Efficacy of Different Concentrations of NAA on Selected Ornamental Woody Shrubs Cuttings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
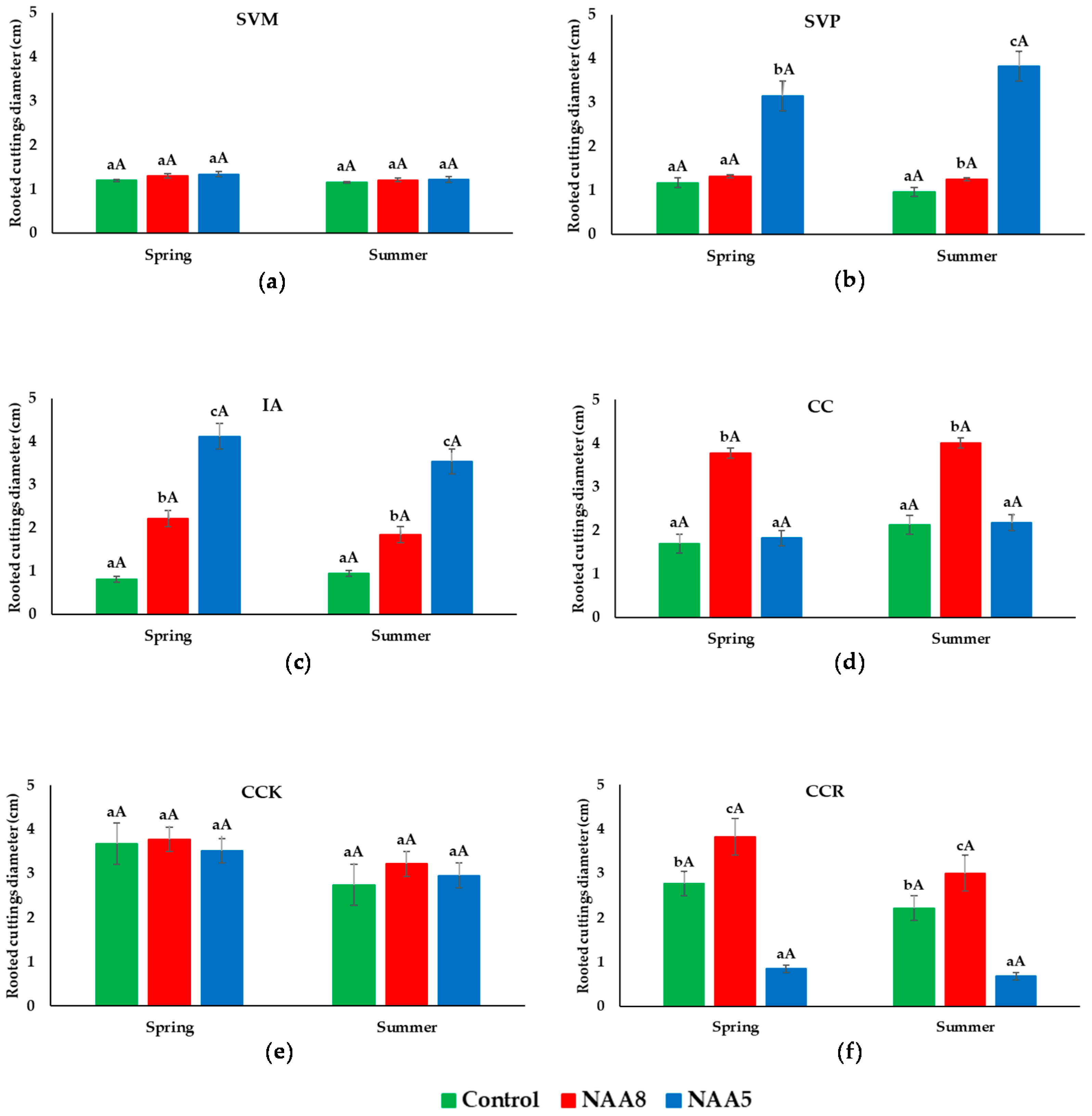
- Syringa vulgaris ‘Mme Lemoine’ (SVM): double white flowers, light green heart-shaped leaves, grows up to 2.5–3 m and 3 m wide.
- Syringa vulgaris ‘President Grevy’ (SVP): double lavender-blue flowers, light green heart-shaped leaves, grows up to 3–3.5 m and 2.5 m wide.
- Ilex aquifolium (IA): cluster white flowers, produces red fruits, glossy green prickly leaves, grows up to 10–25 m and 2 m wide.
- Cotinus coggygria (CC): smoky pink flowers, green or reddish-purple leaves, grows up to 3–5 m and 4 m wide.
- Cotinus coggygria ‘Kanari’ (CCK): white flowers, green leaves, grows up to 2.5–4 m and 2.5–4 m wide.
- Cotinus coggygria ‘Royal Purple’ (CCR): feathery pink flowers, wine purple leaves, grows up to 3–5 m and 4.5–6 m wide.
