Shading of Medical Plants Affects the Phytochemical Quality of Herbal Extracts
Abstract
:1. Introduction
2. Material and Method
2.1. Plant Material and Growing Conditions
2.2. Light Interception by Nets
2.3. Reagents and Chemicals
2.4. Plant Material and Extraction
2.5. Determination of Content of Total Phenols in Extracts
2.6. Determination of Content of Total Flavonoids in Extracts
2.7. Antioxidant Activity of Extracts—DPPH Test
2.8. Statistical Analysis
3. Results
3.1. Growing Conditions
3.2. Content of Total Extactive Substances (TES)
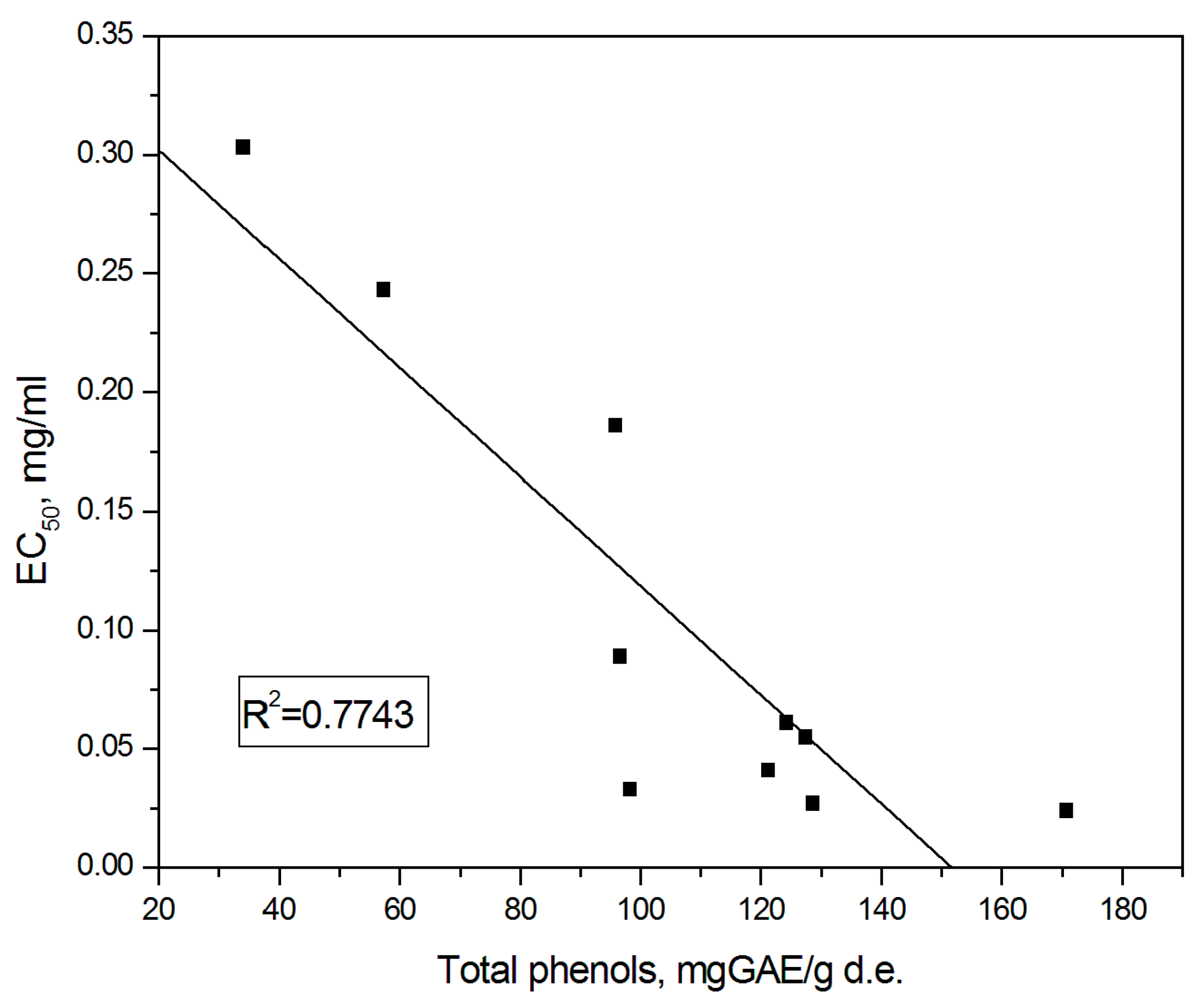
3.3. Total Phenolic Content
3.4. Total Flavonoid Content
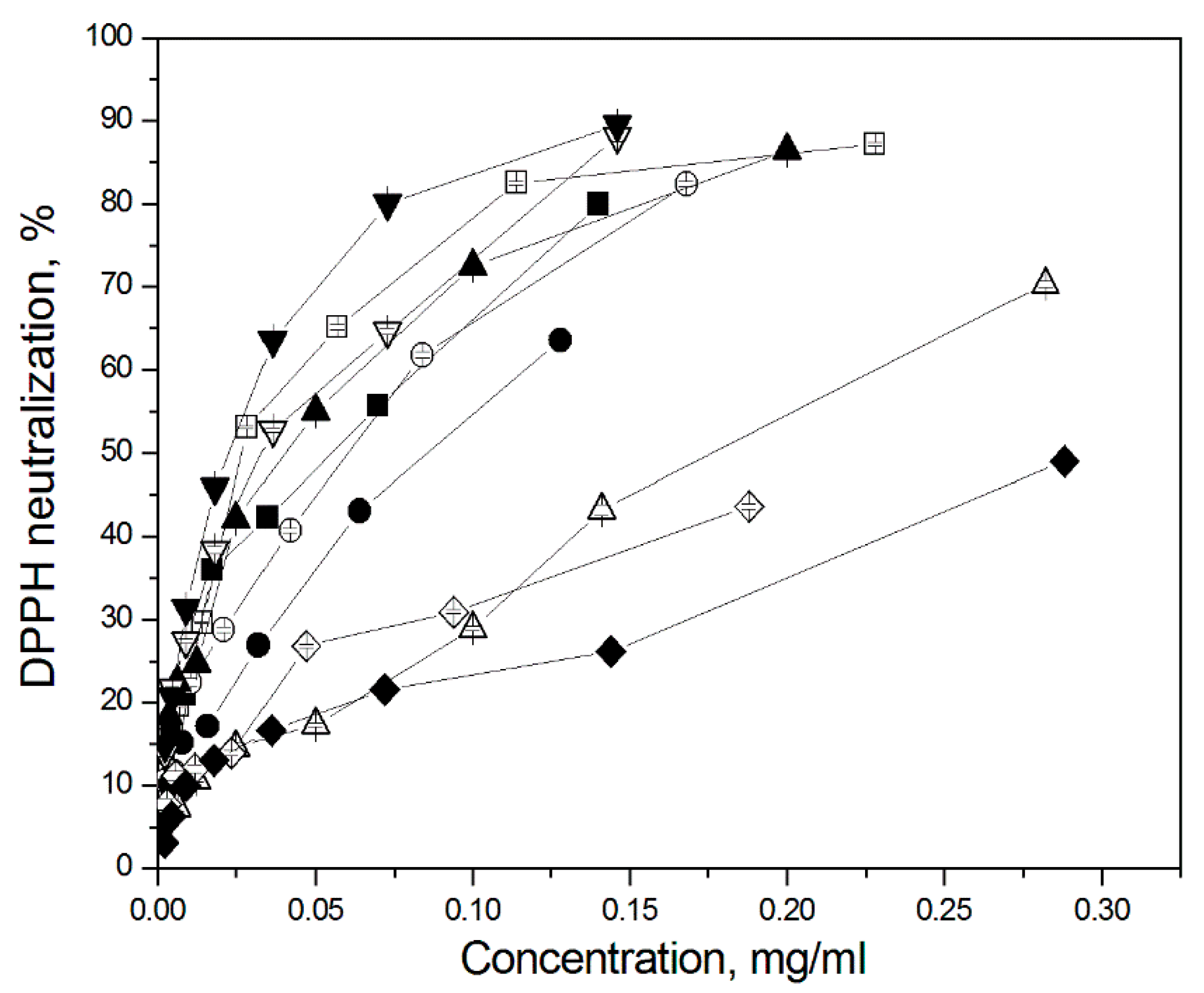
3.5. Total Antioxidant Capacity (TAC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop. Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Raja, R.R. Medicinally potential plants of Labiatae (Lamiaceae) Family: An Overview. Res. J. Med. Plants 2012, 6, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Lie-Fen, S.; Chiu-Ping, L.; Shih-Chang, C. Metabolomics in Herbal Medicine Research. In The Handbook of Plant Metabolomics; Weckwerth, W., Kahl, G., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2013; Chapter 8; pp. 155–174. [Google Scholar]
- Ahmad, N.; Fazal, H.; Ahmad, I.; Abbasi, B.H. Free radical scavenging (DPPH) potential in nine Mentha species. Toxicol. Ind. Health 2012, 28, 83–89. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jifon, J. Influence of photoselective shade nettings on postharvest quality of vegetables. In Preharvest Modulation of Postharvest Fruits and Vegetable Quality; AAP-CRC Press: Boca Raton, FL, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 121–138. [Google Scholar]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Schmitz, O.J.; Meckelmann, S.W. Chemical characterization of eight herbal liqueurs by means of liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2020, 1631, 461560. [Google Scholar] [CrossRef]
- Karabegović, I.T.; Vukosavljević, P.V.; Novaković, M.M.; Gorjanović, S.T.; Džamić, A.M.; Lazić, M.L. Influence of the storage on bioactive compounds and sensory attributes of herbal liqueur. Dig. J. Nanomater. Biostruct. 2012, 7, 1587–1598. [Google Scholar]
- Sarikurkcu, C.; Ozer, M.S.; Calli, N.; Popović-Djordjević, J. Essential oil composition and antioxidant activity of endemic Marrubium parviflorum subsp. oligodon. Ind. Crop. Prod. 2018, 119, 209–213. [Google Scholar] [CrossRef]
