Exogenous Application of Proline and L-Cysteine Alleviates Internal Browning and Maintains Eating Quality of Cold Stored Flat ‘Maleki’ Peach Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peach Fruit and Treatment
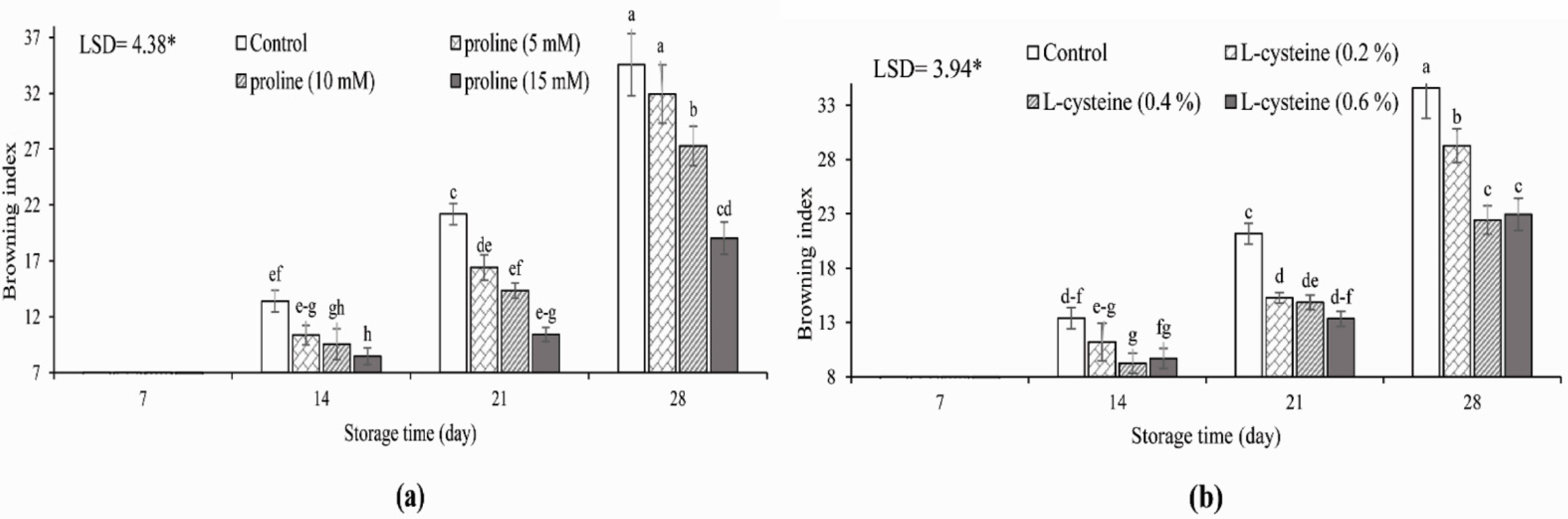
2.2. Browning Index
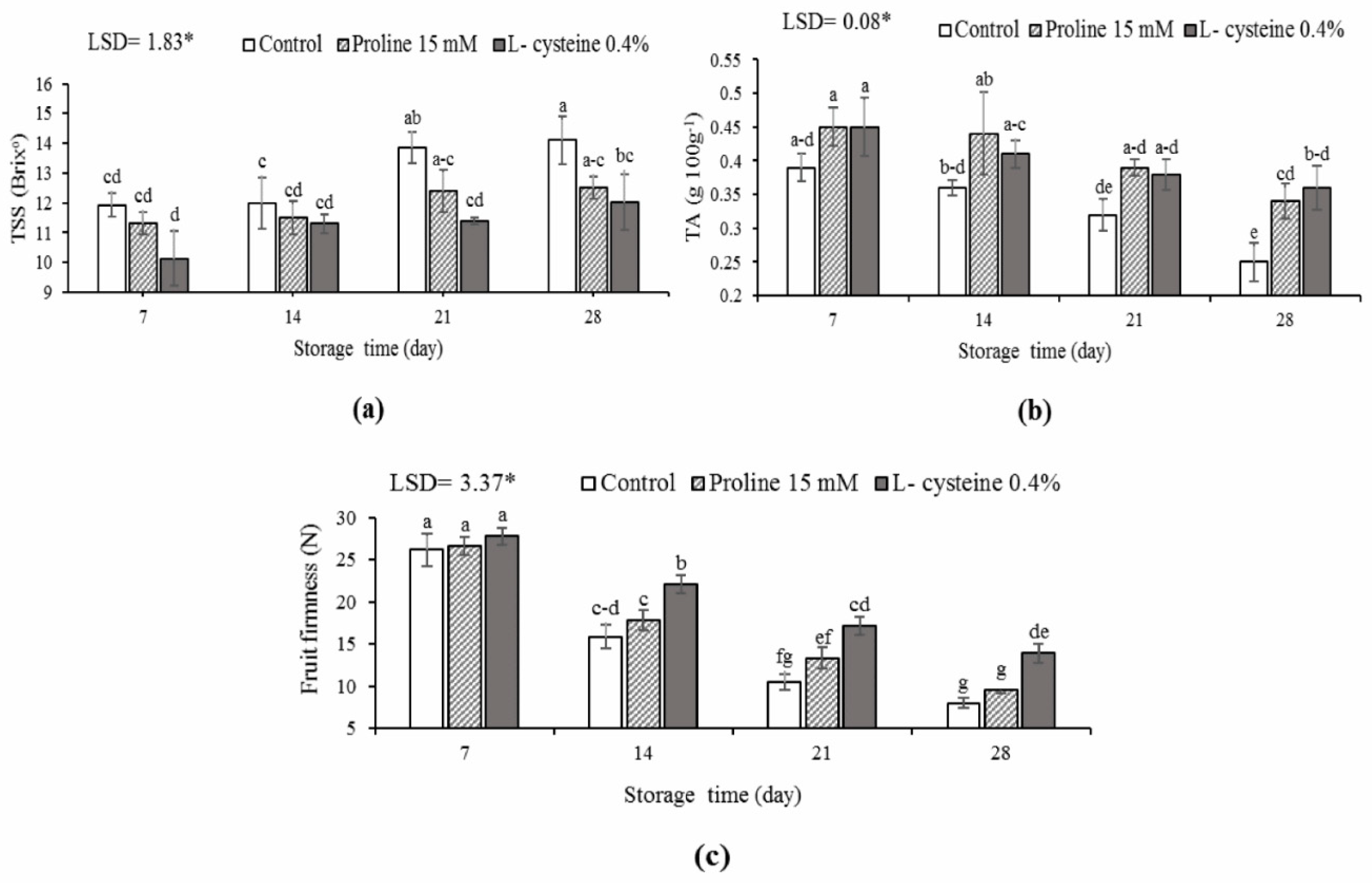
2.3. Firmness, TSS and TA
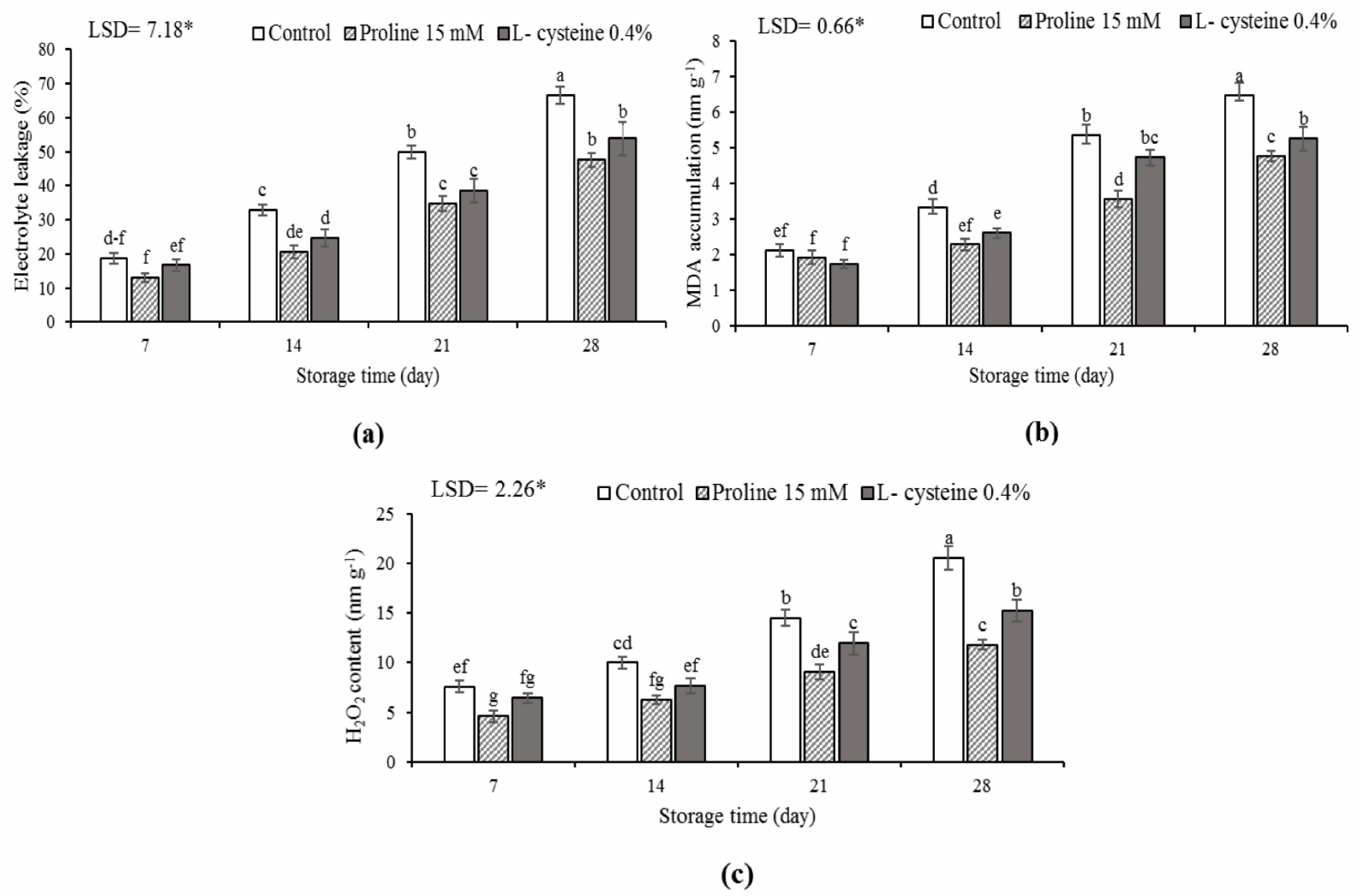
2.4. Electrolyte Leakage, MDA and H2O2 Accumulation
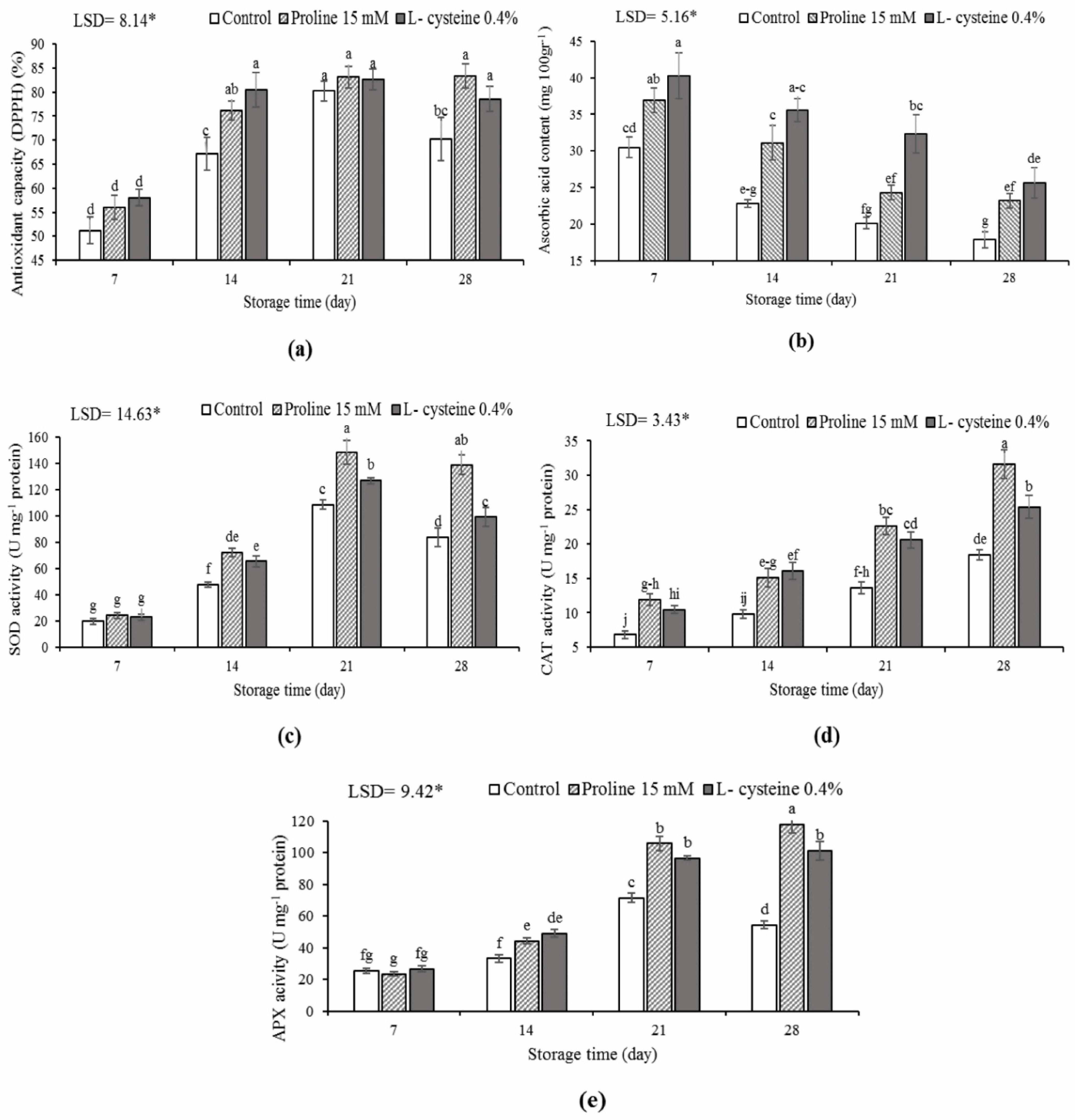
2.5. DPPH Scavenging Activity, Ascorbic Acid Content, and the Activity of Antioxidant Enzymes
2.6. Total Phenol, Flavonoids and Proline Content
2.7. PAL and PPO Activity
2.8. Statistical Analysis
3. Results
3.1. Browning Index
3.2. TSS, TA Content and Fruit Firmness
3.3. DPPH Scavenging Activity, Ascorbic Acid Content, and the Activity of Antioxidant Enzymes
3.4. Electrolyte Leakage, MDA and H2O2 Accumulation
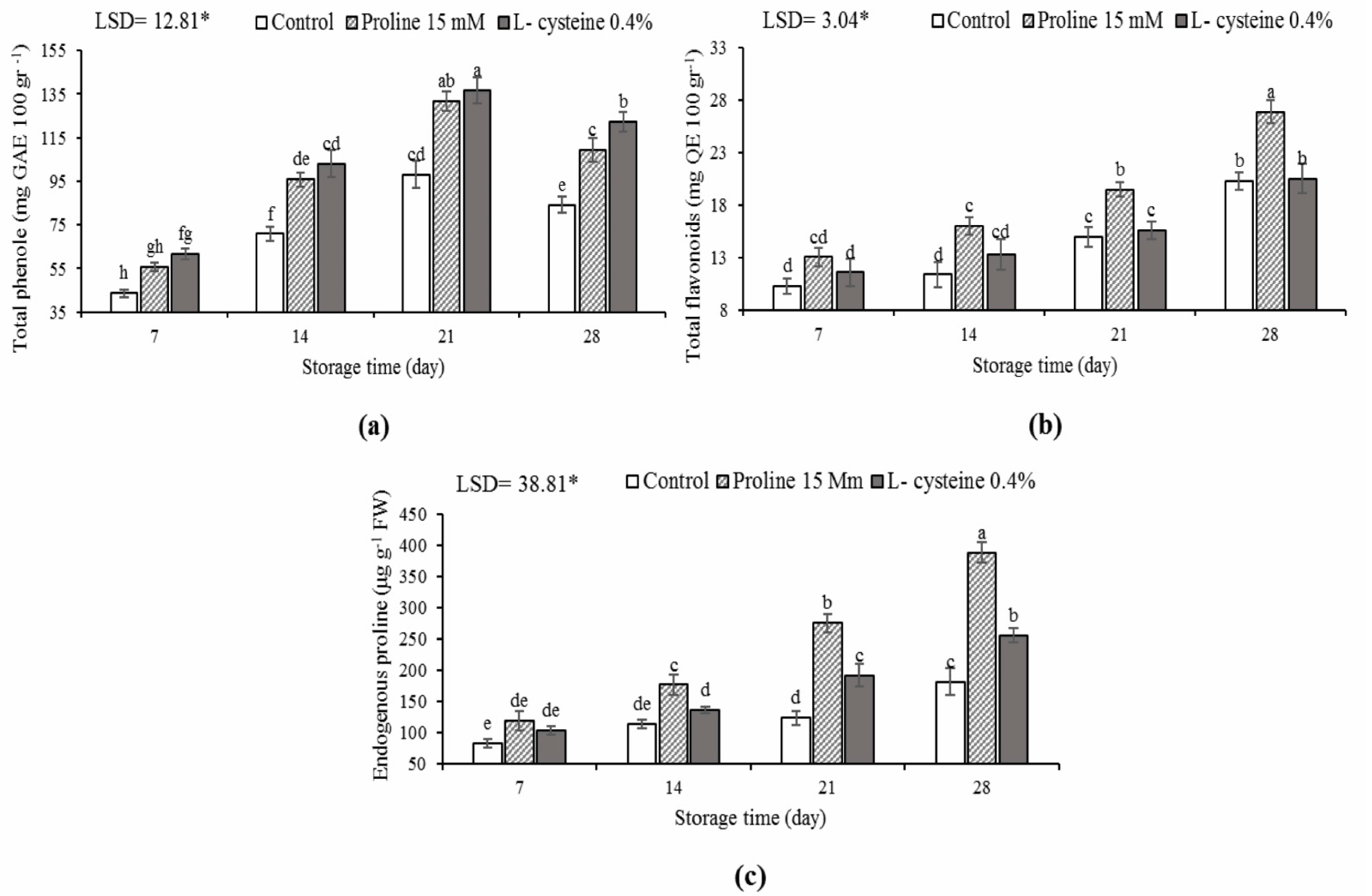
3.5. Total Phenol, Flavonoids and Endogenous Proline Content
3.6. PAL and PPO Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Gómez, A.; Navarro-Martínez, A.; Martínez-Hernández, G.B. Active Paper Sheets Including Nanoencapsulated Essential Oils: A Green Packaging Technique to Control Ethylene Production and Maintain Quality in Fresh Horticultural Products—A Case Study on Flat Peaches. Foods 2020, 9, 1904. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid treatment alleviates chilling injury in peach fruit by promoting sugar and ethylene metabolism. Food Chem. 2021, 338, 128005. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Oria, R.; Arias, E. Inhibitory effect of microwaved thinned nectarine extracts on polyphenol oxidase activity. Food Chem. 2016, 197, 603–610. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Cao, J.; Li, Y. Effect of chilling temperatures on physiological properties, phenolic metabolism and antioxidant level accompanying pulp browning of peach during cold storage. Sci. Hortic. 2019, 255, 175–182. [Google Scholar] [CrossRef]
