Abstract
This research investigated the effects of continuous elevated CO₂ (20%, (v/v)) application or a 3 day CO₂ pretreatment followed by modified atmosphere (MA) or micro-perforated (MP) packaging on the postharvest quality of asparagus. The combination of CO₂ pretreatment with MA packaging (Pre-MA) inhibited the yellowing of asparagus and fresh weight loss (FWL), whereas stem firmness slightly increased with all elevated CO₂ treatments. CO₂ pretreatments increased antioxidant activity in the stem, but not in the tip, in contrast to the continuous flow CO₂ (Flow-CO₂) treatment. The phenolic and flavonoid contents increased in the elevated CO₂ pretreatments and Flow-CO₂ treatment. The elevated CO₂ treatments, especially Flow-CO₂, inhibited the development of microorganisms, and the treated asparagus did not decay. Pre-MA and Flow-CO₂ treatments were more effective in maintaining the visual quality and retarding the off-odor of asparagus. Furthermore, significant correlations between sensory quality characteristics and physiological-biochemical attributes were recognized; three principal components were extracted and they explained 86.4% of asparagus characteristics. The results confirmed the importance of visual quality, off-odor, firmness, color parameters, SSC and total phenolic content. In conclusion, elevated CO₂ pretreatment followed by MA packaging (Pre-MA) was beneficial for extending asparagus cold storage shelf life, and Flow-CO₂ was the best treatment for inhibiting postharvest decay.
1. Introduction
In recent years, the consumption of green asparagus (Asparagus officinalis L.) has been increasing due to its good eating quality, special flavor, and abundance of nutritional value. Unfortunately, rapid quality deterioration, including toughening, off-odor development, shriveling, and fresh weight loss due to higher respiration [1] and metabolic activities occurs. The rapid postharvest loss of green asparagus quality poses a challenge to the development of effective methods to retard the decline of quality and prolong shelf life. The application of modified atmosphere packaging (MAP) at low temperature as an effective technology has been reported to be beneficial for extending the shelf life and maintaining the quality of vegetables and fruits by reducing respiration rate and fresh weight loss, delaying ripening and minimizing physiological disorders and decays [2,3]. In lotus (Nelumbo nucifera), MAP treatments delayed the browning of roots, which involves changes in phenols [4]. The ambient gas levels in postharvest storage are important as they relate to respiration rate and physiological changes [5]. Prestorage or continuous elevated CO₂ treatments have been used for many fruits and vegetables to improve quality, inhibit the development of microbial groups and prolong shelf life. Short-term application of high CO₂ maintained the solids content and firmness of white asparagus spears, which is related to change of metabolic activity and respiration rate [6]. In grapes (Vitis vinifera L.), high CO₂ (20 kPa) pretreatment improved the appearance of bunches and maintained berry quality [7], reduced total decay and induced the accumulation of three small heat shock proteins (HSPs) [8]. Nutrients such as soluble sugar, soluble protein, and free amino acid content were also increased by elevated CO₂ in kidney beans (Phaseolus vulgaris) [9]. Treatment with 20% CO₂ prolonged the strawberry (Fragaria X ananassa Duch.) storage period to 12 days, reduced the energy charge related to the decline of NADH/NAD⁺ and caused the accumulation of γ-aminobutyric acid (GABA) [10]. Fungal decay was prevented, and no fermentation was observed with 20% CO₂ pretreatment for two days of fresh goji (Lycium spp.) berries [11]. High CO₂ pretreatment has also been used for white asparagus, which confirmed that the effects of higher CO₂ (10%) on biochemical and textural properties were determined by the storage temperature [6]. In a previous study, we reported that vacuum packages supplemented with 60% (v/v) CO₂ induced a higher soluble solids content, lower firmness, greener color, and less weight loss, but more off-odor [12]. Considering the low risk and high benefit of high CO₂ treatment in many fruits and vegetables, the use of prestorage or continuous treatments with elevated CO₂ has been proposed as a suitable tool to control the development of microorganisms and maintain green asparagus quality. However, few studies have been carried out on the effect of elevated CO₂ treatment on green asparagus postharvest quality and changes in phytochemical component and antioxidant activity.
This work aimed to analyze the effectiveness of continuous 20% CO₂ treatment and CO₂ pretreatment followed by MAP in controlling the development of microbial groups and its effect on quality attributes, antioxidant ability, and physiological-biochemical characteristics during green asparagus storage period at 4 °C.
2. Materials and Methods
2.1. Plant Materials, CO₂ Treatments, Packaging Material and Storage Conditions
Green asparagus (Asparagus officinalis L. cv. ‘Welcome’) was cultivated and provided by a local farm (Yanggu-gun, Gangwon-do, Korea, lat. 38°12’33.00” N, long. 127°13’3.00” E) in May 2019. Green asparagus spears were harvested and transported to the Postharvest Physiology and Distribution Laboratory, Kangwon National University, on the same day and stored in a 4 °C refrigerator. Healthy and uniform (20–30 g/spear, 1.4 ± 0.1 cm diameter and 24.0 ± 1.0 cm length) green asparagus spears were selected and randomized for elevated CO₂ treatments in this study.
A passive modified atmosphere (MA) package with a 10,000 cc/m²∙day⁻¹∙atm⁻¹ oxygen transmission rate (OTR) (Dae Ryung Precision Packaging Industry Co., Ltd., Gwangju-si, Korea) and a micro-perforated (MP, 34 holes on 10,000 cc/m²∙day⁻¹∙atm⁻¹ MA films perforated by drilling with 0.6 mm diameter tips) package were selected to package asparagus spears. A 20% (v/v) carbon dioxide (CO₂, released from dry ice) treatment was applied in a sealed plastic box (30 × 21 × 15 cm) An air pump (DK-3000, Dae-Kwang Electronics Co., Ltd, Korea) was used to control the CO₂ concentration and CO₂ concentration was measured every 3 hours.
Initially, selected asparagus spears were randomly divided into two groups: pretreated with 20% CO₂ for 3 days and 20% CO₂ continuous application until the last day of cold storage (20 days). The 20% CO₂ pretreatment group was further divided into two groups: packaged with MA (Pre-MA) and MP (Pre-MP) packages. Spears treated with continuous 20% CO₂ (Flow-CO₂) were stored in a sealed plastic box (30–21–15 cm). Untreated groups packaged with MA (Cont-MA) and MP (Cont-MP) packages were used as the controls. All of the groups were stored at 4 ± 0.5 °C and 85 ± 5% relative humidity (RH) for 20 days. Five replicates of 20 green asparagus spears each were used for each packaging group.
