Chemical Variability in the Composition of Zhumeria majdae (Rech. F. & Wendelbo) Essential Oil According to Storage Time and Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Essential Oil Isolation Procedure
2.2. Essential Oil Storage Conditions
2.3. Essential Oil Analysis
2.4. Correlation, Principal Component, Cluster and Statistical Analyses
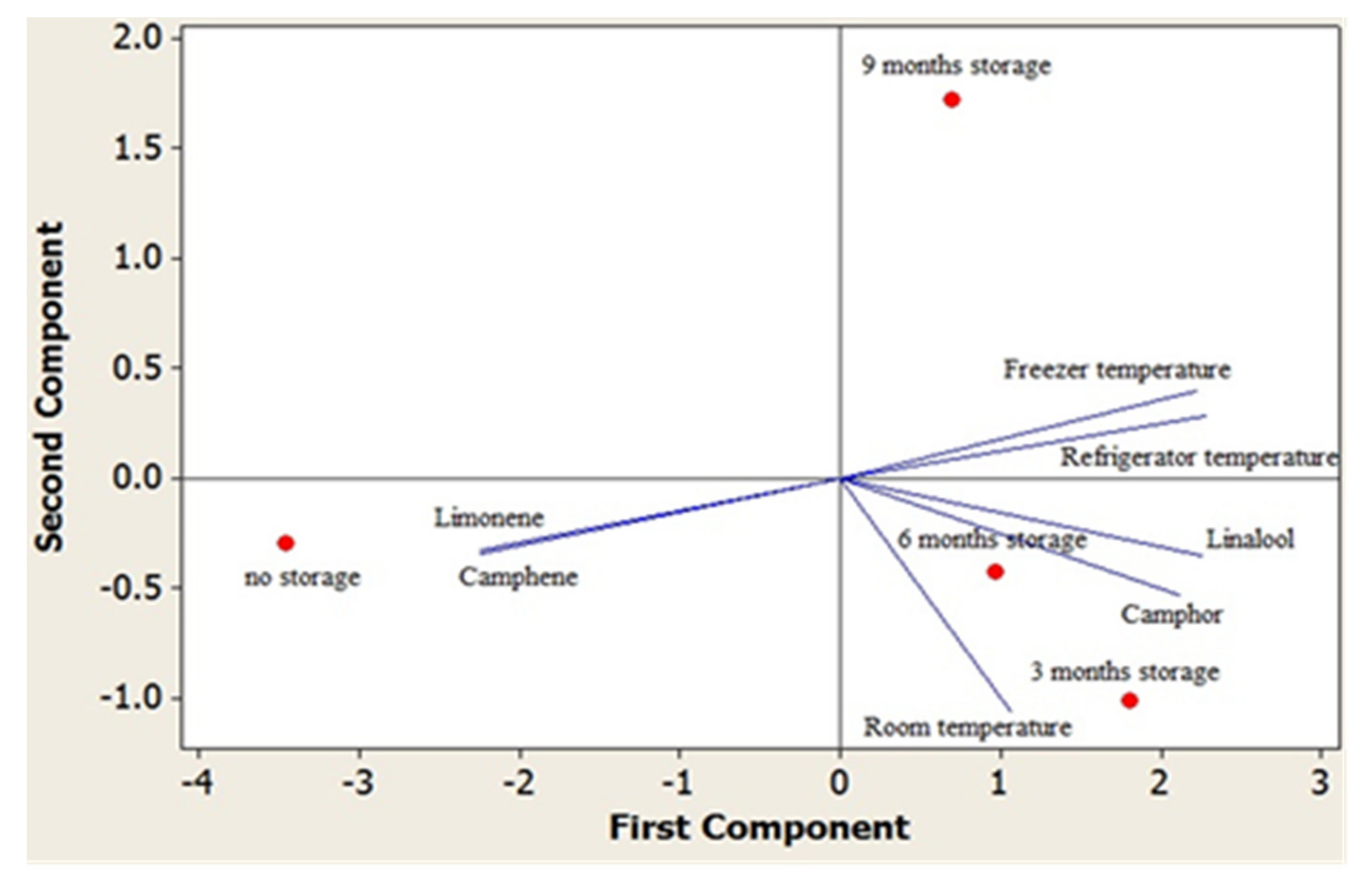
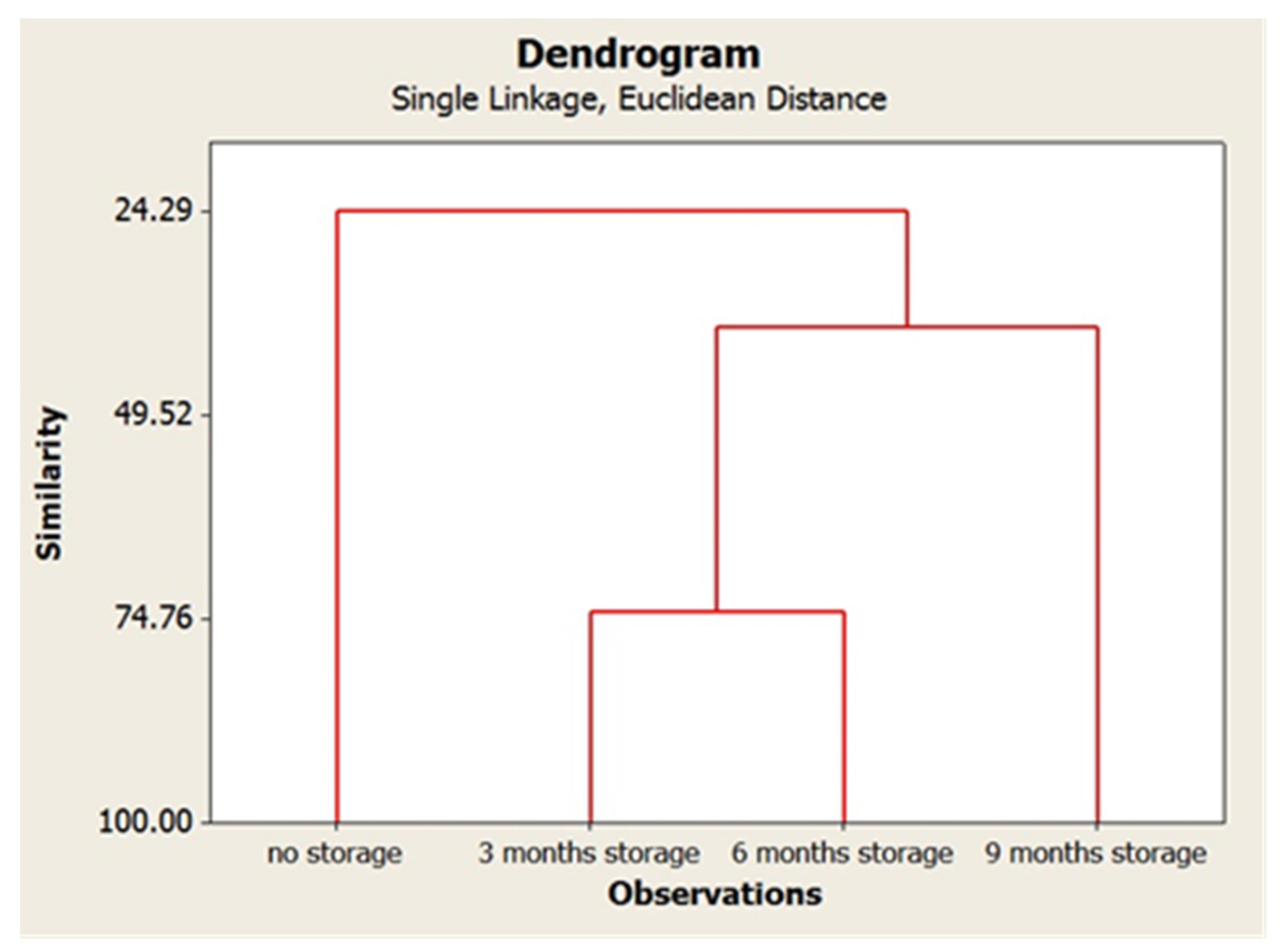
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Omidbaigi, R. Production and Processing of Medicinal Plants 1; Behnashr Press: Khorasan Razavi, Iran, 2005; p. 347. [Google Scholar]
- Hosseinzadeh, H.; Ramezani, M.; Fadishei, M.; Mahmoudi, M. Antinociceptive, anti-inflammatory and acute toxicity effects of Zhumeria majdae extracts in mice and rats. Phytomedicine 2002, 9, 135–141. [Google Scholar] [CrossRef]
- Sharififar, F.; Mozaffarian, V.; Moshafi, M.H.; Dehghan–Nudeh, G.; Parandeh–Rezvani, J.; Mahdavi, Z. Chemical composition and biological activities of Zhumeria majdae Resh. F. & wendelbo. Jundishapur J. Nat. Pharm. Prod. 2008, 3, 8–18. [Google Scholar]
- Zargari, A. Medicinal Plants; Tehran University Publications: Tehran, Iran, 1995. [Google Scholar]
- Izaddoost, M.; Rustaiyan, A.; Niknejad, A. Phytochemical Study of Zhumeria majdae; Biochemical Society Transactions: London, UK, 1983; pp. 1023–1027. [Google Scholar]
- Moein, S.; Moein, M.R. Relationship between antioxidant properties and phenolics in Zhumeria Majdae. J. Med. Plants Res. 2010, 4, 517–521. [Google Scholar]
- Naghibi, F.; Mosadegh, M.; Mohammadi, M.S.; Ghorbani, A.B. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2010, 4, 63–79. [Google Scholar]