- Shaoying, Z.; Lishun, Z.; Xuyuan, D. Combined treatment of carbon monoxide and chitosan reduced peach fruit browning and softening during cold storage. Int. J. Food Sci. Nutr. 2015, 4, 477. [Google Scholar] [CrossRef]
- Huan, C.; Han, S.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Yu, Z. Postharvest hot air and hot water treatments affect the antioxidant system in peach fruit during refrigerated storage. Postharvest Biol. Technol. 2017, 126, 1–14. [Google Scholar] [CrossRef]
- Yang, C.; Chen, T.; Shen, B.; Sun, S.; Song, H.; Chen, D.; Xi, W. Citric acid treatment reduces decay and maintains the postharvest quality of peach (Prunus persica L.) fruit. Food Sci. Nutr. 2019, 7, 3635–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, B.; Capotorto, I.; Ventura, M.; Cefola, M. Evaluation of L-cysteine as anti-browning agent in fresh-cut lettuce processing. J. Food Process. Preserv. 2015, 39, 985–993. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U. Postharvest L-cysteine application delayed pericarp browning, suppressed lipid peroxidation and maintained antioxidative activities of litchi fruit. Postharvest Biol. Technol. 2016, 121, 135–142. [Google Scholar] [CrossRef]
- Li, T.; Wu, Q.; Zhou, Y.; Yun, Z.; Duan, X.; Jiang, Y. L-Cysteine hydrochloride delays senescence of harvested longan fruit in relation to modification of redox status. Postharvest Biol. Technol. 2018, 143, 35–42. [Google Scholar] [CrossRef]
- Richard-Forget, F.C.; Goupy, P.M.; Nicolas, J.J. Cysteine as an inhibitor of enzymic browning. 2. Kinetic studies. J. Agric. Food Chem. 1992, 40, 2108–2113. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Nawaz, A.; Shahid, M. Postharvest application of antibrowning chemicals modulates oxidative stress and delays pericarp browning of controlled atmosphere stored litchi fruit. J. Food Biochem. 2019, 43, 12746. [Google Scholar] [CrossRef]