2.2. Microbiology Analysis
Microbiological analysis was performed on initial samples (Initial day), after 3 days of pretreatment (Treated day 3), and after 20 days of storage according to Wang [12] with some modifications. Fresh asparagus (2.0 g) was mixed with 18 mL sterilized distilled water using a stomacher blender (Powermixer, B&F Korea, Gimpo-si, Korea) set at the highest speed (level 10, 200 rpm) for 3 min. Then, the mixture was diluted by a factor of 1000. Next, 1.0 mL of the dilution was dropped on a microbiology Petri film plate (3 M Co., St Paul, MN, USA). Aerobic bacteria, yeast and mold, and Escherichia coli were cultivated for 72 h at 35 °C, 72 h at 25 °C, and 24 h at 35 °C, respectively. The development of total aerobic bacteria (TAB), yeast and mold (Y&M), and E. coli were measured using Petrifilm Plate Reader (3M Co., St. Paul, MN, USA). The number of microorganisms was represented by the base 10-logarithm of the colony-forming unit concentration (log CFU·g⁻¹).
2.3. Changes in Quality Parameters of Green Asparagus
Firmness (N) was measured at two locations on each asparagus spear, at 5 cm from the tip and 8 cm from the base using a rheometer (Compac-100, Sun Scientific Co. Led.,Tokyo, Japan) with a probe (Ø 3.0 mm) at 1.0 mm/sec speed.
Green asparagus color variables were measured using a color-difference meter (Model CR-400, Konica Minolta Sensing, Inc., Japan) at 5 cm from the tip and at 8 cm from the stem for lightness (L*), Chroma (C*), redness a*, blueness b* and hue angle (h°). The total color difference was represented as ΔE*, and calculated as:
where L, a and b were the color parameters of fresh asparagus spears without treatment (Initial day).
The total chlorophyll content was measured following Yoon [13] with slight modifications. Frozen asparagus samples (1.0 g) were chopped and mixed in 10 mL methanol and then incubated at 4 °C for 48 h to extract chlorophyll. The absorbances at 642.5 nm (A642.5) and 660 nm (A660) were measured using a UV–VIS spectrophotometer (UV mini model 1240, Shimadzu, Japan). The total chlorophyll content was calculated using the following formula:
Soluble solids content (SSC) was measured by a pocket refractometer (PAL-1, Atago, Tokyo, Japan). Asparagus samples were chopped up and extruded with gauze wrapping. The asparagus solution was directly dripped onto a pocket refractometer and the SSC result was indicated as °Brix at ambient temperature [13].
Fresh weight loss was measured according to the following formula:
Fresh asparagus was weighed and put in an oven (OF-21E, Jeio Tech Co., Ltd., Daejeon, Korea) for 10 min at 103 °C, dried to constant weight at 72 °C, and re-weighed. The water content of asparagus spears on the final day of storage at 4 °C was determined as follows:
Sensory qualities, including visual quality and off-odor of green asparagus, were assessed by five skilled members from the “Postharvest Physiology and Distribution Laboratory” [13]. The visual quality of the asparagus was assessed throughout the entire storage period and scored on a scale of 1 to 5 (1 = worst: yellowing, decay, shrinking, woodiness, bract opening, 2 = bad, 3 = good, 4 = better, 5 = best: an at-harvest appearance, no decay or defects, dark green, no shrinking or bract opening). Off-odor was assessed after 20 days of storage and scored on a scale of 0 to 5 (0 = no off-odor and 5 = strong off-odor). Asparagus with a visual quality score equal to or greater than 3 and an off-odor score equal to or less than 3.0 was determined to be marketable.
2.4. The Effect of High CO₂ on Phenol, Flavonoids and DPPH of Green Asparagus
Asparagus (2.0 g) was homogenized in 1% HCl-methanol (v/v) solution, the homogenate was placed in a 25 mL tube with distilled water and incubated for 20 min in the dark, and then it was filtered with 110 mm filter paper. The absorbance of the solution was measured at 280 nm and 325 nm [14]. The contents of total phenol and flavonoids were calculated as follows:
Antioxidant activity was measured by the DPPH (α,α-diphenyl-β-picrylhydrazyl) method [15]. Green asparagus samples (2.0 g) were ground at 4 °C in 20 mL of methanol and then filtered with 110 mm filter paper, 1.0 mL of the filtered solution was mixed with 1.0 mL of a 0.4 mM DPPH in methanol solution, and the mixture was incubated in darkness for 30 min before measuring the absorbance at 516 nm.
2.5. Gas Conditions and Respiration Rate
By inserting a needle into the packages through a septum, 1.0 mL gas samples from the headspace of packages were collected. Carbon dioxide (CO₂) concentration in different treatments was measured with an infrared CO₂/O₂ analyzer (Model Check Mate 9900, PBI-Dansensor, Ringsted, Denmark). Ethylene (C₂H₄) concentration was measured with a GC-2010 Shimadzu gas chromatograph (GC-2010, Shimadzu Corporation, Japan) [12], equipped with BP 20 Wax column (30 m × 0.25 mm × 0.25 um, SGE analytical science, Australia) and a flame ionization detector (FID). The detector and injector operated at 127 °C, the oven was at 50 °C, and the carrier gas (N₂) flow rate was 0.67 mL·s⁻¹. CO₂ and C₂H₄ concentration changes in MA and MP packages were determined on the first and third days of cold storage and subsequently were measured every 5 days up to 20 days of storage at 4 °C. The change in CO₂ concentration of pretreated, continuous and control groups was measured every 1 hour at ambient temperature and determined in five duplicate packages. The respiration rate was expressed as mL CO₂ kg⁻¹ h⁻¹.
2.6. Statistical Data Analysis
The software Microsoft Excel 2019, R program (Version 4.0.2, 2020-06) and IBM SPSS Statistics (24, IBM Corp., Armonk, NY, USA) was used to statistically analyze the data. The effects of elevated CO₂, MA/MP packaging, and CO₂ × package interaction were analyzed using two-way ANOVA. All results are expressed as the means (n = 5) and their standard error (SE). Significant differences were tested with ANOVA (one-way analysis of variance) and Duncan’s Multiple Range Test at α < 0.05. All experiments were performed with at least three independent repetitions and repeated three times. Principal component analysis (PCA) scores and correlation coefficients between sensory quality and physiological and biochemical performance were analyzed using Rstudio version 4.0.2 (Team 2020), with distance measurements by Pearson’s correlation coefficient test.