- Lakićević, S.; Popović Djordjević, J.; Pejin, B.; Djordjević, A.; Matijašević, S.; Lazić, M. An insight into chemical composition and bioactivity of ‘Prokupac’ red wine. Nat. Prod Res. 2020, 34, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Mikulic-Petkovsek, M. Changes in beneficial bioactive compounds in eight traditional herbal liqueurs during a one-month maceration process. J. Sci. Food Agric. 2020, 100, 343–353. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Vázquez-Araújo, L.; Salgado, J.M.; Domínguez, J.M.; Diéguez, C.S. Optimization of the process of aromatic and medicinal plant maceration in grape marc distillates to obtain herbal liqueurs and spirits. J. Sci. Food Agric. 2016, 96, 4760–4771. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Phenolic compounds and aroma-impact odorants in herb liqueurs elaborated by maceration of aromatic and medicinal plants in grape marc distillates. J. Inst. Brew. 2016, 122, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Ayatullah Leghari, S.K.; Shaukat, K.; Khattak, M.I.; Panezai, M.A. Influence of sun and shade on the growth, yield and quality of Vitis vinifera L. (grapes) under semi-arid environmental conditions. App. Ecol. Envir. Res. 2019, 17, 8847–8864. [Google Scholar]
- Ilic, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Šunić, L.; Fallik, E. Effect of coloured shade-nets on plant leaf parameters and tomato fruit quality. J. Sci. Food Agric. 2015, 95, 2660–2667. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Šunić, L.; Fallik, E. Effect of shading by colour nets on plant development, yield and fruit quality of sweet pepper grown under plastic tunnels and open field. Zemdirb. Agric. 2017, 104, 53–62. [Google Scholar] [CrossRef]
- Selahle, K.M.; Sivakumar, D.; Jifon, J.; Soundy, P. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chem. 2015, 173, 951–956. [Google Scholar] [CrossRef]
- Mashabela, M.N.; Selahle, K.M.; Soundy, P.; Crosby, K.M.; Sivakumar, D. Bioactive compounds and fruit quality of green sweet pepper grown under different colored shade netting during postharvest storage. J. Food Sci. 2015, 16, 2612–2618. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Dimitrijević, A.; Stanojević, L.; Cvetković, D.; Mastilović, J.; Kevrešan, Ž. Effect of coloured shade-nets on yield and quality of lettuce (Lactuca sativa L.) during summer production. Sci. Hortic. 2017, 226, 389–397. [Google Scholar] [CrossRef]