- Gorny, J.R.; Hess-Pierce, B.; Cifuentes, R.A.; Kader, A.A. Quality changes in fresh-cut pear slices as affected by controlled atmospheres and chemical preservatives. Postharvest Biol. Technol. 2002, 24, 271–278. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Sobrino-López, A.; Soledad Tapia, M.; Martín-Belloso, O. Browning inhibition in fresh-cut ‘Fuji’apple slices by natural antibrowning agents. J. Food Sci. 2006, 71, S59–S65. [Google Scholar] [CrossRef]
- Ghidelli, C.; Mateos, M.; Rojas-Argudo, C.; Pérez-Gago, M.B. Extending the shelf life of fresh-cut eggplant with a soy protein–cysteine based edible coating and modified atmosphere packaging. Postharvest Biol. Technol. 2014, 95, 81–87. [Google Scholar] [CrossRef]
- Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Beneficial impact of exogenous arginine, cysteine and methionine on postharvest senescence of broccoli. Food Chem. 2021, 338, 128055. [Google Scholar] [CrossRef] [PubMed]
- Zouari, M.; Hassena, A.B.; Trabelsi, L.; Rouina, B.B.; Decou, R.; Labrousse, P. Exogenous proline-mediated abiotic stress tolerance in plants: Possible mechanisms. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–121. [Google Scholar] [CrossRef]
- Trovato, M.; Forlani, G.; Signorelli, S.; Funck, D. Proline metabolism and its functions in development and stress tolerance. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 41–72. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kumar, V.; Burritt, D.J.; Fujita, M.; Mäkelä, P.S. Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants. In Proline Metabolism and Its Functions in Development and Stress Tolerance; Springer Nature: Cham, Switzerland, 2019; pp. 41–72. [Google Scholar] [CrossRef]