3. Results
3.1. Changes in Quality Parameters of Green Asparagus
3.1.1. Fresh Weight Loss (FWL)
Fresh weight loss developed in all treatments, with the increase greater in MP packages than in MA packages during the entire storage period (Figure 1). Fresh weight loss of green asparagus was 2.10 ± 0.06% in Cont-MP and 1.55 ± 0.09% in Pre-MP, whereas in MA it was 0.58 ± 0.05% in Cont-MA and 0.48 ± 0.02% in Pre-MA, all of which significantly differed. Flow-CO₂ treatment showed the highest FWL of 2.30 ± 0.05%, which was probably due to the lack of a film package during cold storage. It is worth noting that although the weight loss of different treatments was significantly different, all treatments had weight loss below 2.5% and were very similar to each other. FWL was well below the limit of marketable acceptance of 8%. In addition, elevated CO₂ inhibited FWL with a lower loss than that of the control. Significant differences were found between Cont-MP and Pre-MP. Pre-MA had the lowest FWL after 20 days of storage at 4 °C. The inhibition of FWL by high CO₂ pretreatments was also observed with goji berries, with significantly lower weight loss than that of control fruit [11]. Previous studies have reported that water content is important for modifying the quality and storage period of fruits and vegetables [16,17].
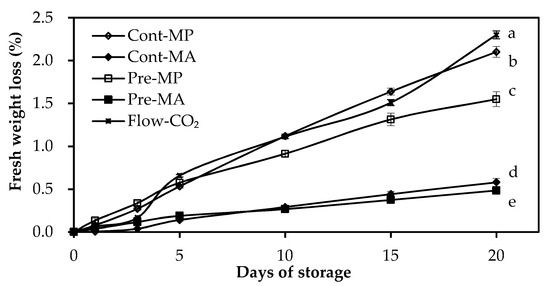
Figure 1.
Fresh weight loss (%) during green asparagus cold storage as influenced by elevated CO₂ treatments. Values are means of five repetitions ± standard errors (SE). Different letters over bars indicate significant differences between treatments by Duncan’s Multiple Range Test (α < 0.05).
3.1.2. Firmness
Compared to the initial samples, asparagus firmness increased after 20 days of storage (Figure 2A). Considering the controls and all CO₂ treatments, a notable increase in firmness was observed in the stem with the initial value of 12.3 N. Compared to the control, Pre-MP and Flow-CO₂ treatments significantly increased the firmness on the last storage day and showed the highest firmness stem (~19.3 N). A significant difference in asparagus stem firmness was observed with elevated CO₂ pretreatment. The increase in firmness was less in MA than MP packages. This suggested that Pre-MA was more efficient at inhibiting the development of firmness during postharvest storage. This was in keeping with the results that the asparagus spear water content was lower in the Flow-CO₂ than the control and CO₂ pretreatments (data not shown). A similar negative relationship between firmness and water content in carrots has been reported [18]. The increase in firmness in asparagus was correlated with an increase in crude fibers, the development of lignin and the activity of phenylalanine ammonia-lyase [19]. Likewise, our results revealed significant differences in firmness only in the stem between the elevated CO₂ treatments and the control. This result suggests that CO₂ treatment influenced the wound response from cutting damage. Verlinden [20] reported a higher respiration rate, higher moisture loss and enhanced CO₂ diffusion during the wound response in the asparagus stem, which may lead to higher firmness.
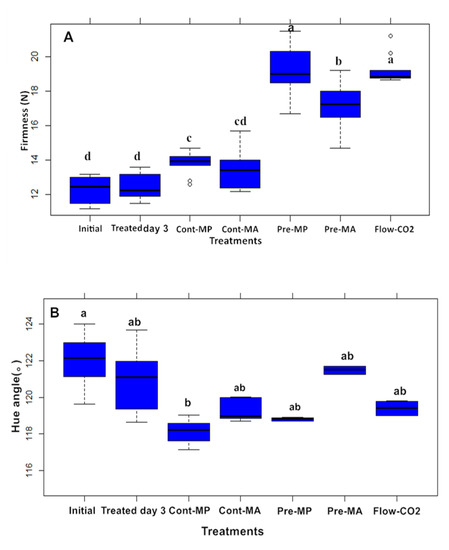
Figure 2.
Effects of elevated CO₂ treatments on (A) firmness and (B) hue angle in asparagus stem on the initial day, treated day 3 and after 20 days of storage at 4 °C. The different letters represent significant differences by Duncan’s Multiple Range Test (α < 0.05 level). Box plots consist of the median, the lowest and the highest values, the outliers, and the approximate quartiles of our parameters.
3.1.3. Color Parameters
Color is one of the most important factors for postharvest biology and sensory evaluation of green asparagus. Yellowing (i.e., lower hue angle) of green asparagus indicates degradation of quality during storage [21]. Yellowing was obvious and showed a trend between the initial day and after 20 days of storage (Figure 2B) with only Cont-MP lower than the Initial value. The deviation from the raw material total color was represented as ΔE* and the tip was significantly less than the other treatments at 20 days in the Flow-CO₂ treatment, and it did not differ from CO₂-treated day 3 (Table 1). For stems, the lowest color difference was observed for the Pre-MP treatment at 20 days, with a value of 5.19, which was less than the treated day 3 values.

Table 1.
The means of chlorophyll content and total color deviation (ΔE*) obtained from tip and stem of green asparagus in initial, treated day 3 and on the 20th day of storage at 4 °C.
The green color is mainly determined by chlorophyll content [22]. The chlorophyll content was consistent with the hue angle value in all treatments (Table 1). Initial day samples had the highest chlorophyll content and subsequently exhibited a progressive decrease to the last day of cold storage. Interestingly, lower chlorophyll content was observed in the controls at the start and after cold storage. There was a significant difference among all the treatments; the Pre-MA treatment showed the highest chlorophyll content, and the Cont-MP treatment had the lowest content. The breakdown of chlorophyll is closely related to the remobilization of chloroplast proteins, lipids and metals during senescence [23]. An inhibitory effect of elevated CO₂ on the decrease in chlorophyll content of broccoli florets after harvest has been reported [24]. High CO₂ treatment affected anthocyanin stability and color expression in strawberries by changing the pH, while MA packaging with 15% CO₂ treatment maintained the anthocyanin stability of strawberries [25]. In the current study, Pre-MA treatment evidenced greater inhibition of yellowing during green asparagus cold storage. The mechanism of elevated CO₂ and MA package effects may have been partially based on a decrease in chlorophyllase activity that delays the degradation of chlorophyll because of the decrease in metabolic activity. This is consistent with the results that high CO₂ delayed chlorophyll degradation and anthocyanin accumulation by inhibiting chlorophyllase activity and downregulating the expression of FaChl b reductase, FaPAO and FaRCCR, which are related to chlorophyll state in postharvest storage of strawberry fruits [26]. Likewise, cold shock delayed the degradation of chlorophyll by suppressing the activity of chlorophyllase in cucumber [27].