- Buthelezi, M.N.D.; Soundy, P.; Jifon, J.; Sivakumar, D. Spectral quality of photo-selective nets improves phytochemicals and aroma volatiles in coriander leaves (Coriandrum sativum L.) after postharvest storage. J. Photochem. Photobiol. B Biol. 2016, 161, 328–334. [Google Scholar] [CrossRef]
- Buthelezi, M.N.D. Effect of Photo-Selective Netting on Postharvest Quality and Bioactive Compounds in Three Selected Summer Herbs (Coriander, Marjoram and Oregano). Master’s Thesis, Department of Crop Sciences, Faculty of Science Tshwane University of Technology, Pretoria, South Africa, 2015. [Google Scholar]
- Milenković, L.; Ilić, Z.; Šunić, L.; Tmušić, N.; Lalević, D.; Stanojević, L.J.; Stanojević, L.; Cvetković, D. Modification of light intensity influence essential oils content, composition and antioxidant activity of thyme, marjoram and oregano. Saudi J. Biol. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Stanojević, L.J.; Stanojević, J.; Milenković, L.; Šunić, L.J.; Cvetković, D.; Babic, M.; Ilić, S.Z. Aroma profile and antioxidant activity of basil aqueous extracts affect by light modification. LWT Food Sci. Technol. 2021, in press. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, A.K.; Prasad, K.; Bahadur, A.; Rai, M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J. Food Compos. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Stanojević, L.J.P.; Stanković, M.Z.; Nikolić, V.D.; Nikolić, L.J.B. Antioxidative and antimicrobial activities of Hieraciumpilosella L. extracts. J. Serb. Chem. Soc. 2008, 73, 531–540. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Šunić, L.J.; Barać, S.; Cvetković, D.; Stanojević, L.J.; Kevrešan, Ž.; Mastilović, J. Bioactive constituents of red and green lettuce grown under colour shade nets. Emir. J. Food Agric. 2019, 31, 937–944. [Google Scholar] [CrossRef]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Milenković, L.; Stanojević, J.; Cvetković, D.; Stanojević, L.; Lalević, D.; Šunić, L.; Fallik, E.; Ilić, S.Z. New technology in basil production with high essential oil yield and quality. Ind. Crops Prod. 2019, 140, 111718. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Tmušić, N.; Stanojević, L.J.; Stanojević, J.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT Food Sci. Technol. 2021, 153, 112210. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; AlimpićAradski, A.; Kolarević, S.; Vuković-Gačić, B.; Oalđe, M.; Živković, J.; Šavikin, K.; Marin, P.D. Antineurodegenerative, antioxidant and antibacterial activities and phenolic components of Origanummajorana L. (Lamiaceae) extracts. J. Appl. Bot. Food Qual. 2018, 91, 126–134. [Google Scholar]
