Abstract
Brassica oleracea is an important vegetable species which belongs to the genus Brassica and the mustard family Brassicaceae Burnett. Strong heterosis in B. oleracea is displayed in yield, quality, disease resistance, and stress tolerance. Heterosis breeding is the main way to improve B. oleracea varieties. Male sterile mutants play an important role in the utilization of heterosis and the study of development and regulation in plant reproduction. In this paper, advances in the research and application of male sterility in B. oleracea were reviewed, including aspects of the genetics, cytological characteristics, discovery of genes related to male sterility, and application of male sterility in B. oleracea. Moreover, the main existing problems and prospect of male sterility application in B. oleracea were addressed and a new hybrids’ production strategy with recessive genic male sterility is introduced.
1. Introduction
Brassica oleracea L. is an important vegetable, fodder, and ornamental diploid (2n = 18) species which belongs to the genus Brassica and mustard family Brassicaceae Burnett. B. oleracea probably originates from the Western Mediterranean region, Great Britain and Northern-Central China. According to the habit of the plant and its edible parts, B. oleracea can be divided into seven different varieties or cultivars: Capitata Group (cabbage, savoy cabbage, red cabbage; B. oleracea var. capitata), Acephala Group (kale, borecole, collards; B. oleracea var. acephala) and Tronchuda Group (Portuguese cabbage, seakale cabbage), Italica Group (purple sprouting, sprouting broccoli; B. oleracea var. italica), Botrytis Group (broccoli, cauliflower, broccoflower, calabrese; B. oleracea var. botrytis), Gongylodes Group (kohlrabi, knol-kohl; B. oleracea var. gongylodes), Gemmifera Group (sprouts, Brussels sprouts; B. oleracea var. gemmifera), and Alboglabra Group (Chinese kale, Chinese broccoli, gai lan, kai lan; B. oleracea var. alboglabra) [1,2]. B. oleracea vegetables are rich in nutrients [3], have strong adaptability and resistance to stress environments, and are widely cultivated all over the world. According to the Food and Agriculture Organization (FAO) statistics, the global cabbage, and other brassicas harvest area in 2018 was 2.41 million hm2 (http://faostat.fao.org/), and cabbage is one of the main vegetables consumed in Europe, North and South America, Asia, and Oceania.
B. oleracea vegetables are typically cross-pollinated crops. Across B. oleracea strong heterosis is displayed on yield, quality, disease resistance, and stress tolerance [4]. Heterosis breeding is the main way of improving B. oleracea varieties. At present, most B. oleracea varieties used in agriculture are hybrids. In heterosis breeding, self-incompatible and male sterile lines can be used to produce B. oleracea hybrid seeds. Self-incompatibility genes are ubiquitous across B. oleracea. When using self-incompatible lines to produce hybrids, the hybrid seeds can be obtained from both parents, so seed yield is high [5]. Before the 21st century, B. oleracea hybrids were mainly produced using self-incompatible lines, such as Jingfeng No.1, the first cabbage hybrid in China. However, this method has some shortcomings. For example, it is difficult to attain a 100% hybridization rate. Inbred lines will degenerate after multiple generations of selfing events and propagating inbred lines through artificial pollination during the bud stage is costly [5].
Male sterility refers to the degeneration or loss of function of male organs in bisexual plants. When male sterile lines are used to produce hybrids, the hybridization rate can reach 100%. Moreover, the maintenance of male sterile lines can be self-compatible lines that are propagated by bees, which could save labor and reduce the overall production cost [5]. Due to these reasons, scientists have always placed great importance on male sterility in B. oleracea. In order to provide a reference for and facilitate future study and application of male sterility in B. oleracea crop breeding, this review summarizes the main advances to date regarding the male sterility issue in B. oleracea. The main problems that presently exist for breeding with male sterility and the prospect for application of male sterility in B. oleracea are also addressed.
2. Types and Genetic Characteristics of Male Sterility in B. oleracea
Ever since the German botanist Joseph Gottlieb Kolreuter first reported the male sterility in plants back in 1763, the male-sterile phenomenon has been found across 43 families, 162 genera, and 320 species of plants [6,7]. According to the genetic characteristics of male sterile genes, male sterility can be divided into genic male sterility (GMS) and cytoplasmic male sterility (CMS). GMS is controlled by nuclear genes and CMS is controlled by mitochondrial genes and a smaller subset of nuclear genes [8].
2.1. Genic Male Sterility (GMS)
GMS in B. oleracea includes mainly dominant genic male sterility (DGMS) and recessive genic male sterility (RGMS). At present, most GMS in B. oleracea crops were found to be controlled by recessive nuclear genes. For example, 83121A is a spontaneous male sterile mutant in cabbage. Genetic analysis showed that male sterility was controlled by a recessive gene in cabbage. The 83121A line exhibits normal vegetative development, has fully open flowers, well-developed nectaries, and normal pistils. However, the anthers of 83121A are severely degraded and have no pollen grains [9,10]. RGMS does not have a typical maintenance line, and at most, only 50% of male sterile plants can be obtained from test cross progeny.