- Ehtemami, Z.; Shafaroodi, H.; Asgarpanah, J. Effect of essential oil of Zhumeria majdae on morphine tolerance and dependence in mice. Chin. J. Integr. Med. 2020, 26, 683–687. [Google Scholar] [CrossRef]
- Arman, M.; Yousefzadi, M.; Ebrahimi, S.N. Antimicrobial activity and composition of the essential oil from Zhumeria majdae Rech. f. & Wendelbo. J. Essent. Oil Bear. Plants 2009, 12, 630–634. [Google Scholar]
- Fallah, M.; Farzaneh, M.; Yousefzadi, M.; Ghorbanpour, M.; Mirjalili, M.H. In vitro mass propagation and conservation of a rare medicinal plant, Zhumeria majdae Rech. f & Wendelbo (Lamiaceae). Biocatal. Agric. Biotechnol. 2019, 17, 318–325. [Google Scholar]
- Mirzakhani, M.; Ekrami, M.; Moini, S. Chemical composition, total phenolic content and antimicrobial activities of Zhumeria majdae. J. Food Bioprocess Eng. 2018, 2, 1–8. [Google Scholar]
- Sanei–Dehkordi, A.; Soleimani–Ahmadi, M.; Akbarzadeh, K.; Salim Abadi, Y.; Paksa, A.; Gorouhi, M.A.; Mohammadi-Azni, S. Chemical composition and mosquito larvicidal properties of essential oil from leaves of an Iranian indigenous plant Zhumeria Majdae. J. Essent. Oil Bear. Plants 2016, 19, 1454–1461. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Khosravi, R.; Sendi, J.; Mahboubi, M.; Kosari, A.A. Chemical composition of essential oil from Zhumeria majdae Rech. F. & Wendelbo and its bioactivities against Tribolium castaneum Herbst (Tenebrionidae) larvae. J. Essent. Oil Bear. Plants 2014, 17, 824–831. [Google Scholar]
- Omidpanah, N.; Valifard, M.; Esmaeili, M.; Yousefi, R.; Moghadam, A. Antioxidant and antibacterial properties of the essential oils of two Iranian Medicinal Plants: Zhumeria majdae and Salvia mirzayanii. J. Adv. Med. Sci. Appl. Technol. 2015, 1, 51–60. [Google Scholar] [CrossRef][Green Version]