- Benchikha, N.; Menaceur, M.; Barhi, Z. Extraction and antioxidant activities of two species Origanum plant containing phenolic and flavonoid compounds. J. Fundam. Appl. Sci. 2013, 5, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Bergquist, S.A.M.; Gertsson, U.E.; Nordmark, L.Y.G.; Olsson, M.E. Ascorbic acid, carotenoids, and visual quality of baby spinach as affected by shade netting and postharvest storage. J. Agric. Food Chem. 2007, 55, 8444–8451. [Google Scholar] [CrossRef] [PubMed]
- Čadanović-Brunet, J.; Djilas, S.; Ćetković, G.; Tumbas, V.; Mandić, A.; Čadanović, V. Antioxidant activities of different Teucrium monthanum L. extracts. Int. J. Food Sci. Technol. 2006, 41, 667–673. [Google Scholar] [CrossRef]
- Mihailovic-Stanojevic, N.; Belscak-Cvitanovic, A.; Grujic-Milanovic, J.; Ivanov, M.; Jovovic, D.; Bugarski, D.; Miloradovic, Z. Antioxidant and antihypertensive activity of extract from Thymus serpyllum L. in experimental hypertension. Plant Food Hum. Nutr. 2013, 68, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.S.; Battistin, A.; Pauletti, G.; Rota, L.; Serafini, L.A. Antioxidant properties of essential oils from Mentha species evidenced by electrochemical methods. Rev. Bras. Plantas Med. 2009, 11, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Sodré, A.C.B.; Luz, J.M.Q.; Haber, L.L.; Marques, M.O.M.; Rodrigues, C.R.; Blank, A.F. Organic and mineral fertilization and chemical composition of lemon balm (Melissa officinalis) essential oil. Rev. Bras. Farmacogn. 2012, 22, 40–44. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Esteves, E.; Mansinhos, I.; Gonçalves, S.; Pérez-Santín, E.; Galego, L.; Romano, A. Influence of elaboration process on chemical, biological, and sensory characteristics of E uropean pennyroyal liqueurs. J. Sci. Food Agric. 2021, 101, 4076–4089. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Rodríguez-Solana, R.; Cortés-Diéguez, S.M.; Domínguez, J.M. Study of the suitability of two hop cultivars for making herb liqueurs: Volatile composition, sensory analysis, and consumer study. Eur. Food Res. Technol. 2013, 237, 775–786. [Google Scholar] [CrossRef]
