungaray, M.L.C. Phenolic content and antibacterial activity of extracts of Hamelia patens obtained by different extraction methods. Braz. J. Microbiol. 2018, 49, 656–661. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Zeng, X.; Xi, Y.; Jiang, W. Protective roles of flavonoids and flavonoid-rich plant extracts against urolithiasis: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 2125–2135. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Salem, M.Z.M.; Behiry, S.; El-Hefny, M. Inhibition of Fusarium culmorum, Penicillium chrysogenum and Rhizoctonia solani by n-hexane extracts of three plant species as a wood-treated oil fungicide. J. Appl. Microbiol. 2019, 126, 1683–1699. [Google Scholar] [CrossRef]
- Siramon, P.; Ohtani, Y.; Ichiura, H. Chemical composition and antifungal property of Eucalyptus camaldulensis leaf oils from Thailand. Rec. Nat. Prod. 2013, 7, 49–53. [Google Scholar]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int. J. Microbiol. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Harish, S.; Saravanakumar, D.; Radjacommare, R.; Ebenezar, E.G.; Seetharaman, K. Use of plant extracts and biocontrol agents for the management of brown spot disease in rice. BioControl 2007, 53, 555–567. [Google Scholar] [CrossRef]
- Sabo, V.A.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crop. Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Bujor, O.-C.; Popa, V.I. Chapter 3—Phenolic natural compounds and their influence on physiological processes in plants. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–58. [Google Scholar]
- Hassan, S.; El-Bebany, A.; Salem, M.Z.M.; Komeil, D. Productivity and post-harvest fungal resistance of hot pepper as affected by potassium silicate, clove extract foliar spray and nitrogen application. Plants 2021, 10, 662. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Sas-Paszt, L.; Górnik, K.; Ali, H.M.; Salem, M.Z.M. Vegetative Growth, Yield, and Fruit Quality of Guava (Psidium guajava L.) cv. Maamoura as Affected by Some Biostimulants. Bioresources 2021, 16, 7379–7399. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Salem, M.Z.M.; Kordy, A.M.; Salem, A.Z.M.; Behiry, S.I. Antiviral, antifungal, and insecticidal activities of Eucalyptus bark extract: HPLC analysis of polyphenolic compounds. Microb. Pathog. 2020, 147, 104383. [Google Scholar] [CrossRef]
- Cadahia, E.; Conde, E.; García-Vallejo, M.C.; De Simón, B.F. Tannin composition of Eucalyptus camaldulensis, E. globulus and E. rudis. Part I. Wood. Holzforschung 1997, 51, 119–124. [Google Scholar] [CrossRef]
- Singab, A.-N.; Ayoub, N.; Al-Sayed, E.; Martiskainen, O.; Sinkkonen, J.; Pihlaja, K. Phenolic constituents of Eucalyptus camaldulensis Dehnh, with potential antioxidant and cytotoxic activities. Rec. Nat. Prod. 2011, 5, 271–280. [Google Scholar]
- Elansary, H.O.; Salem, M.Z.M.; Ashmawy, N.; Yessoufou, K.; El-Settawy, A.A. In vitro antibacterial, antifungal and antioxidant activities of Eucalyptus spp. leaf extracts related to phenolic composition. Nat. Prod. Res. 2017, 31, 2927–2930. [Google Scholar] [CrossRef]
- Bashir, U.; Tahira, J.J. Evaluation of Eucalyptus camaldulensis against Fusarium solani. Int. J. Agric. Biol. 2012, 14, 675–677. [Google Scholar]
- Raza, W.; Ghazanfar, M.U.; Iftikhar, Y.; Ahmed, K.S.; Haider, N.; Rasheed, M.H. Management of early blight of tomato through the use of plant extracts. Management 2016, 1, 1123–1133. [Google Scholar]
- Bajwa, R. Antifungal activity of allelopathic plant extracts VI: In vitro control of fungal pathogens by aqueous leaf extracts of Eucalyptus. Mycopath 2005, 1, 7–12. [Google Scholar]
- Okwu, D.E.; Awurum, A.N.; Okoronkwo, J.I. Phytochemical composition and in vitro antifungal activity screening of extracts from citrus plants against Fusarium oxysporum of okra plant (Hibiscus esculentus). Pest. Technol. 2007, 1, 145–148. [Google Scholar]