- Imani, Z.; Asgarpanah, J.; Hashemi, F.; Hezaveh, J.H. Composition and antifungal activity of Zhumeria majdae essential oil. Curr. Med. Mycol. 2015, 1, 13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Emami, R.; Ghaneian, M.R.; Jebali, J. Antimicrobial activity and toxicity of Zhumeria majdae essential oil and its capsulated form. J. Community Health Res. 2015, 3, 242–252. [Google Scholar]
- Buchbauer, G. Biological activities of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 235–280. [Google Scholar]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, M.R.; Saraiva, J.R.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crop. Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Said, Z.B.O.S.; Haddadi-Guemghar, H.; Boulekbache-Makhlouf, L.; Rigou, P.; Remini, H.; Adjaoud, A.; Khoudja, N.K.; Madani, K. Essential oils composition, antibacterial and antioxidant activities of hydrodistillated extract of Eucalyptus globulus fruits. Ind. Crop. Prod. 2016, 89, 167–175. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.; Akhtar, M.S. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.L.; Matos, F.J.A. Analgesic and anti–inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti–inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Perry, N.S.; Bollen, C.; Perry, E.K.; Ballard, C. Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial. Pharmacol. Biochem. Behav. 2003, 75, 651–659. [Google Scholar] [CrossRef]
- Nychas, G.H.; Tassou, C.C.; Skandamis, P. Antimicrobials from herbs and spices. In Natural Antimicrobials for the Minimal Processing of Foods; Roller, S.M., Ed.; Wood Head Publishers: New York, NY, USA, 2003; pp. 176–200. [Google Scholar]
- Nieto, G. Biological activities of three essential oils of the Lamiaceae family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef]