2.2. Experimental Design and Rooting Conditions
2.3. Data Evaluation
2.4. Statistical Analysis
3. Results
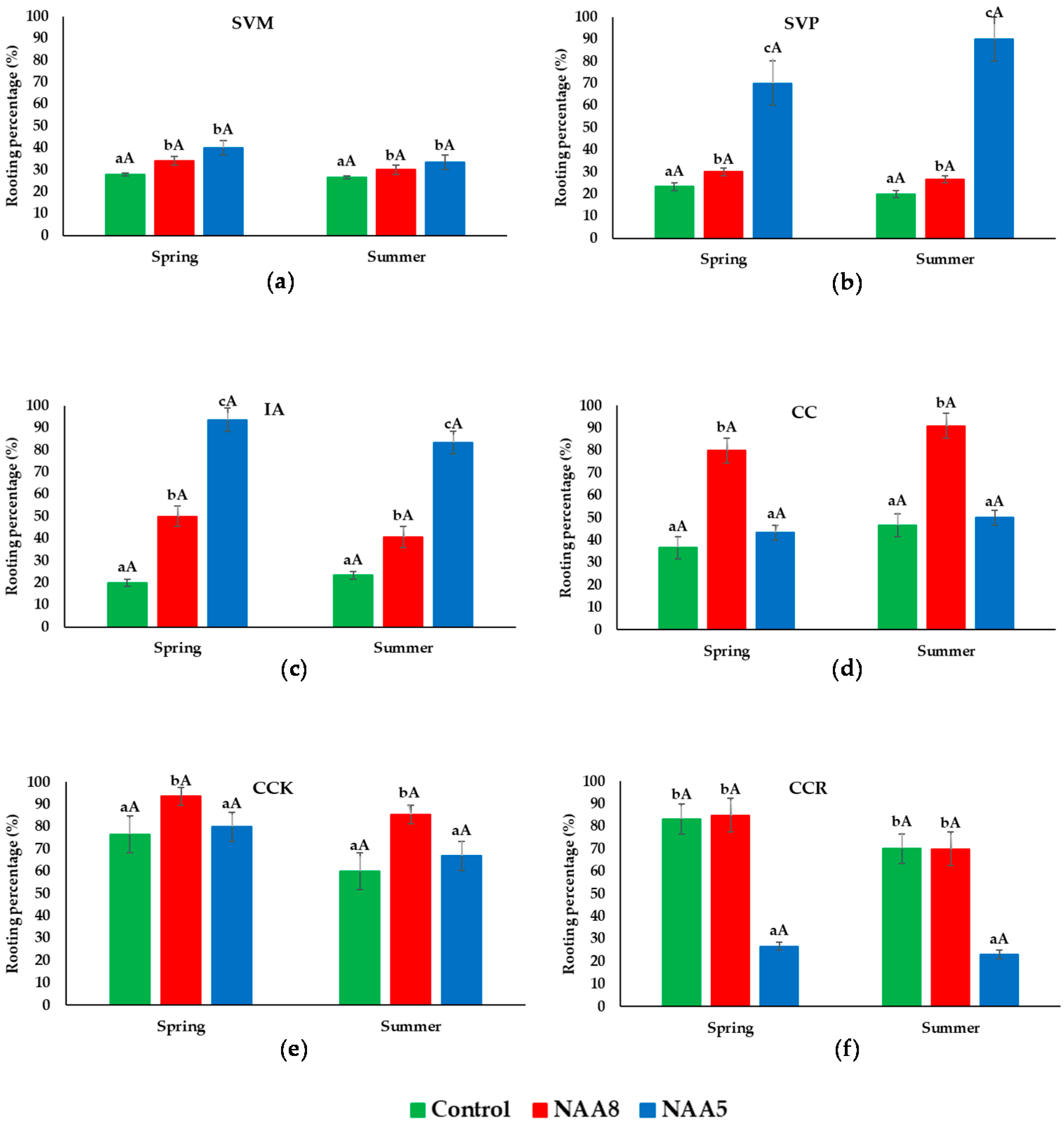
3.1. Rooting Percentage of Cuttings
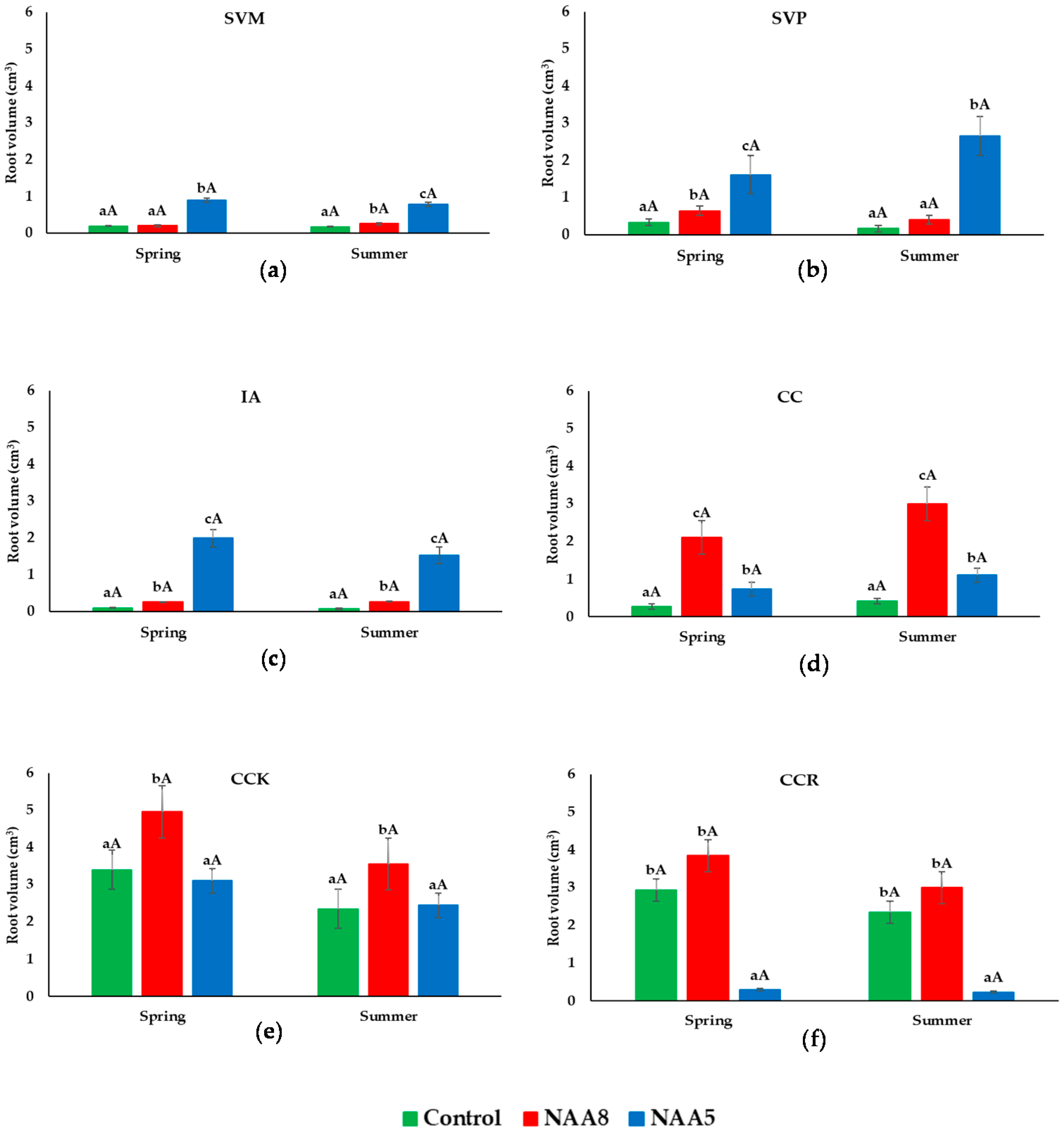
3.2. Root Volume
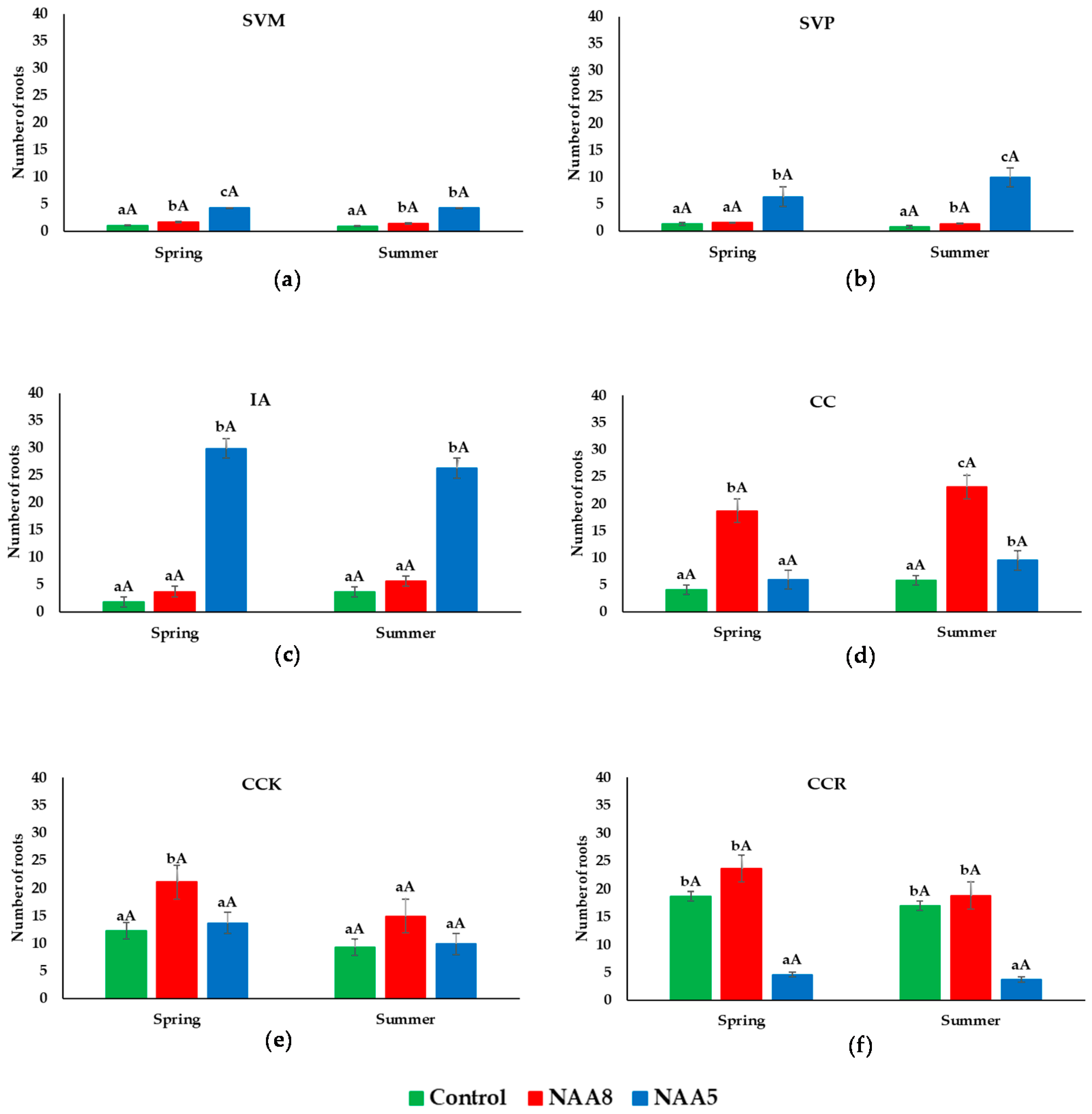
3.3. Number of Roots
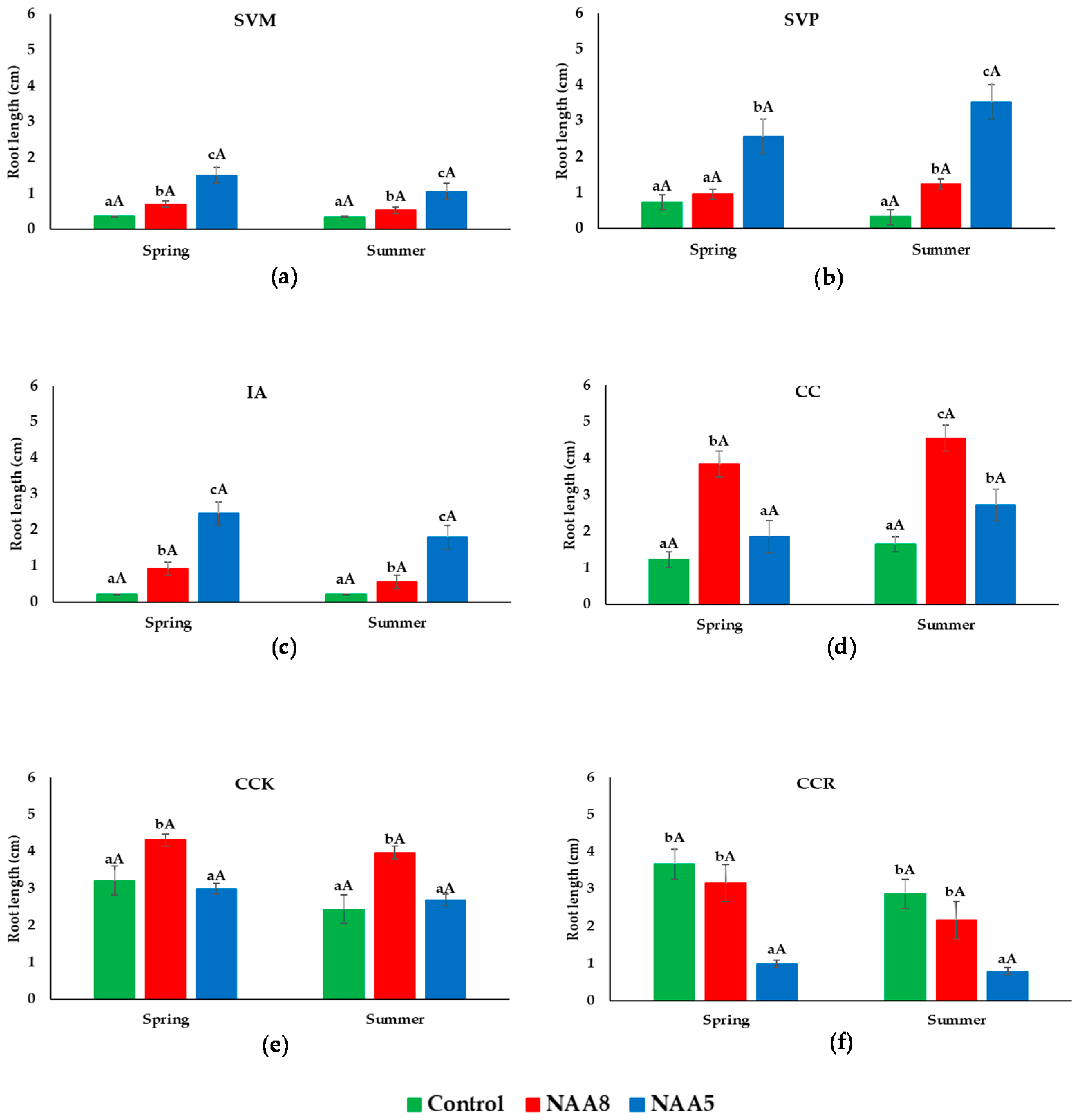
3.4. Root Length
3.5. Diameter of Cuttings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naik, E.K. Success rate of different ornamental cuttings based on different growing media. J. Pharmacogn. Phytochem. 2018, 7, 2479–2482. [Google Scholar]
- Swaroop, K. Unit-1 Woody Ornamentals Trees; Indira Gandhi National Open University: New Delhi, India, 2021. [Google Scholar]
- Ruchala, S.L. Propagation of Several Native Ornamental Plants; Master’s Thesis, The University of Maine, Orono, ME, USA, 2002; p. 448. [Google Scholar]
- Mateescu, R. Ornamental Tree and Shrubs; M.A.S.T. Publishing: Bucharest, Romania, 2002; pp. 28–64. [Google Scholar]
- Brown, C.L.; Sommer, H.E. Vegetative propagation of dicotyledonous trees. In Tissue Culture in Forestry; Springer: Dordrecht, The Netherlands, 1982; pp. 109–149. [Google Scholar] [CrossRef]
- Stuepp, C.A.; Wendling, I.; Xavier, A.; Zuffellato-Ribas, K.C. Vegetative propagation and application of clonal forestry in Brazilian native tree species. Pesqui. Agropecu. Bras. 2018, 53, 985–1002. [Google Scholar] [CrossRef]
- Husen, A.; Iqbal, M.; Siddiqui, S.N.; Sohrab, S.S.; Masresha, G. Effect of indole-3-butyric acid on clonal propagation of mulberry (Morus alba L.) stem cuttings: Rooting and associated biochemical changes. In Proceedings of the National Academy of Sciences, India Section B: Biological Sciences; Springer: Dordrecht, The Netherlands, 2017; Volume 87, pp. 161–166. [Google Scholar] [CrossRef]
- Kaviani, B.; Negahdar, N. Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark, an endangered ornamental shrub. S. Afr. J. Bot. 2017, 111, 326–335. [Google Scholar] [CrossRef]
- Kumar, K.V.; Fatmi, U. Effects of IBA and NAA on shoot growth of cuttings of various ornamental plants in water as rooting medium. J. Pharmacogn. Phytochem. 2021, 10, 685–687. [Google Scholar]
- Kaushik, S.; Shukla, N. A review on effect of IBA and NAA and their combination on the rooting of stem cuttings of different ornamental crops. J. Pharmacogn. Phytochem. 2020, 9, 1881–1885. [Google Scholar]