- Egea, T.; Signorini, M.A.; Ongaro, L.; Rivera, D.; de Castro, C.O.; Bruschi, P. Traditional alcoholicbeverages their value in the local culture of the Alta Valle del Reno, a mountain borderland betweenTuscany and Emilia-Romagna. J. Ethnobiol. Ethnomed. 2016, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Bhowmik, R. Health benefits of plant derived bioactive secondary metabolites as dietary constituents. SF J. Clin. Pharm. Res. 2020, 2, 1002. [Google Scholar]
| Month | Number of Summer Days (over 25 °C in June; over 30 °C for July and August) | Average Temperature Difference from Multiannual Average (°C) | Sum of Insolation Difference from Multiannual Average (h) |
|---|---|---|---|
| June | 27 | 0.8 | −51.4 |
| July | 10 | −0.2 | −72.5 |
| August | 28 | 2.0 | −1.9 |
| Time (h) | PAR * (μmol m−2 s−1) | Solar Radiation (W m−2) | Temperature °C | Relative Humidity % | ||||
|---|---|---|---|---|---|---|---|---|
| Unshading | Reduction by Shading % | Unshading | Shading | Unshading | Reduction by Shading % | Unshading | Reduction by Shading % | |
| 6:00 | 182.5 | 31.2 | 162.5 | 40.5 | 16.7 | 0.0 | 74.7 | −4.1 |
| 9:00 | 1325.6 | 46.0 | 513.8 | 281.0 | 24.7 | −0.4 | 71.8 | 0.0 |
| 12:00 | 2242.2 | 49.1 | 874.5 | 459.5 | 31.4 | −2.2 | 47.3 | −2.1 |
| 15:00 | 1684.1 | 51.9 | 790.5 | 351.0 | 31.5 | −3.4 | 48.2 | −1.2 |
| 18:00 | 672.0 | 53.9 | 375.5 | 90.9 | 28.3 | −1.0 | 50.4 | −0.2 |
| Plant Species | TES (g/100g f.p.m. *) | |
|---|---|---|
| Non Shading | Shading Pearl Nets (50%) | |
| A—Thyme (Thymus vulgaris L.) | 10.26 b ± 0.09 | 6.30 g ± 0.09 |
| B—Marjoram (Origanum majorana L.) | 7.06 e ± 0.08 | 5.33 h ± 0.07 |
| C—Oregano (Origanum vulgare L.) | 12.72 a ± 0.07 | 9.02 c ± 0.08 |
| D—Lemon balm (Melissa officinalis L.) | 6.48 f ± 0.12 | 6.48 f ± 0.05 |
| E—Peppermint (Mentha piperita L.) | 8.38 d ± 0.08 | 6.47 f ± 0.14 |
| ANOVA | ||
| Plant | p < 0.01 | |
| Shading | p < 0.01 | |
| Plantx shading | p < 0.01 | |
| Plant Species | TP(mg GAE/g.d.e. *) | |
|---|---|---|
| Non Shading | Shading Pearl Nets (50%) | |
| A—Thyme (Thymus vulgaris L.) | 128.64 b ± 0.50 | 127.42 b ± 0.82 |
| B—Marjoram (Origanum majorana L.) | 124.27 c ± 0.68 | 96.55 ef ± 0.89 |
| C—Oregano (Origanum vulgare L.) | 95.81 f ± 0.41 | 121.22 d ± 0.57 |
| D—Lemon balm (Melissa officinalis L.) | 98.22 e ± 0.78 | 170.58 a ± 1.36 |
| E—Peppermint (Mentha piperita L.) | 57.31 g ± 1.31 | 34.08 h ± 1.59 |
| ANOVA | ||