- Jesus, A.S.D.; Blank, A.F.; Alves, M.F.; Arrigoni–Blank, M.D.F.; Lima, R.N.; Alves, P.B. Influence of storage time and temperature on the chemical composition of the essential oil of Hyptis pectinata L. Poit. Rev. Bras. Plantas Med. 2016, 18, 336–340. [Google Scholar] [CrossRef]
- Moghaddam, M.; Omidbaigi, R.; Sefidkon, F. Changes in content and chemical composition of Tagetes minuta oil at various harvest times. J. Essent. Oil Res. 2007, 19, 18–20. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Demuner, A.J.; Almeida Barbosa, L.C.; Gonçalves Magalhaes, C.; Da Silva, C.J.; Alvares Maltha, C.R.; Lelis Pinheiro, A. Seasonal variation in the chemical composition and antimicrobial activity of volatile oils of three species of Leptospermum (Myrtaceae) grown in Brazil. Molecules 2011, 16, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, A.; Hosseini, S.M.; Karami, A.; Afsharifar, A.; Sharifi Olounabadi, A.R. Variation in essential oil composition of Rydingia michauxii at the three developmental stages. Nat. Prod. Res. 2021, 35, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Ghasemi Pirbalouti, A.; Mehdizadeh, L.; Pirmoradi, M.R. Changes in composition and essential oil yield of Ocimum ciliatum at different phenological stages. Eur. Food Res. Technol. 2015, 240, 199–204. [Google Scholar] [CrossRef]
- Nguyen, H.; Campi, E.M.; Jackson, W.R.; Patti, A.F. Effect of oxidative deterioration on flavor and aroma components of Lemon oil. Food Chem. 2009, 112, 388–393. [Google Scholar] [CrossRef]
- Mohtashami, S.; Rowshan, V.; Tabrizi, L.; Babalar, M.; Ghani, A. Summer savory (Satureja hortensis L.) essential oil constituent oscillation at different storage conditions. Ind. Crop. Prod. 2018, 111, 226–231. [Google Scholar] [CrossRef]