- Mirihagalla, M.K.P.N.; Fernando, K.M.C. Effect of Aloe vera gel for inducing rooting of stem cuttings and air layering of plants. J. Dry Zone Agric. 2020, 6, 13–26. [Google Scholar]
- Anfang, M.; Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, Y.; Jaskani, M.J.; Asif, M.; Qasim, M. Application of plant growth regulators in ornamental plants: A review. Pak. J. Agric. Sci. 2017, 54, 327–333. [Google Scholar] [CrossRef]
- Toungos, M.D. Plant growth substances in crop production: A Review. IJIABR 2018, 6, 1–8. [Google Scholar]
- Mendel, P.; Schiavo-Capri, E.; Lalge, A.B.; Vyhnanek, T.; Kalousek, P.; Trojan, V.; Havel, L.; Filippi, A.; Braidot, E. Evaluation of selected characteristics in industrial hemp after phytohormonal treatment. Pak. J. Agric. Sci. 2020, 57, 1–7. [Google Scholar]
- Pop, T.I.; Pamfil, D.; Bellini, C. Auxin control in the formation of adventitious roots. Not. Bot. Horti. Agrobot. Cluj-Napoca 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef]
- Park, S.H.; Elhiti, M.; Wang, H.; Xu, A.; Brown, D.; Wang, A. Adventitious root formation of in vitro peach shoots is regulated by auxin and ethylene. Sci. Hortic. 2017, 226, 250–260. [Google Scholar] [CrossRef]
- OuYang, F.; Wang, J.; Li, Y. Effects of cutting size and exogenous hormone treatment on rooting of shoot cuttings in Norway spruce [Picea abies (L.) Karst.]. N. For. 2015, 46, 91–105. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, D.; Han, Y.; Wu, Z.; Zhang, Z. Effects of Naphthylacetic acid on rootage of cutting twigs of the Hydrangea macrophylla. J. Zhongkai Univ. Agric. Eng. 2016, 29, 27–29. [Google Scholar]
- Su, G.; Cao, Y.; Li, C.; Yu, X.; Gao, X.; Tu, P.; Chai, X. Phytochemical and pharmacological progress on the genus Syringa. Chem. Cent. J. 2015, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Z.; Sun, Y.; Yang, B.; Wang, Q.; Kuang, H. Traditional uses, phytochemistry and pharmacology of genus Syringa: A comprehensive review. J. Ethnopharmacol. 2021, 266, 113465. [Google Scholar] [CrossRef] [PubMed]
- Peterken, G.F.; Lloyd, P.S. Ilex aquifolium L. J. Ecology. 1967, 55, 841–858. [Google Scholar] [CrossRef]