| Plant | p < 0.01 | |
| Shading | p < 0.01 | |
| Plant × shading | p < 0.01 | |
| Plant Species | TF (mg RE/g.d.e. *) | |
|---|---|---|
| Non Shading | Shading Pearl Nets (50%) | |
| A—Thyme (Thymus vulgaris L.) | 27.51 a ± 0.31 | 6.67 e ± 0.77 |
| B—Marjoram (Origanum majorana L.) | 15.92 b ± 0.72 | 1.60 g ± 0.55 |
| C—Oregano (Origanum vulgare L.) | 12.15 c ± 0.12 | 10.43 d ± 0.20 |
| D—Lemon balm (Melissa officinalis L.) | 3.17 f ± 0.28 | 6.88 e ± 0.28 |
| E—Peppermint (Mentha piperita L.) | 3.92 f ± 0.22 | 3.87 f ± 0.49 |
| ANOVA | ||
| Plant | p < 0.01 | |
| Shading | p < 0.01 | |
| Plant × shading | p < 0.01 | |
| Plant Species | EC50 Values (mg∙mL−1 *) | |
|---|---|---|
| Non Shading | Shading Pearl Nets (50%) | |
| A—Thyme (Thymus vulgaris L.) | 0.027 a ± 0.0001 | 0.055 d ± 0.0006 |
| B—Marjoram (Origanum majorana L.) | 0.061 e ± 0.0003 | 0.089 f ± 0.0005 |
| C—Oregano (Origanum vulgare L.) | 0.186 g ± 0.0021 | 0.041 c ± 0.0002 |
| D—Lemon balm (Melissa officinalis L.) | 0.033 b ± 0.0003 | 0.024 a ± 0.0002 |
| E—Peppermint (Mentha piperita L.) | 0.243 h ± 0.0040 | 0.303 i ± 0.0040 |
| ANOVA | ||
| Plant | p < 0.01 | |
| Shading | p < 0.01 | |
| Plant × shading | p < 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tmušić, N.; Ilić, Z.S.; Milenković, L.; Šunić, L.; Lalević, D.; Kevrešan, Ž.; Mastilović, J.; Stanojević, L.; Cvetković, D. Shading of Medical Plants Affects the Phytochemical Quality of Herbal Extracts. Horticulturae 2021, 7, 437. https://doi.org/10.3390/horticulturae7110437
Tmušić N, Ilić ZS, Milenković L, Šunić L, Lalević D, Kevrešan Ž, Mastilović J, Stanojević L, Cvetković D. Shading of Medical Plants Affects the Phytochemical Quality of Herbal Extracts. Horticulturae. 2021; 7(11):437. https://doi.org/10.3390/horticulturae7110437
Chicago/Turabian StyleTmušić, Nadica, Zoran S. Ilić, Lidija Milenković, Ljubomir Šunić, Dragana Lalević, Žarko Kevrešan, Jasna Mastilović, Ljiljana Stanojević, and Dragan Cvetković. 2021. "Shading of Medical Plants Affects the Phytochemical Quality of Herbal Extracts" Horticulturae 7, no. 11: 437. https://doi.org/10.3390/horticulturae7110437
APA StyleTmušić, N., Ilić, Z. S., Milenković, L., Šunić, L., Lalević, D., Kevrešan, Ž., Mastilović, J., Stanojević, L., & Cvetković, D. (2021). Shading of Medical Plants Affects the Phytochemical Quality of Herbal Extracts. Horticulturae, 7(11), 437. https://doi.org/10.3390/horticulturae7110437