- Najafian, S. Storage conditions affect the essential oil composition of cultivated balm mint herb (Lamiaceae) in Iran. Ind. Crop. Prod. 2014, 52, 575–581. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Ghasemi Pirbalouti, A.; Moghaddam, M. Storage stability of essential oil of cumin (Cuminum cyminum L.) as a function of temperature. Int. J. Food Prop. 2017, 20, 1742–1750. [Google Scholar] [CrossRef]
- Rowshan, V.; Bahmanzadehgan, A.; Saharkhiz, M.J. Influence of storage conditions on the essential oil composition of Thymus daenensis Celak. Ind. Crop. Prod. 2013, 49, 97–101. [Google Scholar] [CrossRef]
- Ehlert, P.A.D.; Ming, L.C.; Marques, M.O.M.; Fenandes, D.M.; Rocha, W.A.; Luz, J.M.Q.; Silva, R.F. Influência do horário de colheita sobre o rendimento e composição do óleo essencial de erva–cidreira brasileira [Lippia alba (Mill.) NE Br.]. Rev. Bras. Plantas Med. 2013, 15, 72–77. [Google Scholar] [CrossRef]
- Silva, F.; Park, K.J.; Magalhães, P.M.; Martins, G.N.; Gama, E.V.S. Avaliação do teor de óleo essencial de Baccharis trimera (Less.) DC. em diferentes embalagens durante o armazenamento. Rev. Bras. Plantas Med. 2013, 15, 54–58. [Google Scholar] [CrossRef][Green Version]
- Jacxsens, L.; Devlieghere, F.; Debevere, J. Temperature dependence of shelf life as affected by microbial proliferation and sensory quality of equilibrium modified atmosphere packaged fresh produce. Postharvest Biol. Technol. 2002, 26, 59–73. [Google Scholar] [CrossRef]
- Ding, C.K.; Chachin, K.; Yasunori, H.; Yasunori, U.; Yasunori, I. Effects of storage temperatures on physiology and quality of loquat fruit. Postharvest Biol. Technol. 1998, 14, 309–315. [Google Scholar] [CrossRef]