- Tsaktsira, M.; Chavale, E.; Kostas, S.; Pipinis, E.; Tsoulpha, P.; Hatzilazarou, S.; Ziogou, F.T.; Nianiou-Obeidat, I.; Iliev, I.; Economou, A.; et al. Vegetative Propagation and ISSR-Based Genetic Identification of Genotypes of Ilex aquifolium ‘Agrifoglio Commune’. Sustainability 2021, 13, 10345. [Google Scholar] [CrossRef]
- Matić, S.; Stanić, S.; Mihailović, M.; Bogojević, D. Cotinus coggygria Scop.: An overview of its chemical constituents, pharmacological and toxicological potential. Saudi J. Biol. Sci. 2016, 23, 452–461. [Google Scholar] [CrossRef]
- Antal, D.S.; Ardelean, F.; Jijie, R.; Pinzaru, I.; Soica, C.; Dehelean, C. Integrating Ethnobotany, Phytochemistry, and Pharmacology of Cotinus coggygria and Toxicodendron vernicifluum: What Predictions can be Made for the European Smoketree? Front. Pharmacol. 2021, 12, 746. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Pacholczak, A.; Ilczuk, A. Smoke tree (Cotinus coggygria Scop.) propagation and biotechnology: A mini-review. S. Afr. J. Bot. 2018, 114, 232–240. [Google Scholar] [CrossRef]
- Negahdar, N.; Hashemabadi, D.; Kaviani, B. An improved procedure for in vivo and in vitro propagation of Buxus hyrcana, an ornamental shrub critically at risk of extinction. BioTechnologia 2019, 100, 417–428. [Google Scholar] [CrossRef]
- Monder, M.J.; Pacholczak, A. Rhizogenesis and concentration of carbohydrates in cuttings harvested at different phenological stages of once-blooming rose shrubs and treated with rooting stimulants. Biol. Agric. Hortic. 2020, 36, 53–70. [Google Scholar] [CrossRef]
- Hamidon, A.; Shah, R.M.; Razali, R.M.; Lob, S. Effect of different types and concentration of rooting hormones on Momordica cochinensis (Gac Fruit) Root Vine Cuttings. Malays. Appl. Biol. 2020, 49, 127–132. [Google Scholar]
- Kashyap, U.; Chandel, A.; Sharma, D.; Bhardwaj, S.; Bhargava, B. Propagation of Jasminum parkeri: A Critically Endangered Wild Ornamental Woody Shrub from Western Himalaya. Agronomy 2021, 11, 331. [Google Scholar] [CrossRef]
- Ignatova, G.A. Use of growth promoter activators for rooting decorative cultures. Bull. Agrar. Sci. 2018, 3, 43–47. [Google Scholar] [CrossRef]