- Kumar, N.; Pal, M.; Singh, A.; SaiRam, R.K.; Srivastava, G.C. Exogenous proline alleviates oxidative stress and increase vase life in rose (Rosa hybrida L.‘Grand Gala’). Sci. Hortic. 2010, 127, 79–85. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed. Res. Int. 2014, 2014, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, N.; Gupta, K.; Bhandhari, K.; Kumar, S.; Thakur, P.; Nayyar, H. Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol. Mol. Biol. Plants 2011, 17, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Mohammadrezakhani, S.; Hajilou, J.; Rezanejad, F.; Zaare-Nahandi, F. Assessment of exogenous application of proline on antioxidant compounds in three Citrus species under low temperature stress. J. Plant Interact. 2019, 14, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Fathi, H.; Dejampour, J.; Jahani, U.; Zarrinbal, M. Tree and fruit characterization of peach genotypes grown under Ardabil and East Azarbaijan environmental conditions in Iran. Crop. Breed. J. 2013, 3, 31–43. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Xu, Y.; Limwachiranon, J.; Li, D.; Ban, Z.; Luo, Z. Effect of exogenous nitro oxide on chilling tolerance, polyamine, proline, and γ-aminobutyric acid in bamboo shoots (Phyllostachys praecox f. prevernalis). J. Agric. Food Chem. 2017, 65, 5607–5613. [Google Scholar] [CrossRef] [PubMed]
- Naser, F.; Rabiei, V.; Razavi, F.; Khademi, O. Effect of calcium lactate in combination with hot water treatment on the nutritional quality of persimmon fruit during cold storage. Sci. Hortic. 2018, 233, 114–123. [Google Scholar] [CrossRef]
- Chen, J.Y.; He, L.H.; Jiang, Y.M.; Wang, Y.; Joyce, D.C.; Ji, Z.L.; Lu, W.J. Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol. Plant 2008, 132, 318–328. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1992, 207, 604–611. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Dehghan, G.; Khoshkam, Z. Tin (II)–quercetin complex: Synthesis, spectral characterisation and antioxidant activity. Food Chem. 2012, 131, 422–426. [Google Scholar] [CrossRef]