- Maalekuu, K.; Elkind, Y.; Leikin–Frenkel, A.; Lurie, S.; Fallik, E. The relationship between water loss, lipid content, membrane integrity and LOX activity in ripe pepper fruit after storage. Postharvest Biol. Technol. 2006, 42, 248–255. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Food Res. Int. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Najafian, S.; Hosseinifarahi, M.; Gholipour, S. The effect of time and temperature on shelf life of essential oil composition of Teucrium polium L. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Swisher, H.E.; Swisher, L.H. Specialy Citrus Products. In Citrus Science and Technology; Nagy, P.E.S., Shaw, M.K., Veldhuis, M.K., Eds.; The AVI Publishing Co. Inc.: Westport, CT, USA, 1997; Volume 1, p. 301. [Google Scholar]
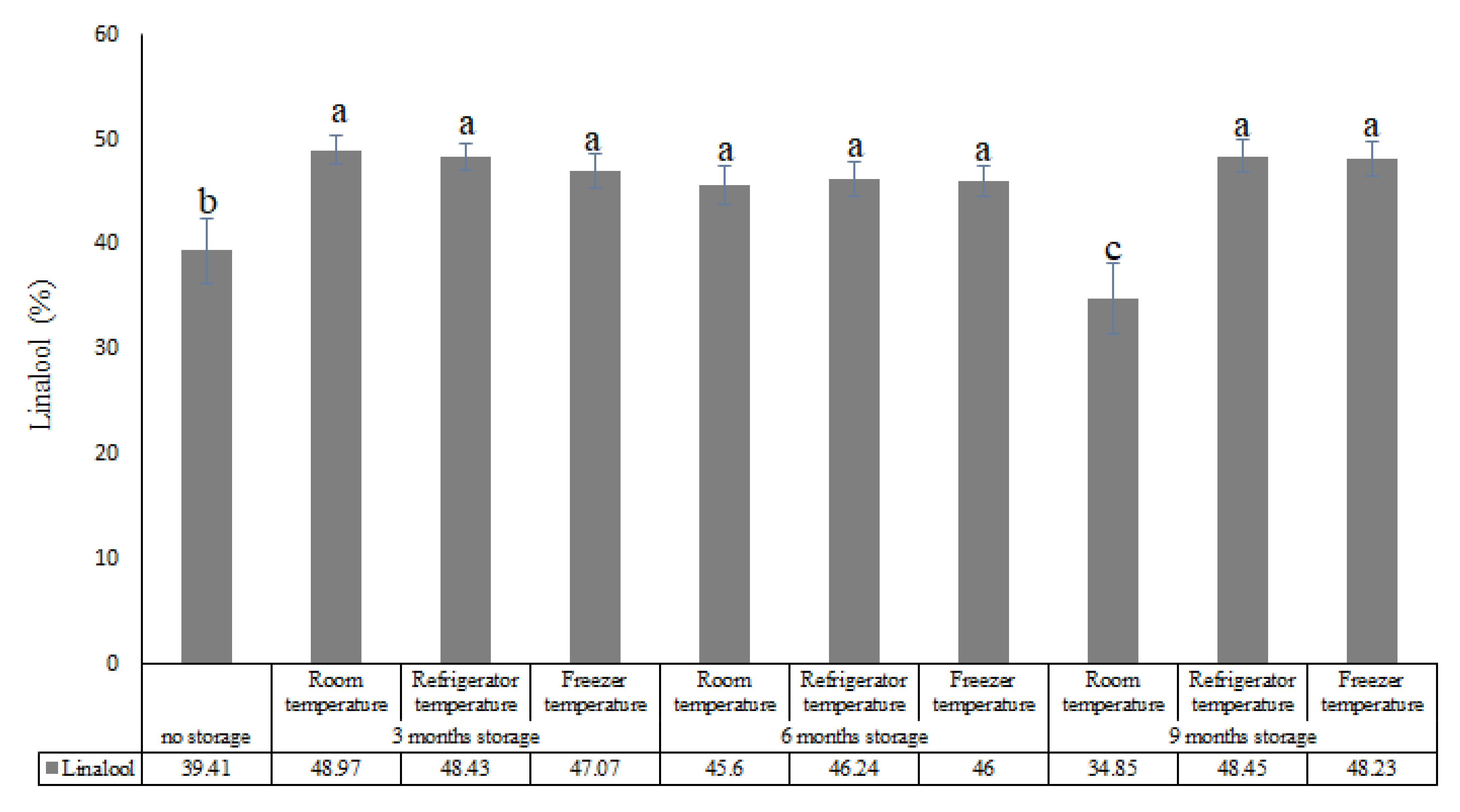
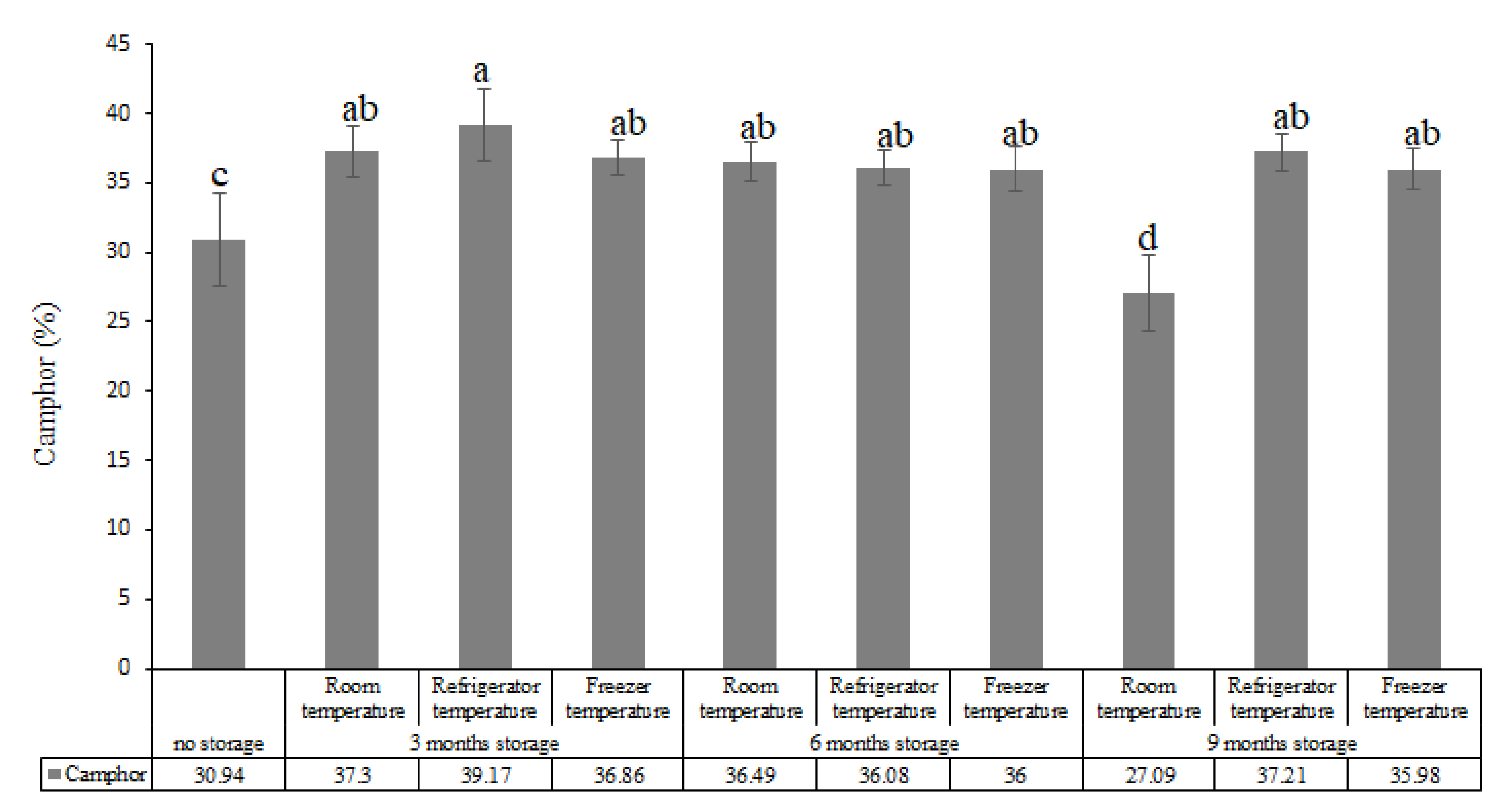
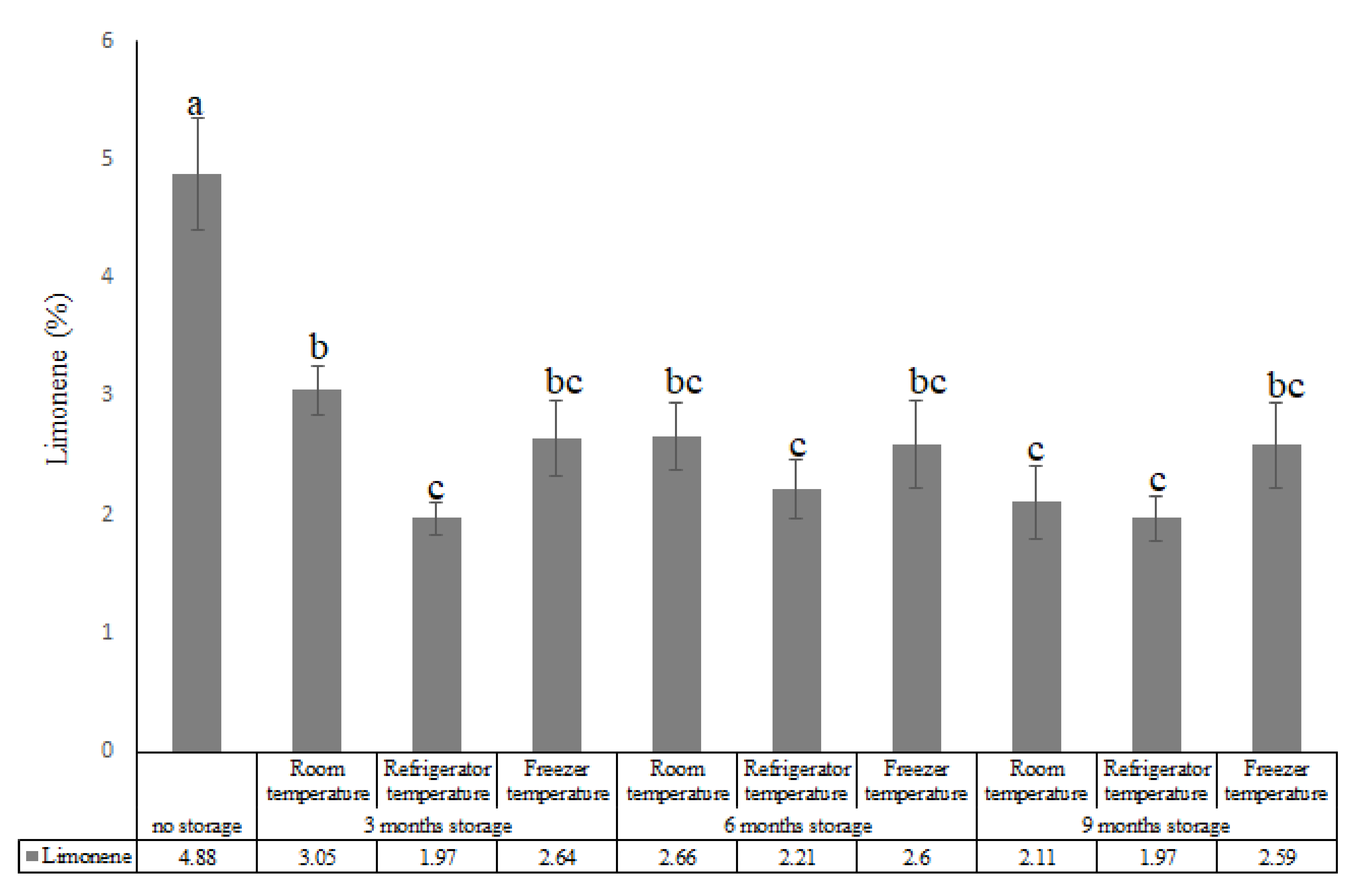
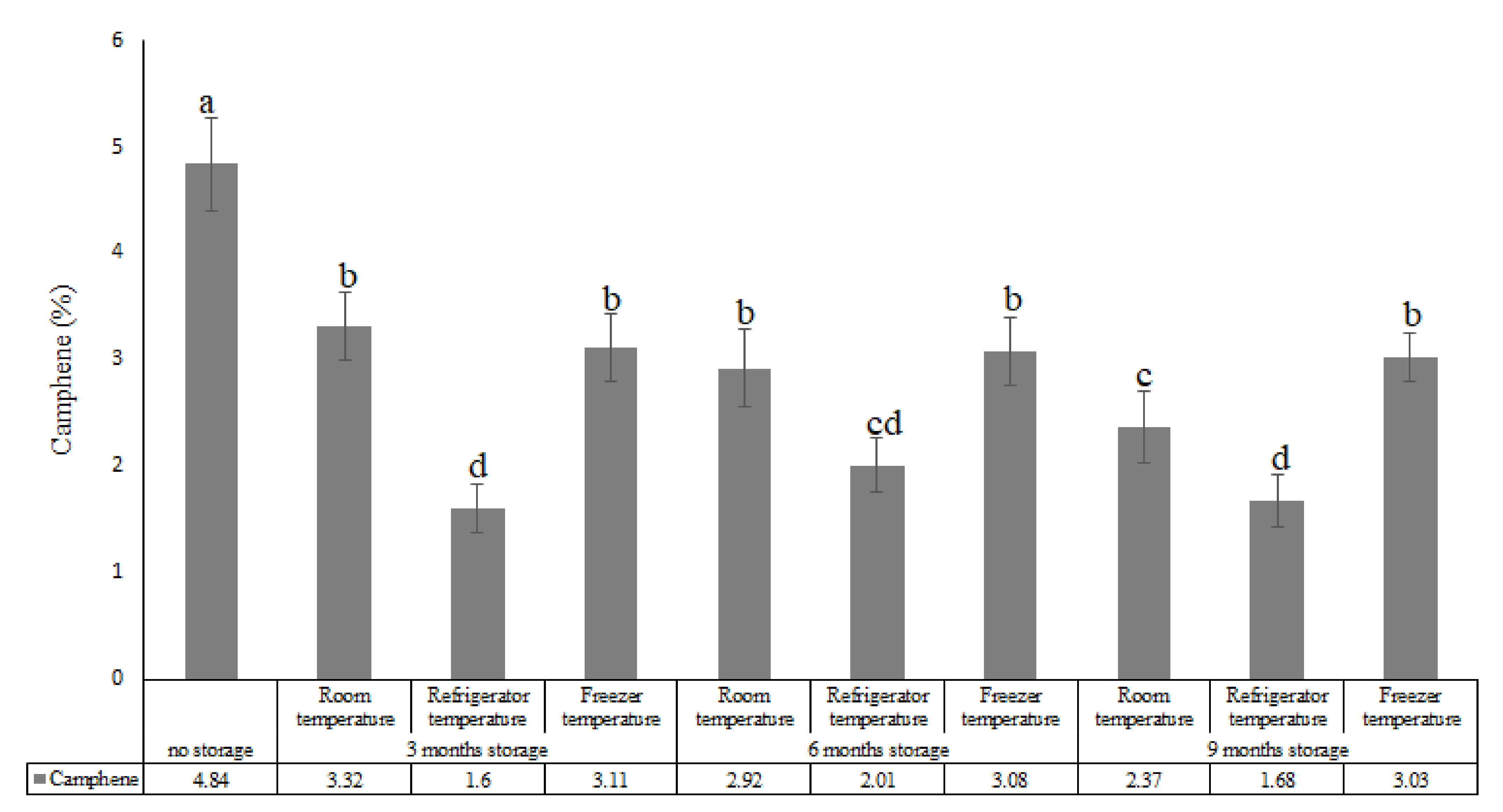
| Compounds (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Compound | RIa | RIb | No Storage | After 3 Months | After 6 Months | After 9 Months | ||||||
| RT | R | F | RT | R | F | RT | R | F | |||||
| 1 | α-Pinene | 938 | 932 | 2.38 * | 1.36 | 0.53 | 1.34 | 1.26 | 0.73 | 1.34 | 1.77 | 0.61 | 1.33 |
| 2 | Camphene | 935 | 946 | 4.84 | 3.32 | 1.60 | 3.11 | 2.92 | 2.01 | 3.08 | 2.37 | 1.68 | 3.03 |
| 3 | β-Pinene | 977 | 974 | 0.21 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 4 | 3-Octanone | 981 | 979 | 1.86 | 1.11 | 0.76 | 0.94 | 0.95 | 0.87 | 0.93 | 0.77 | 0.74 | 0.91 |
| 5 | Myrcene | 988 | 988 | 1.03 | ND | ND | 0.38 | 0.37 | 0.27 | 0.37 | 0.33 | ND | ND |
| 6 | n-Octanal | 989 | 989 | 0.10 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 7 | p-Cymene | 1022 | 1020 | ND | ND | ND | ND | 0.21 | 0.14 | 0.10 | ND | ND | ND |
| 8 | Limonene | 1032 | 1024 | 4.88 | 3.05 | 1.97 | 2.64 | 2.66 | 2.21 | 2.60 | 2.11 | 1.97 | 2.59 |