3.1.4. Soluble Solids Content (SSC)
Total soluble solids content (SSC) are mainly sugars, which tended to decrease towards the end of shelf life in all of the samples [28]. A low soluble solids content was observed in all treatments and significantly differed among treatments (Table 2). On treated day 3, SSC had decreased to 5.58 °Brix compared to the initial day samples at 6.19 °Brix. The decrease was probably related to respiration, which is the key factor in converting soluble solids into energy [29]. There was no significant difference between the control (5.30 °Brix), Flow-CO₂ (5.23 °Brix) and Pre-MA (5.03 °Brix) treatments after 20 days of storage. However, the lowest SSC was in the Pre-MP treatment, with a content of 4.37 °Brix on the last day of cold storage. Flow-CO₂ treatment without film packages slowed the decrease in the SSC. Li and Zhang [30] reported that the water loss of green asparagus also contributed to an increasing soluble solids content, which is consistent with the water content of asparagus in this study (data not shown). A similar result was reported with grapes, in which high CO₂ treatment inhibited the decrease in the SSC due to an inhibition of normal postharvest metabolic activity [31].

Table 2.
Effects of elevated CO₂ treatments on green asparagus soluble solids content (SSC), total phenolic and flavonoid contents, and DPPH-radical scavenging activity, on the initial day, treated day 3 and after 20 days of storage at 4 °C.
3.2. The Effect of High CO₂ on Phenol, Flavonoids and DPPH of Green Asparagus
Phenolic compounds such as flavonoids and anthocyanin are important naturally occurring antioxidant compounds from many plants. The phenolic content could be influenced by biotic and abiotic factors [32,33]. Moreover, the antioxidant capacity of phenolic compounds is related to the ability to scavenge DPPH radicals in an assay [34]. In this study, total phenol and flavonoids content and DPPH radical scavenging activity were measured on the initial day, treated day 3 and after 20 days of storage (Table 2). The total phenolic and flavonoid content in the initial day samples were 0.72 U·g⁻¹ and 0.68 U·g⁻¹, respectively. Total phenolic and flavonoid content increased in the CO₂ pretreatments on treated day 3, which showed the highest absorbance values of 0.82 U·g⁻¹ and 0.91 U·g⁻¹, respectively. At 20 days, the levels of total phenolics under elevated CO₂ pretreatments had decreased compared to treated day 3 but increased compared to the initial day. The lowest total phenolic and flavonoid contents were observed in the Cont-MP treatment, with values of 0.67 U·g⁻¹ and 0.58 U·g⁻¹, respectively. The difference between the control groups and all elevated CO₂ treatments was pronounced, although no difference was found between elevated CO₂ pretreatments and Flow-CO₂ treatment in total phenolics but not in flavonoids. Although elevated CO₂ treatments slowed the loss of phenolic components, a decrease occurred after longer storage in all elevated CO₂ treatments and control groups which is similar to changes in strawberry [35].
Postharvest CO₂ enrichment stimulated antioxidant activities and phenolic content in lettuce [36]. The beneficial effect may be because elevated CO₂ treatment is a kind of abiotic stress promoting the synthesis and accumulation of phenolic as a physiological response. Previously, CO₂ storage had marked effects on phenolic metabolites and quality, while modified atmospheres (MA) had a positive effect on phenolic-related quality [37]. High CO₂ may allow for the removal of free radicals, which are associated with an increase of antioxidant capacity [38]. The DPPH radical scavenging activity of green asparagus showed a similar change in pattern as the phenolic and flavonoid contents in response to the different treatments (Table 2). The highest DPPH value of 92.67 was a significant increase after CO₂ pretreatment. Flow-CO₂ treatment sustained the antioxidant activity through 20 days, with a DPPH value of 91.18. Based on these points, the Flow-CO₂ treatment was noted for improving asparagus qualities and maintaining the higher bioactive compound levels for long-term postharvest storage.
3.3. Gas Conditions in Packages and Respiration Rate
MA and MP packages involve the process of changing the atmospheric gas composition surrounding the green asparagus, especially carbon dioxide and oxygen concentration, by controlling gas exchange between the inside of the packages and the ambient air. The gas levels exhibited different concentration changes between MA packages and MP packages caused by their difference in oxygen transmission rates (OTRs). As shown in Figure 3A, the CO₂ concentration increased rapidly on the first day of cold storage in all packages and subsequently remained stable until a small decrease at 15 days of storage. MA packages showed higher CO₂ concentrations than MP packages throughout the whole storage period, 4–6% versus 0–2%, respectively. A CO₂ level above 10% and O₂ level below 3% can produce injuries to green asparagus at the optimal temperature [39,40]. Compared to the gas composition in MP packages, a better gas composition was observed in MA packages. The recommended gas concentration for green asparagus storage at 5 °C is 5–10% O₂, similar to ambient composition [41], and a CO₂ level of 4–6% probably decreases the respiration rate and inhibits anaerobic metabolism. The concentration of ethylene in all packages first increased (Figure 3B), and was followed by a decrease, finally becoming stable. It is possible that this was because elevated CO₂ reduced the respiration rate and inhibited the biosynthesis and negative effects of ethylene.
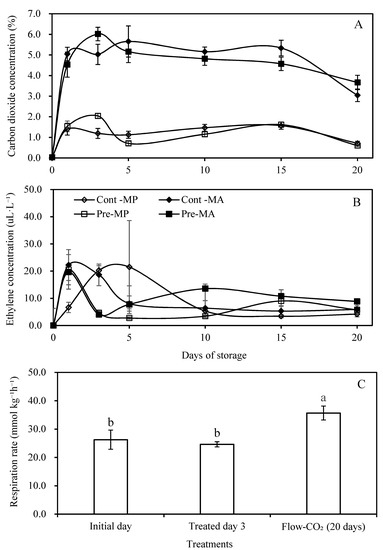
Figure 3.