- Terada, M.; Watanabe, Y.; Kunitomo, M.; Hayashi, E. Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenylhydrazine method. Anal. Biochem. 1978, 84, 604–608. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Rao, J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Sánchez, E.; López-Lefebre, L.R.; García, P.C.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Proline metabolism in response to highest nitrogen dosages in green bean plants (Phaseolus vulgaris L. cv. Strike). J. Plant Physiol. 2001, 158, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.B.T.; Ketsa, S.; van Doorn, W.G. Relationship between browning and the activities of polyphenol oxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol. Technol. 2003, 30, 187–193. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Sci. Hortic. 2013, 156, 73–85. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, S.; Meng, X.; Li, B. Physiological and biochemical responses in peach fruit to oxalic acid treatment during storage at room temperature. Food Chem. 2007, 104, 156–162. [Google Scholar] [CrossRef]
- Sodchit, C.; Kongbangkerd, T.; Phun, W.N. Prevention of enzymatic browning of postharvest longan fruit by N-acetyl-L-cysteine and 4-hexylresorcinol. Songklanakarin. J. Sci. Technol. 2008, 30, 31–35. [Google Scholar]
- Cabezas-Serrano, A.B.; Amodio, M.L.; Colelli, G. Effect of solution pH of cysteine-based pre-treatments to prevent browning of fresh-cut artichokes. Postharvest Biol. Technol. 2013, 75, 17–23. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, R.; Li, W.; Song, J.; Cheng, F. Improved postharvest preservation effects of Pholiota nameko mushroom by sodium alginate–based edible composite coating. Food Bioproc. Technol. 2019, 12, 587–598. [Google Scholar] [CrossRef]
- Banin Sogvar, O.; Razavi, F.; Rabiei, V.; Gohari, G. Postharvest application of L-cysteine to prevent enzymatic browning of “Stanley” plum fruit during cold storage. J. Food Process. Preserv. 2020, 44, 14788. [Google Scholar] [CrossRef]