| 9 | (E)-β-Ocimene | 1045 | 1044 | 0.24 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 10 | γ-Terpinene | 1060 | 1054 | 0.55 | ND | ND | 0.32 | ND | 0.31 | 0.30 | ND | ND | ND |
| 11 | cis-Linalool oxide | 1067 | 1067 | 0.44 | ND | ND | ND | 0.21 | 0.21 | 0.20 | ND | ND | ND |
| 12 | trans-Linalool oxide | 1069 | 1084 | 0.26 | ND | ND | ND | 0.19 | ND | ND | ND | ND | ND |
| 13 | Terpinolene | 1090 | 1086 | 1 | ND | ND | 0.49 | 0.33 | 0.40 | 0.47 | ND | ND | 0.43 |
| 14 | Linalool | 1100 | 1095 | 39.41 | 48.20 | 48.43 | 47.07 | 45.60 | 46.24 | 46 | 34.85 | 48.45 | 48.23 |
| 15 | Camphor | 1140 | 1141 | 30.94 | 37.30 | 39.17 | 36.86 | 36.49 | 36.08 | 36 | 27.09 | 37.21 | 35.98 |
| 16 | Borneol | 1164 | 1165 | ND | 2.03 | 2.10 | 1.89 | 1.95 | 2.19 | 1.99 | 1.42 | 2.03 | 1.81 |
| 17 | Terpinen-4-ol | 1174 | 1174 | 0.63 | ND | 0.40 | 0.27 | 0.19 | 0.44 | 0.25 | ND | 0.49 | ND |
| 18 | α-Terpineol | 1185 | 1186 | 3.67 | ND | ND | 0.35 | 0.42 | 0.22 | 0.32 | ND | ND | ND |
| 19 | Nerol | 1231 | 1227 | ND | ND | ND | 0.77 | ND | ND | 0.81 | ND | 1.05 | 1.09 |
| 20 | Neral | 1240 | 1235 | 0.77 | 0.1 | 0.37 | 0.22 | 0.2 | 0.4 | 0.2 | 0.12 | 0.2 | 0.2 |
| 21 | (Z)-Anethole | 1249 | 1249 | 0.44 | ND | 0.50 | ND | 0.08 | 0.54 | ND | ND | 0.50 | ND |
| 22 | Geraniol | 1252 | 1252 | 0.2 | 1.90 | 0.74 | 1.37 | 2.06 | 2.14 | 2.18 | 1.99 | 2.11 | 2.08 |
| 23 | Geranial | 1268 | 1264 | 1.67 | 0.2 | 0.77 | 0.48 | 0.29 | 0.78 | 0.39 | 0.28 | 0.45 | 0.37 |
| 24 | Thymol | 1291 | 1289 | 0.09 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 25 | (Z)-jasmone | 1400 | 1392 | 0.29 | ND | 0.26 | 0.26 | 0.23 | 0.27 | 0.26 | 0.24 | 0.26 | 0.23 |
| 26 | (E)-Caryophyllene | 1417 | 1417 | 2.17 | 1.75 | 1.35 | 1.10 | 1.69 | 1.53 | 1.17 | 1.93 | 1.47 | 1.22 |