Changes in carbon dioxide (A) and ethylene (B) concentrations in modified atmosphere (MA) and micro-perforated (MP) packages during the postharvest storage, and the respiration rate (C) of green asparagus as influenced by high CO₂ pre and continuous treatments. Values are means of five repetitions ± standard errors (SE). Different letters over bars indicate significant differences among all treatments by Duncan’s Multiple Range Test (α < 0.05).
The respiration rate measured on the initial day was 26.26 mL kg⁻¹ h⁻¹ CO₂, while elevated CO₂ pretreatment showed a value of 24.62 mL kg⁻¹h⁻¹ CO₂ (Figure 3C). Comparing the respiration rate of the Flow-CO₂ treatment on the final day (35.67 mL kg⁻¹ h⁻¹ CO₂) to the initial value, the significant difference could be ascribed to the long period of storage and the influence of elevated CO₂ since the respiration rate increased. Asparagus is classified as perishable due to a high respiration rate of 60 mg kg⁻¹ h⁻¹ CO₂ (at 5 ℃) and rapid degradation of quality [5,42].
3.4. Microorganism Analysis
Compared to the initial value of 3.26 log CFU·g-1, the growth of total aerobic bacteria (TAB) was inhibited by elevated CO₂ pretreatment with 2.10 log CFU·g⁻¹ (Table 3). Bacterial growth is critical in the spoilage of green asparagus, as significant differences in aerobic bacterial count between the initial and final storage days occurred. After 20 days of storage, TABs significantly increased, especially in Cont-MP, which had the highest count of 5.76 log CFU·g⁻¹. Likewise, regardless of the packages, there was no significant difference in TABs between the CO₂ pretreatments and control groups. However, Flow-CO₂ with a TAB count of 2.16 log CFU·g⁻¹ was not significantly different from the initial or treated day 3 samples, which indicated that the Flow-CO₂ treatment controlled the growth of these microorganisms and maintained lower counts up to the 20th day at 4 °C. The initial E. coli counts of green asparagus were 2.71 log CFU·g⁻¹, and the counts increased through 20 days of storage in all treatments. Cont-MP and Cont-MA reached 4.31 and 4.20 log CFU·g⁻¹, respectively. Treated day 3 did not show a sterilization effect on E. coli with 3.50 log CFU·g⁻¹. However, a significant effect of elevated CO₂ pretreatments on E. coli was observed with lower counts after 20 days of storage at 4 °C than at treated day 3 (Table 3). Likewise, 3.01 log CFU·g⁻¹ was observed under the Flow-CO₂ treatment on the last day of storage (20 days). The initial yeast and mold (Y&M) count before and after elevated CO₂ pretreatment was 0.00 log CFU·g⁻¹ (Table 3). In contrast, Y&M increased significantly in the Cont-MP treatment on the last day of cold storage, with 2.10 log CFU·g⁻¹. Notably, all elevated CO₂ treatments showed an encouraging lack of Y&M with counts of 0.00 log CFU·g⁻¹. This was consistent with the result that high CO₂ was beneficial in controlling microbial growth in fresh-cut mango (Mangifera indica) cubes [43]. According to a previous study, short-term exposure to high levels of CO₂ modified the pathogenesis-related protein defense response and the low molecular mass chitinase, which renders grapes less susceptible to fungal infection [44]. It has also shown that elevated CO₂ retards the growth of microorganisms, which is lethal to spores and damages the cell wall of the microorganisms [45]. Likewise, the development of microorganisms inhibited by high CO₂ is probably due to its dissolution in the aqueous phase of food products, which causes intracellular acidification, inhibition of enzymatically catalyzed reactions and enzyme synthesis, and interaction with the cell membrane [46].

Table 3.
Effects of elevated CO₂ pretreatments in MA and MP packages and continuous CO₂ treatments on the growth of microorganisms in green asparagus stored at 4 °C up to 20 days.
3.5. Sensory Quality and Relative Impacts of Asparagus Quality Attributes
On the last storage day, asparagus quality characteristics were assessed. Figure 4 illustrates the relationships among important quality traits. Visual quality, off-odor, fresh weight loss, and stem firmness (F-Stem) were affected significantly by elevated CO₂ and different packages. In contrast, tip firmness (F-Tip), hue angle in the tip (h°-Tip) and stem (h°-Stem), soluble solids content (SSC), and water content were not affected by elevated CO₂ and different packaging treatments. Visual quality and off-odor are major factors that determine consumption. p-values in this study demonstrated that elevated CO₂ treatments and different packages had significant effects on nearly every quality characteristic (Table 4). The elevated CO₂ main effect, package main effect and CO₂ × package interaction significantly affected fresh weight loss of green asparagus whereas no effect (p ≥ 0.05) was observed for water content. However, the elevated CO₂ main effect and interaction of CO₂ × package effects did affect tip hue angle (h°-Tip), and tip firmness (F-Tip).
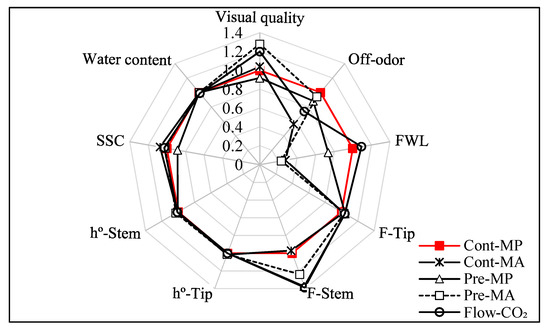
Figure 4.
Spider graphs illustrating the relative impacts of elevated CO₂ treatments followed by MP or MA packaging on green asparagus quality characteristics after 20 days of storage at 4 ℃. FWL: fresh weight loss; F-Tip: Tip firmness; F-Stem: Stem firmness; h°-Tip: Tip hue angle; h°- Stem: Stem hue angle; SSC: soluble solids content

Table 4.
Analysis of variance results for the effects of elevated CO₂ × packaging (MP or MA) interaction green asparagus quality attributes after 20 days of storage at 4 ℃.
Visual quality was estimated according to the extent of shrinkage, water loss, yellowing, bract opening, and fungal attack. In the present study, marketable visual quality was found in the Pre-MA and Flow-CO₂ treatments with scores of 3.2 and 3.0, respectively. Regarding the packages, the control groups lost retail value, and there was no significant difference between the control and Pre-MP treatments. The off-odor scores in the Cont-MA and Flow-CO₂ treatments were low and maintained commercial acceptability (off-odor score below 3.0), with scores of 1.9 and 2.5, respectively, after 20 days of storage. Regardless of packages, elevated CO₂ pretreatments lost marketable value with a strong off-odor. The accumulation of odor in green asparagus is caused by methanol and acetaldehyde, which are related to anaerobic respiration, microorganism decay and secondary metabolism [47,48]. Modified atmosphere (MA) packages reduced the production of off-odor by modifying the gas composition of packages and maintaining the adopted CO₂ (5–12%) content [5]. This is consistent with the growth of microorganisms in this study. Considering the better visual quality and slight off-odor, the notable efficiency of Flow-CO₂ treatment on asparagus quality and shelf life was confirmed in this study.