- Suekawa, M.; Fujikawa, Y.; Esaka, M. Exogenous proline has favorable effects on growth and browning suppression in rice but not in tobacco. Plant Physiol. Biochem. 2019, 142, 1–7. [Google Scholar] [CrossRef]
- Öztürk, L.; Demir, Y. In vivo and in vitro protective role of proline. Plant Growth Regul. 2002, 38, 259–264. [Google Scholar] [CrossRef]
- Khan, A.S.; Ullah, W.; Malik, A.U.; Ahmad, R.; Saleem, B.A.; Rajwana, I.A. Exogenous applications of boron and zinc influence leaf nutrient status, tree growth and fruit quality of Feutrell’s early (Citrus reticulata Blanco). Pak. J. Agri. Sci. 2012, 49, 113–119. [Google Scholar]
- Barman, K.; Asrey, R.; Pal, R.K.; Kaur, C.; Jha, S.K. Influence of putrescine and carnauba wax on functional and sensory quality of pomegranate (Punica granatum L.) fruits during storage. J. Food Sci. Technol. 2014, 51, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Rao, T.R. Effect of honey and L-cysteine as antioxidants on the quality attributes of fresh-cut carambola (Averrhoa carambola L.) stored at two different temperatures. Int. J. Postharvest Technol. Innov. 2013, 24, 362–381. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Wang, Y.; Hu, S.; Zheng, Y.; Jin, P. Genome-wide identification of heat shock transcription factors and potential role in regulation of antioxidant response under hot water and glycine betaine treatments in cold stored peaches. J. Sci. Food Agric. 2021, 42, 11392. [Google Scholar] [CrossRef]
- Yıldız, H.; Baysal, T. Effects of alternative current heating treatment on Aspergillus niger, pectin methylesterase and pectin content in tomato. J. Food Eng. 2006, 75, 327–332. [Google Scholar] [CrossRef]