| 27 | α-Humulene | 1453 | 1452 | ND | ND | ND | ND | 0.17 | 0.21 | 0.15 | ND | ND | ND |
| 28 | Caryophyllene oxide | 1589 | 1582 | ND | ND | 0.38 | 0.25 | 0.30 | 0.78 | 0.48 | 0.38 | 0.80 | 0.51 |
| 29 | β-Eudesmol | 1646 | 1649 | 3.23 | ND | ND | ND | ND | ND | 0.20 | ND | ND | ND |
| Total | 99.99 | 99.99 | 100 | 99.99 | 99.67 | 99.95 | 99.89 | 99.67 | 99.95 | 99.89 | |||
| Linalool | Camphor | Limonene | Camphene | |
|---|---|---|---|---|
| Linalool | 1 | |||
| Camphor | 0.986 * | 1 | ||
| Limonene | −0.824 ns | −0.731 ns | 1 | |
| Camphene | −0.820 ns | −0.725 ns | 1 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karami, A.; Tashani, F.; Tahmasebi, A.; Maggi, F. Chemical Variability in the Composition of Zhumeria majdae (Rech. F. & Wendelbo) Essential Oil According to Storage Time and Temperature. Horticulturae 2021, 7, 463. https://doi.org/10.3390/horticulturae7110463
Karami A, Tashani F, Tahmasebi A, Maggi F. Chemical Variability in the Composition of Zhumeria majdae (Rech. F. & Wendelbo) Essential Oil According to Storage Time and Temperature. Horticulturae. 2021; 7(11):463. https://doi.org/10.3390/horticulturae7110463
Chicago/Turabian StyleKarami, Akbar, Fatemeh Tashani, Aminallah Tahmasebi, and Filippo Maggi. 2021. "Chemical Variability in the Composition of Zhumeria majdae (Rech. F. & Wendelbo) Essential Oil According to Storage Time and Temperature" Horticulturae 7, no. 11: 463. https://doi.org/10.3390/horticulturae7110463
APA StyleKarami, A., Tashani, F., Tahmasebi, A., & Maggi, F. (2021). Chemical Variability in the Composition of Zhumeria majdae (Rech. F. & Wendelbo) Essential Oil According to Storage Time and Temperature. Horticulturae, 7(11), 463. https://doi.org/10.3390/horticulturae7110463