- Kentelky, E. The analysis of rooting and growth peculiarities of Juniperus species propagated by cuttings. Bull. UASVM Hortic. 2011, 68, 380–385. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, A.; Yadav, R.; Yadav, G. Role of different rooting media and auxins for rooting in floricultural crops: A review. Int. J. Chem. Stud. 2019, 7, 1778–1783. [Google Scholar]
- Monder, M.J.; Pacholczak, A. Rhizogenesis and contents of polyphenolic acids in cuttings of old rose cultivars treated with rooting stimulants. Acta Hortic. 2017, 1232, 99–104. [Google Scholar] [CrossRef]
- Zamir, R.; Rab, A.; Sajid, M.; Khattak, G.S.S.; Khalil, S.A.; Shah, S.T. Effect of Different Auxins on Rooting of Semi Hard and Soft Wood Cuttings of Guava (Psidium guajava L.) CV. Safeda. Nucleus 2017, 54, 46–51. [Google Scholar]
- Jeberean, M.G.; Bala, M.; Berar, C.; Tota, C.E.; Silivasan, M. Research on the influence of rooting stimulants at Parthenocissus quinquefolia in different crop conditions. Int. Multidiscip. Sci. GeoConference 2017, 7, 915–919. [Google Scholar] [CrossRef]
- Gil, C.S.; Jung, H.Y.; Lee, C.; Eom, S.H. Blue light and NAA treatment significantly improve rooting on single leaf-bud cutting of Chrysanthemum via upregulated rooting-related genes. Sci. Hortic. 2020, 274, 109650. [Google Scholar] [CrossRef]
- Tsaktsira, M.; Alevropoulos, A.; Tsoulpha, P.; Scaltsoyiannes, V.; Scaltsoyiannes, A.; Iliev, I. Inter-and intra-genetic variation on rooting ability of Ilex aquifolium L. Varieties and cultivars. Propag. Ornam. Plants 2018, 18, 131–138. [Google Scholar]
- Swarts, A.; Matsiliza-Mlathi, B.; Kleynhans, R. Rooting and survival of Lobostemon fruticosus (L) H. Buek stem cuttings as affected by season, media and cutting position. S. Afr. J. Bot. 2018, 119, 80–85. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, J.P.; Sonkar, M.K.; Mishra, Y.; Shirin, F. Relationship of Season and Cuttings’ Diameter with Rooting Ability of Culm-Branch Cuttings in Bambusa tulda and Bambusa nutans. J. Plant Growth Regul. 2021, 1–8. [Google Scholar] [CrossRef]
- Koroch, A.; Juliani, H.R.; Kapteyn, J.; Simon, J.E. In vitro regeneration of Echinacea purpurea from leaf explants. PCTOC 2002, 69, 79–83. [Google Scholar] [CrossRef]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious root formation: New insights and perspectives. Annu. Plant Rev. 2009, 37, 127–156. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, J.; Tan, Q.; Zhao, M.; Zhou, T.; Cao, F. The effects of exogenous hormones on rooting process and the activities of key enzymes of Malus hupehensis stem cuttings. PLoS ONE 2017, 12, e0172320. [Google Scholar] [CrossRef][Green Version]