3.6. Correlations and Principal Components Analysis
The correlations between sensory quality characteristics and physiological and biochemical attributes after 20 days of storage at 4 °C are shown in Figure 5. A significant positive association between visual quality and soluble solids content (SSC), firmness (F), hue angle (h°), chlorophyll content (Chl), and DPPH in the tip (DPPH-Tip). Negative correlations between visual quality and off-odor, water content (WC), total phenolic content in stem (TP-Stem), total flavonoid content (Fla), and DPPH in the stem (DPPH-Stem) occurred. Likewise, the off-odor showed negative correlations with SSC, h°, Chl, and Fla. This was consistent with the performance of green asparagus in different treatments, i.e., the greener the color, the higher the soluble solids content and chlorophyll, the better the visual quality value and the less the off-odor. Another interesting negative correlation between firmness and WC and SSC was observed. A change in firmness is related to the change of cell structure caused by the transformation of SSC and storage sugars in cell walls [49]. The changes in cell structure affect the transpiration of plants which relates to water content and fresh weight loss [27]. In addition, negative correlations were observed between FWL and h° and Chl. Another important finding was the association between sensory quality and physiological-biochemical characteristics between tips and stems of asparagus, which could be related to the at-harvest wounding of the stem (the relations between the DPPH and TP).
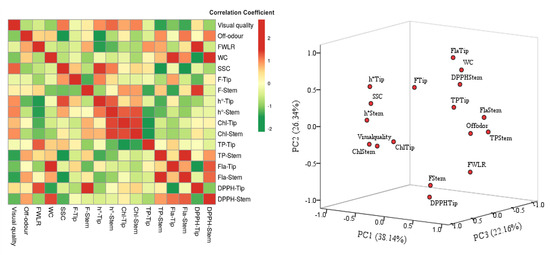
Figure 5.
Pearson’s correlation coefficient heatmap matrices and principal component analysis (PCA) among the responses of sensory, physiological quality attributes, and biochemical components in asparagus stored at 4 °C for 20 days of shelf life. FWL: fresh weight loss, WC: water content, SSC: soluble solids content, F-Tip: firmness in the tip, F-Stem: firmness in the stem, h°-Tip: Hue angle in the tip, h°-Stem: Hue angle in the stem, Chl-Tip: chlorophyll content in the tip, Chl-Tip: chlorophyll content in the stem, TP-tip: total phenolic content in the tip, TP-tip: total phenolic content in the stem, Fla-Tip: total flavonoid content in the tip, Fla-Tip: total flavonoid content in the stem, DPPH-Tip: DPPH radical scavenging activity in the tip, DPPH-Tip: DPPH radical scavenging activity in the stem.
The sensory quality of green asparagus was evaluated according to color, smell, decay, shrinkage, bract crack and others [50]. The association between sensory quality and the physiological situation was consistent with the sensory evaluation. These results are in agreement with Lu [51] who showed significant positive correlations between overall quality and firmness, SSC, TP, and negative correlations to the odor of ‘Akihime’ strawberry. Subsequently, principal component analysis (PCA) was performed and the results showed that the first three components (PC1, PC2, PC3) explained a total of 86.64% of the parameters of asparagus characteristic (Figure 5). The PC1, PC2, PC3 explained 38.14%, 26.34%, 22.16% of the variance, respectively. The visual quality, off-odor, TP-stem, Fla-Stem SSC, h° were clustered into the positive/negative of PC1, and the PC2 included FWL, DPPH, WC, Fla-Tip, F-Tip. Only F-Stem, TP-Tip, Chl-Tip variance belonged in PC3. The results confirmed the importance of visual quality, off-odor, firmness, color parameters, SSC and total phenolic content.
4. Conclusions
This study investigated the effects of elevated CO₂ pretreatment followed by modified atmosphere (Pre-MA) or micro-perforated (Pre-MP) packaging and continuous elevated CO₂ (Flow-CO₂) treatment on the quality characteristics of green asparagus during storage at 4 °C. The development of microbial groups was inhibited by elevated CO₂ treatments, especially the Flow-CO₂ treatment, which showed the lowest counts of microorganisms. Although higher firmness was observed, elevated CO₂ pretreatments and Flow-CO₂ treatment resulted in better sensory quality, strong inhibition of microorganisms, high content of the nutritional and antioxidant activity, a higher content of SSC, phenolics, chlorophyll, and DPPH, and lower respiration rates. Furthermore, the results from the heatmap coefficient analysis and principal component analysis indicated that sensory quality, physiological attributes, and antioxidant activity responded differently depending on the specific treatments. The elevated CO₂ treatments caused changes in physiological attributes and content of biochemical components that determined the sensory qualities of green asparagus. Overall, Flow-CO₂ or Pre-MA treatments had a significant effect on the qualities of green asparagus and could be useful for green asparagus cold storage.
Author Contributions
Conceptualization and methodology, L.-X.W., I.-L.C., and H.-M.K.; Experiments performance and data curation, L.-X.W.; writing, L.-X.W.; writing—review and editing, H.-M.K. All authors read and agreed to the published version of the manuscript.
Funding
This study was supported by the IPET through Export Promotion Technology Development Program, funding from the Ministry of Agriculture, Food and Rural Affairs (Nos 117035-03).
Acknowledgments
The authors would like to thank lab members for their assistance.
Conflicts of Interest
The author declares no conflict of interest.