- Laghabenamrouche, S.; Madani, K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crop. Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- Sawalha, S.M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of main phenolic compounds in sweet and bitter orange peel using CE–MS/MS. Food Chem. 2009, 116, 567–574. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K. Phenols in citrus peel byproducts. Concentrations of Hydroxycinnamates and Polymethoxylated flavones in citrus peel molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Favela-Hernández, J.M.J.; González-Santiago, O.; Ramírez-Cabrera, M.A.; Esquivel-Ferriño, P.C.; Camacho-Corona, M.D.R. Chemistry and pharmacology of Citrus sinensis. Molecules 2016, 21, 247. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Kalidhar, S.; Rani, G.; Yadav, L. Chemical examination of Citrus sinensis flavedo variety pineapple. Indian J. Pharm. Sci. 2009, 71, 677–679. [Google Scholar] [CrossRef]
- Saleem, M.; Farooq, A.; Ahmad, S.; Shafiq, N.; Riaz, N.; Jabbar, A.; Arshad, M.; Malik, A. Chemical constituents of Citrus sinensis var. Shukri from Pakistan. J. Asian Nat. Prod. Res. 2010, 12, 702–706. [Google Scholar] [CrossRef]
- Gopukumar, S.; Alexander, P.; Jainamboo, M.; Praseetha, P. Phytochemical screening and FT-IR analysis of Ficus benghalensis fruits. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1529–1534. [Google Scholar]
- Mousa, O.; Vuorela, P.; Kiviranta, J.; Wahab, S.; Hiltunen, R. Bioactivity of certain Egyptian ficus species. J. Ethnopharmacol. 1994, 41, 71–76. [Google Scholar] [CrossRef]
- Kannabiran, K.; Gayathri, M. Antimicrobial activity of Hemidesmus indicus, Ficus bengalensis and Pterocarpus marsupium roxb. Indian J. Pharm. Sci. 2009, 71, 578–581. [Google Scholar] [CrossRef]
- Rao, K.B.; Ojha, V.; Preeti; Kumar, G.; Karthik, L. Phytochemical composition and antioxidant activity of Ficus benghalensis (Moraceae) leaf extract. J. Biol. Act. Prod. Nat. 2014, 4, 236–248. [Google Scholar] [CrossRef]
- Afzal, T.; Ali, Q.; Malik, A. Phenolic compounds proliferation by HPLC: To find out antibacterial activities in Ficus benghalensis plant extract. Int. J. Bot. Stud. 2020, 5, 140–144. [Google Scholar]
- Behiry, S.I.; Okla, M.K.; Alamri, S.; El-Hefny, M.; Salem, M.Z.M.; Alaraidh, I.A.; Ali, H.M.; Al-Ghtani, S.M.; Monroy, J.C.; Salem, A.Z.M. Antifungal and antibacterial activities of wood treated with Musa paradisiaca L. peel extract: HPLC analysis of Phenolic and Flavonoid contents. Processes 2019, 7, 215. [Google Scholar] [CrossRef]
- Huang, J.; Chung, W. Management of vegetable crop diseases with plant extracts. Adv. Plant Dis. Manag. 2003, 37, 153–163. [Google Scholar]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.; Barreiro, M.F. Phenolic acids, cinnamic acid, and ergosterol as cosmeceutical ingredients: Stabilization by microencapsulation to ensure sustained bioactivity. Microchem. J. 2019, 147, 469–477. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, B.; Cheng, D.; Li, J.; Huang, F.; Lu, Y. Dyeing characteristics and function ability of tussah silk fabric with oak bark extract. Text. Res. J. 2017, 87, 1806–1817. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.-R.; Zhang, Y.-H.; Wang, X.-P.; Wang, J.-Y.; Ning, Z.-X.; Ruan, Q.-J.; Zhang, Y.-S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Rodríguez, G.V.; Chen, C.-Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Inclusion complexation of catechin by β-cyclodextrins: Characterization and storage stability. LWT 2017, 86, 555–565. [Google Scholar] [CrossRef]
- Çam, M.; Içyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Moser, P.; Telis, V.R.N.; Neves, N.D.A.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.G.; Da Rocha, G.O.; De Andrade, J.B. Occurrence of the potent mutagens 2-nitrobenzanthrone and 3-nitrobenzanthrone in fine airborne particles. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).