- Punetha, P.; Rawat, T.; Bohra, M.; Trivedi, H. Effects of various concentrations of GA3 and NAA on cuttings of hydrangea under shade net conditions. J. Hill Agric. 2018, 9, 260–264. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Topacoglu, O.; Sevik, H.; Guney, K.; Unal, C.; Akkuzu, E.; Sivacioglu, A. Effect of rooting hormones on the rooting capability of Ficus benjamina L. cuttings. Šumarski List 2016, 140, 39–44. [Google Scholar] [CrossRef]
- Asănică, A.; Tudor, V.; Sumedrea, D.; Teodorescu, R.I.; Peticilă, A.; Iacob, A. The propagation of two red and black currant varieties by hardwood cuttings combining substrate and rooting stimulators. Sci. Papers Series B Hortic. 2017, 11, 175–181. [Google Scholar]
- Celik, H.; Cakir, S.; Celik, D.; Altun, B. Effects of leaf size and IBA on rooting and root quality of Japanese privet, rock cotoneaster and ornamental pomegranate mini-cuttings. Acta Hortic. 2018, 1263, 253–260. [Google Scholar] [CrossRef]
- Wulandari, R.; Hasanah, Y.; Meiriani, M. Growth Response of Two Pepper (Piper nigrum L.) Stem Cuttings on Application of IBA (Indole Butyric Acid) and NAA (Naphthalene Acetic Acid). Indones. J. Agric. Res. 2018, 1, 87–95. [Google Scholar] [CrossRef]
- Ada, R.; Angela, C.; Enrico, R.; Cristina, B.; Camillo, B. The weak cytokinins N, N′-bis-(1-naphthyl) urea and N, N′-bis-(2-naphthyl) urea may enhance rooting in apple and mung bean. PCTOC 2005, 83, 179–186. [Google Scholar] [CrossRef]
- Trofimuk, L.P.; Kirillov, P.S.; Egorov, A.A. Application of biostimulants for vegetative propagation of endangered Abies gracilis. J. For. Res. 2020, 31, 1195–1199. [Google Scholar] [CrossRef]
- Tikendra, L.; Amom, T.; Nongdam, P. Effect of phytohormones on rapid In vitro propagation of Dendrobium thyrsiflorum Rchb. f.: An endangered medicinal orchid. Pharmacogn. Mag. 2018, 14, 495. [Google Scholar] [CrossRef]
- Wang, F.; Xin, X.; Wei, H.; Qiu, X.; Liu, B. In vitro regeneration, ex vitro rooting and foliar stoma studies of Pseudostellaria heterophylla (Miq.) Pax. Agronomy 2020, 10, 949. [Google Scholar] [CrossRef]