References
- Mangaraj, S.; Goswami, T.K. Modified atmosphere packaging of fruits and vegetables for extending shelf-life-A review. Fresh Prod. 2009, 3, 1–31. [Google Scholar]
- Wang, J.W.; Jiang, Y.G.; Li, G.D.; Lv, M.; Zhou, X.; Zhou, Q.; Fu, W.W.; Zhang, L.; Chen, Y.F.; Ji, S.J. Effect of low temperature storage on energy and lipid metabolisms accompanying peel browning of ‘Nanguo’ pears during shelf life. Postharvest Biol. Technol. 2018, 139, 75–81. [Google Scholar] [CrossRef]
- Yoon, H.S.; Choi, I.L.; Han, S.J.; Kim, J.Y.; Kang, H.M. Effects of Precooling and Packaging Methods on Quality of Asparagus Spears during Simulated Distribution. Prot. Hortic. Plant Fact. 2018, 27, 7–12. [Google Scholar] [CrossRef]
- Liu, E.C.; Niu, L.F.; Yi, Y.; Wang, L.M.; Ai, Y.W.; Zhao, Y.; Wang, H.X.; Min, T. Expression Analysis of ERFs during Storage under Modified Atmosphere Packaging (High-concentration of CO2) of Fresh-cut Lotus Root. Hortscience 2020, 55, 216–223. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; University of California, Agriculture and Natural Resources: Davis, CA, USA, 2002. [Google Scholar]
- Huyskens-Keil, S.; Herppich, W.B. High CO2 effects on postharvest biochemical and textural properties of white asparagus (Asparagus officinalis L.) spears. Postharvest Biol. Technol. 2013, 75, 45–53. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Romero, I.; Jimenez, J.B.; Orea, J.M.; Gonzalez-Urena, A.; Escribano, M.I.; Merodio, C. Involvement of the phenylpropanoid pathway in the response of table grapes to low temperature and high CO₂ levels. Postharvest Biol. Technol. 2007, 46, 29–35. [Google Scholar] [CrossRef]
- Romero, I.; Casillas-Gonzalez, A.C.; Carrazana-Villalba, S.J.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Impact of high CO2 levels on heat shock proteins during postharvest storage of table grapes at low temperature. Functional in vitro characterization of VVIHSP18.1. Postharvest Biol. Technol. 2018, 145, 108–116. [Google Scholar] [CrossRef]
- Qian, L.; He, S.Q.; Liu, X.W.; Huang, Z.J.; Chen, F.J.; Gui, F.R. Effect of elevated CO2 on the interaction between invasive thrips, Frankliniella occidentalis, and its host kidney bean, Phaseolus Vulgaris. Pest Manag. Sci. 2018, 74, 2773–2782. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Xiao, G.N.; Limwachiranon, J.; Xu, Y.J.; Lu, H.Y.; Yang, D.M.; Luo, Z.S. Effects of elevated CO2 on energy metabolism and gamma-aminobutyric acid shunt pathway in postharvest strawberry fruit. Food Chem. 2018, 265, 281–289. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Christopoulos, M.V.; Tsantili, E. Short-term treatments with high CO2 and low O2 concentrations on quality of fresh goji berries (Lycium barbarum L.) during cold storage. J. Sci. Food Agric. 2017, 97, 5194–5201. [Google Scholar] [CrossRef]
- Wang, L.X.; Choi, I.L.; Lee, J.H.; Kang, H.M. The Effect of High CO2 Treatment and MA Packaging on Asparagus Quality and Shelf Life during Cold Storage. J. Agric. Life Environ. Sci. 2019, 31, 41–49. [Google Scholar]
- Yoon, H.S.; Choi, I.L.; Kang, H.M. Different Oxygen Transmission Rate Packing Films during Modified Atmosphere Storage: Effects on Asparagus Spear Quality. Hortic. Sci. Technol. 2017, 35, 314–322. [Google Scholar]
- Cao, J.K.; Jiang, W.B.; Zhan, Y.M. Experiment Guidance of Postharvest Physiology and Biochemistry of Fruits and Vegetables, 1st ed.; China Light Industry Press: Beijing, China, 2007. [Google Scholar]
- Oboh, G. Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. Lwt-Food Sci. Technol. 2005, 38, 513–517. [Google Scholar] [CrossRef]
- Sharma, H.K.; Kaur, J.; Sarkar, B.C.; Singh, C.; Singh, B.; Shitandi, A.A. Optimization of Pretreatment Conditions of Carrots to Maximize Juice Recovery by Response Surface Methodology. J. Eng. Sci. Technol. 2006, 1, 158–165. [Google Scholar]
- Rashidi, M.; Bahri, M.H.; Abbassi, S. Effects of relative humidity, coating methods and storage periods on some qualitative characteristics of carrot during cold storage. Am.-Eurasian J. Agric. Environ. Sci. 2009, 5, 359–367. [Google Scholar]
- Rashidi, M.; Gholami, M. Prediction of carrot firmness based on water content and total soluble solids of carrot. Afr. J. Agric. Res. 2011, 6, 1831–1834. [Google Scholar]
- An, J.S.; Zhang, M.; Wang, S.J.; Tang, J.M. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. Lwt-Food Sci. Technol. 2008, 41, 1100–1107. [Google Scholar] [CrossRef]
- Verlinden, S.; Silva, S.M.; Herner, R.C.; Beaudry, R.M. Time-dependent Changes in the Longitudinal Sugar and Respiratory Profiles of Asparagus Spears During Storage at 0 °C. J. Am. Soc. Hortic. Sci. 2014, 139, 339–348. [Google Scholar] [CrossRef]
- Kaewsuksaeng, S.; Urano, Y.; Aiamlaor, S.; Shigyo, M.; Yamauchi, N. Effect of UV-B irradiation on chlorophyll-degrading enzyme activities and postharvest quality in stored lime (Citrus latifolia Tan.) fruit. Postharvest Biol. Technol. 2011, 61, 124–130. [Google Scholar] [CrossRef]
- Zhao, S.S.; Yang, Z.; Zhang, L.; Luo, N.; Li, X. Effect of combined static magnetic field and cold water shock treatment on the physicochemical properties of cucumbers. J. Food Eng. 2018, 217, 24–33. [Google Scholar] [CrossRef]
- Christ, B.; Hortensteiner, S. Mechanism and Significance of Chlorophyll Breakdown. J. Plant Growth Regul. 2014, 33, 4–20. [Google Scholar] [CrossRef]
- Yamauchi, N.; Watada, A.E. Chlorophyll and xanthophyll changes in broccoli florets stored under elevated CO2 or ethylene-containing atmosphere. HortScience 1998, 33, 114–117. [Google Scholar] [CrossRef]
- Nakata, Y.; Izumi, H. Microbiological and Quality Responses of Strawberry Fruit to High CO2 Controlled Atmosphere and Modified Atmosphere Storage. Hortscience 2020, 55, 386–391. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.C.; Li, L.; Aghdam, M.S.; Wei, X.X.; Liu, J.Q.; Xu, Y.Q.; Luo, Z.S. Elevated CO2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chem. 2019, 285, 163–170. [Google Scholar] [CrossRef]