- Plotto, A.; Narciso, J.A.; Rattanapanone, N.; Baldwin, E.A. Surface treatments and coatings to maintain fresh-cut mango quality in storage. J. Sci. Food Agric. 2010, 90, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Alandes, L.; Quiles, A.; Pérez-Munuera, I.; Hernando, I. Improving the quality of fresh-cut apples, pears, and melons using natural additives. J. Food Sci. 2009, 74, S90–S96. [Google Scholar] [CrossRef] [PubMed]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance; Humana Press: Totowa, NJ, USA, 2010; pp. 291–297. [Google Scholar] [CrossRef]
- Catalá, A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int. J. Biochem. Cell Biol. 2006, 38, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Molaei, S.; Rabiei, V.; Soleimani, A.; Razavi, F. Exogenous application of glycine betaine increases the chilling tolerance of pomegranate fruits cv. Malase Saveh during cold storage. J. Food Process. Preserv. 2021, 45, 15315. [Google Scholar] [CrossRef]
- Nasibi, F.; Farahmand, H.; Kamyab, A.; Alipour, S. Effects of arginine, cysteine and 5-sulfosalicylic acid on of vase life of tuberose cut flowers. Agric. Commun. 2014, 2, 35–41. [Google Scholar]
- Li, Z.G.; Jin, J.Z. Hydrogen sulfide partly mediates abscisic acid-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells. Plant Cell Tissue Organ. Cult. (PCTOC) 2016, 125, 207–214. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Aghdam, M.S.; Dokhanieh, A.Y.; Hassanpour, H.; Fard, J.R. Enhancement of antioxidant capacity of cornelian cherry (Cornus mas) fruit by postharvest calcium treatment. Sci. Hortic. 2013, 161, 160–164. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S.; Fard, J.R.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hortic. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Naderi, R.; Sarcheshmeh, M.A.A.; Babalar, M. Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol. Technol. 2015, 110, 70–76. [Google Scholar] [CrossRef]
- Molaei, S.; Soleimani, A.; Rabiei, V.; Razavi, F. Impact of chitosan in combination with potassium sorbate treatment on chilling injury and quality attributes of pomegranate fruit during cold storage. J. Food Biochem. 2021, 45, 13633. [Google Scholar] [CrossRef] [PubMed]