- Mirani, A.A.; Abul-Soad, A.A.; Markhand, G.S. In vitro rooting of Dendrobium nobile Orchid: Multiple responses to auxin combinations. Not. Sci. Biol. 2017, 9, 84–88. [Google Scholar] [CrossRef][Green Version]
- Koyama, R.; Aparecido Ribeiro Júnior, W.; Mariani Zeffa, D.; Tadeu Faria, R.; Mitsuharu Saito, H.; Simões Azeredo Gonçalves, L.; Ruffo Roberto, S. Association of indolebutyric acid with Azospirillum brasilense in the rooting of herbaceous blueberry cuttings. Horticulturae 2019, 5, 68. [Google Scholar] [CrossRef]
- Badawy, E.M.; El-Attar, A.B.; El-Khateeb, A.M.A. Effect of collection dates and auxins sources on rooting and growth of Ligustrum ovalifolium Hassk cuttings. Plant Arch. 2020, 20, 9199–9210. [Google Scholar]
- Wang, Z.; Xu, J.; Li, H.; Yu, C.; Yin, Y. Rooting capabilities for Taxodium ’Zhongshanshan’ 302, 118, and 405. J. Zhejiang Univ. 2015, 32, 648–654. [Google Scholar]
- Kishore, G.R. Effect of type of cuttings and concentration of NAA on the rooting performance of jasmine (Jasminum humile). HRS 2016, 5, 86–87. [Google Scholar]
- Salmi, M.S.; Hesami, M. Time of collection, cutting ages, auxin types and concentrations influence rooting Ficus religiosa L. stem cuttings. J. Appl. Environ. Biol. Sci. 2016, 6, 124–132. [Google Scholar]
- Patil, Y.B.; Saralch, H.S.; Chauhan, S.K.; Dhillon, G.P.S. Effect of Growth Hormone (IBA and NAA) on Rooting and Sprouting Behaviour of Gmelina arborea (Roxb.). Indian For. 2017, 143, 81–85. [Google Scholar]
- Mishra, J.P.; Bhadrawale, D.; Rana, P.K.; Mishra, Y. Evaluation of cutting diameter and hormones for clonal propagation of Bambusa balcooa Roxb. J. For. Res. 2019, 24, 320–324. [Google Scholar] [CrossRef]
- Tien, L.; Chac, L.; Oanh, L.; Ly, P.; Sau, H.; Hung, N.; Thanh, V.; Doudkin, R.; Thinh, B. Effect of auxins (IAA, IBA and NAA) on clonal propagation of Solanum procumbens stem cuttings. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 113–120. [Google Scholar]
- Seiar, Y.A. Effect of Growth Regulators on Rooting of Cuttings in Pomegranate (Punica granatum L.) Cv. ‘Bhagwa’. J. Hortic. Sci. 2017, 11, 156–160. [Google Scholar]
- Hasan, A.M.; Mohamed Ali, T.J.; Al-Taey, D.K. Effects of winter foliar fertilizing and plant growth promoters on element and carbohydrate contents on the shoot of navel orange sapling. Int. J. Fruit Sci. 2020, 20, 682–691. [Google Scholar] [CrossRef]