- Zhao, S.S.; Yang, Z.; Zhang, L.; Luo, N.; Wang, C. Effects of different direction of temperature jump treatment on cucumbers. J. Food Process Eng. 2018, 41, e12600. [Google Scholar] [CrossRef]
- Zurawicz, A.; Krzesinski, W.; Knaflewski, M. Changes in Soluble Solid Content in Green Asparagus Spears during Harvest Season. Acta Hortic. 2008, 776, 435–444. [Google Scholar] [CrossRef]
- Nourian, F.; Ramaswamy, H.S.; Kushalappa, A.C. Kinetics of quality change associated with potatoes stored at different temperatures. Lwt-Food Sci. Technol. 2003, 36, 49–65. [Google Scholar] [CrossRef]
- Li, T.H.; Zhang, M. Effects of modified atmosphere package (MAP) with a silicon gum film window on the quality of stored green asparagus (Asparagus officinalis L) spears. Lwt-Food Sci. Technol. 2015, 60, 1046–1053. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Jimenez, J.B.; Romero, I.; Orea, J.M.; Maldonado, R.; Urena, A.G.; Escribano, M.I.; Merodio, C. Effect of high CO2 pretreatment on quality, fungal decay and molecular regulation of stilbene phytoalexin biosynthesis in stored table grapes. Postharvest Biol. Technol. 2006, 42, 209–216. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R.; Stampar, F.; Solar, A. Phenolic response in green walnut husk after the infection with bacteria Xanthomonas arboricola pv. juglandis. Physiol. Mol. Plant Pathol. 2011, 76, 159–165. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Hodges, D.M. Abiotic Stress in harvested fruits and vegetables. In Abiotic Stress in Plants-Mechanisms and Adaptations; Shanker, A., Venkateswarlu, B., Eds.; IntechOpen: London, UK, 2011; p. 442. [Google Scholar]
- Kogure, K.; Goto, S.; Nishimura, M.; Yasumoto, M.; Abe, K.; Ohiwa, C.; Sassa, H.; Kusumi, T.; Terada, H. Mechanism of potent antiperoxidative effect of capsaicin. Biochim. Biophys. Acta 2002, 1573, 84–92. [Google Scholar] [CrossRef]
- Chandra, D.; Lee, J.S.; Hong, Y.P.; Park, M.H.; Choi, A.J.; Kim, J.G. Short-term application of CO2 gas: Effects on physicochemical, microbial, and sensory qualities of “Charlotte” strawberry during storage. J. Food Saf. 2019, 39, e12597. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Micaelli, F.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A.; Quartacci, M.F. Elevated CO2 and salinity are responsible for phenolics-enrichment in two differently pigmented lettuces. Plant Physiol. Biochem. 2017, 115, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bunce, J.A.; Maas, J.L. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agric. Food Chem. 2003, 51, 4315–4320. [Google Scholar] [CrossRef]
- Gariepy, Y.; Raghavan, G.S.V.; Castaigne, F.; Arul, J.; WillemotI, C. Precooling and Modified Atmosphere Storage of Green Asparagus. J. Food Process. Preserv. 1991, 15, 215–224. [Google Scholar] [CrossRef]
- Simón, A.; Gonzalez-Fandos, E. Influence of modified atmosphere packaging and storage temperature on the sensory and microbiological quality of fresh peeled white asparagus. Food Control 2011, 22, 369–374. [Google Scholar] [CrossRef]
- Bartz, J.A.; Brecht, J.K. Postharvest Physiology and Pathology of Vegetables; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Lipton, W.J. Postharvest Biology of Fresh Asparagus. In Horticultural Reviews; Janick, J., Ed.; JohnWiley and Sons: Hoboken, NJ, USA, 2011; pp. 69–155. [Google Scholar]
- Jutatip, P.; Hidemi, I. Shelf Life and Microbial Quality of Fresh-cut Mango Cubes Stored in High CO2 Atmospheres. J. Food Sci. 2005, 70, 6. [Google Scholar]
- Vazquez-Hernandez, M.; Navarro, S.; Sanchez-Ballesta, M.T.; Merodio, C.; Escribano, M.I. Short-term high CO2 treatment reduces water loss and decay by modulating defense proteins and organic osmolytes in Cardinal table grape after cold storage and shelf-life. Sci. Hortic. 2018, 234, 27–35. [Google Scholar] [CrossRef]
- Rao, L.; Bi, X.F.; Zhao, F.; Wu, J.H.; Hu, X.S.; Liao, X.J. Effect of high-pressure CO2 processing on bacterial spores. Crit. Rev. Food Sci. Nutr. 2016, 56, 1808–1825. [Google Scholar] [CrossRef]
- Mohn, G. Modified atmospheres. In The Microbiological Safety and Quality of Foods; Lund, B.M., Baird-Parker, A.C., Gould, G.W., Eds.; Springer Science & Business Media: Gaithersburg, MD, USA; Aspen, CO, USA, 2000; pp. 214–234. [Google Scholar]
- Tudela, J.A.; Marin, A.; Garrido, Y.; Cantwell, M.; Medina-Martinez, M.S.; Gil, M.I. Off-odor development in modified atmosphere packaged baby spinach is an unresolved problem. Postharvest Biol. Technol. 2013, 75, 75–85. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, Y.Y.; Zhang, J.; Liu, W.; Guan, W.Q.; Wang, Z.D. Effects of high CO2 in-package treatment on flavor, quality and antioxidant activity of button mushroom (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2017, 123, 112–118. [Google Scholar] [CrossRef]
- Herppich, W.B.; Huyskens-Keil, S. Cell wall biochemistry and biomechanics of harvested white asparagus shoots as affected by temperature. Ann. Appl. Biol. 2008, 152, 377–388. [Google Scholar] [CrossRef]
- Herppich, W.B.; Huyskens-Keil, S.; Hassenberg, K. Impact of Ethanol Treatment on the Chemical Properties of Cell Walls and Their Influence on Toughness of White Asparagus (Asparagus officinalis L.) Spears. Food Bioprocess Technol. 2015, 8, 1476–1484. [Google Scholar] [CrossRef]
- Lu, H.Y.; Wang, K.D.; Wang, L.; Li, D.; Yan, J.W.; Ban, Z.J.; Luo, Z.S.; Li, L.; Yang, D.M. Effect of superatmospheric oxygen exposure on strawberry (Fragaria x ananassa Fuch.) volatiles, sensory and chemical attributes. Postharvest Biol. Technol. 2018, 142, 60–71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