- Bico, S.L.S.; de Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Chemical dips and edible coatings to retard softening and browning of fresh-cut banana. Int. J. Postharvest Technol. Innov. 2010, 2, 13–24. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Botany 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Heuer, B. Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci. 2003, 165, 693–699. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gohari, G.; Molaei, S.; Kheiry, A.; Ghafouri, M.; Razavi, F.; Lorenzo, J.M.; Juárez-Maldonado, A. Exogenous Application of Proline and L-Cysteine Alleviates Internal Browning and Maintains Eating Quality of Cold Stored Flat ‘Maleki’ Peach Fruits. Horticulturae 2021, 7, 469. https://doi.org/10.3390/horticulturae7110469
Gohari G, Molaei S, Kheiry A, Ghafouri M, Razavi F, Lorenzo JM, Juárez-Maldonado A. Exogenous Application of Proline and L-Cysteine Alleviates Internal Browning and Maintains Eating Quality of Cold Stored Flat ‘Maleki’ Peach Fruits. Horticulturae. 2021; 7(11):469. https://doi.org/10.3390/horticulturae7110469
Chicago/Turabian StyleGohari, Gholamreza, Sanaz Molaei, Azizollah Kheiry, Mahshid Ghafouri, Farhang Razavi, Jose M. Lorenzo, and Antonio Juárez-Maldonado. 2021. "Exogenous Application of Proline and L-Cysteine Alleviates Internal Browning and Maintains Eating Quality of Cold Stored Flat ‘Maleki’ Peach Fruits" Horticulturae 7, no. 11: 469. https://doi.org/10.3390/horticulturae7110469
APA StyleGohari, G., Molaei, S., Kheiry, A., Ghafouri, M., Razavi, F., Lorenzo, J. M., & Juárez-Maldonado, A. (2021). Exogenous Application of Proline and L-Cysteine Alleviates Internal Browning and Maintains Eating Quality of Cold Stored Flat ‘Maleki’ Peach Fruits. Horticulturae, 7(11), 469. https://doi.org/10.3390/horticulturae7110469