- Hasan, A.M.; Al-Falahy, T.H.; Al-Taey, D.K. The effect of cutting diameter and storage method on the rooting and growth of pomegranate cuttings (Salimi and Rawa cultivars). Int. J. Agricult. Stat. Sci. 2020, 16, 1457–1463. [Google Scholar]
- Benbya, A.; Cherkaoui, S.; Gaboun, F.; Chlyah, O.; Delporte, F.; Alaoui, M.M. Clonal propagation of Argania spinosa (L.) skeels: Effects of leaf retention, substrate and cutting diameter. Adv. Hortic. Sci. 2021, 35, 61–72. [Google Scholar] [CrossRef]
- Kabanova, S.A.; Musoni, W.; Zenkova, Z.N.; Danchenko, M.A.; Scott, S.A.; Kabanov, A.N. Selection of Scots pine seedling growth stimulants in extreme conditions of the Northern Kazakhstan steppe zone. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 611, p. 012039. [Google Scholar] [CrossRef]
- Ghosh, A.; Dey, K.; Mani, A.; Bauri, F.K.; Mishra, D.K. Efficacy of different levels of IBA and NAA on rooting of Phalsa (Grewia asiatica L.) cuttings. Int. J. Chem. Stud. 2017, 5, 567–571. [Google Scholar]
- Kumar, S.; Muraleedharan, A.; Kamalakannan, S.; Sudhagar, R.; Sanjeevkumar, K. Effect of rooting hormone on rooting and survival of Nerium (Nerium odorum L.) var. Pink single. Plant Arch. 2020, 20, 3017–3019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kentelky, E.; Jucan, D.; Cantor, M.; Szekely-Varga, Z. Efficacy of Different Concentrations of NAA on Selected Ornamental Woody Shrubs Cuttings. Horticulturae 2021, 7, 464. https://doi.org/10.3390/horticulturae7110464
Kentelky E, Jucan D, Cantor M, Szekely-Varga Z. Efficacy of Different Concentrations of NAA on Selected Ornamental Woody Shrubs Cuttings. Horticulturae. 2021; 7(11):464. https://doi.org/10.3390/horticulturae7110464
Chicago/Turabian StyleKentelky, Endre, Denisa Jucan, Maria Cantor, and Zsolt Szekely-Varga. 2021. "Efficacy of Different Concentrations of NAA on Selected Ornamental Woody Shrubs Cuttings" Horticulturae 7, no. 11: 464. https://doi.org/10.3390/horticulturae7110464
APA StyleKentelky, E., Jucan, D., Cantor, M., & Szekely-Varga, Z. (2021). Efficacy of Different Concentrations of NAA on Selected Ornamental Woody Shrubs Cuttings. Horticulturae, 7(11), 464. https://doi.org/10.3390/horticulturae7110464