The cabbage breeding group of the Chinese Academy of Agricultural Sciences (CAAS) discovered the dominant male sterile plant DGMS79-399-3 in the 1970s. Genetic analysis showed that male sterility of DGMS79-399-3 was controlled by a dominant gene and was affected by other small-effect genes [11]. A few sensitive DGMS plants produced traces of pollen induced at low temperatures. After selfing with the trace pollen, the ratio of male sterile plants to fertile plants in the progeny was 3:1, from which dominant homozygous sterile plants could be obtained. DGMS lines have beneficial economic characteristics, large flowers, and well-developed nectaries [5]. The DGMS has been successfully applied in the production of cabbage hybrids.
2.2. Cytoplasmic Male Sterility (CMS)
Most CMS in B. oleracea was transferred from other cruciferous crops (e.g., Raphanus sativus L. and B. napus L.). The main types of CMS include Ogura (Ogu) CMS, Polima (Pol) CMS, and Nigra (Nig) CMS.
2.2.1. Ogu CMS
Ogu CMS is a completely infertile, naturally mutated type of CMS found in radish (Raphanus raphanistrum subsp. sativus L.) [12]. So far, Ogu CMS is the most widely studied and widely used type of male sterility in cruciferous vegetable breeding. This sterility type is induced by the interaction of a homozygous nuclear gene rfogrfog and a sterile Ogu cytoplasm. Ogu CMS in radish was originally transferred to cabbage by distant hybridization and embryo rescue in order to obtain Ogu CMS R1 [13]. Because the nucleus and cytoplasm are not coordinated, the nectaries and pistils of Ogu CMS R1 do not develop normally, and the leaves of Ogu CMS R1 are yellow at low temperatures. Using asymmetric protoplast fusion technology, radish chloroplasts in Ogu CMS R1 were successfully replaced with broccoli chloroplasts to obtain Ogu CMS R2, which do not turn yellow at low temperatures, but its siliques are deformed and its nectaries degenerate after multiple generations of backcrossing [14]. Following Ogu CMS R2, the company U.S. Asgrow reorganized the mitochondria of Ogu CMS R2, again, through asymmetric protoplast fusion technology and obtained Ogu CMS R3, which has stable sterility, does not turn yellow at low temperatures, and has well-developed siliques [15]. Similarly, the well-known Brassica Ogu-INRA CMS was also obtained by the plant somatic fusion method [16]. These male sterile lines derived from Ogu CMS R3 or Ogu-INRA CMS have been widely used for the production of cabbage hybrids.
2.2.2. Pol CMS
Pol CMS was discovered in a homonymous rapeseed (B. napus L.) variety bred in Poland [17]. Its male sterility was controlled by both cytoplasmic and nuclear genes. Concerning the temperature dependence of the male sterility, Pol CMS lines can be divided into three types: low-temperature, high-temperature, and stable CMS lines [18,19,20,21]. It is easy to find Pol CMS fertility-restored materials in B. napus, B. campestris, and B. juncea. Both two-line and three-line schemes are used to produce the F1 hybrids based on the Pol CMS [21]. The Pol CMS was transferred from B. napus to B. oleracea by using the protoplast fusion method [20]. However, the obtained male sterile plants showed abnormal development of flowers and siliques, as well as incomplete pollen abortion, which meant that the male sterile type could not be used for breeding.
2.2.3. Nig CMS
The F1 of B. nigra (wild mustard) × B. oleracea (broccoli) was treated with colchicine to double the number of chromosomes and was then repeatedly backcrossed with cabbage to obtain Nig CMS [22]. The Nig CMS fertility-restored materials could be found in cabbage and kale. However, most Nig CMS flowers do not open normally and its nectaries degenerate markedly. Moreover, the proportion of male sterile plants in the test cross progeny was only 33.7–60.0%, which means that the male sterile type could not be used for breeding.
3. Cytological Study of Male Sterility in B. oleracea
The main goal of plant male sterility cytology research is to determine the abortion period and abortion mode of microspores, as well as to explore the factors leading to microspore abortion from a cell morphology perspective. A large number of studies have shown that microspore abortion can occur at any stage of microspore development, including from the archesporial cell stage to the mature pollen stage [6]. The peak period of microspore abortion occurs from the tetrad stage to the unicellular stage [6].
The main male sterile stages and characteristics for the four types of male sterility in B. oleracea were analyzed in detail by paraffin section, scanning electron microscopy, and transmission electron microscopy techniques. Microspore abortion of RGMS 83121A occurred at the early unicellular stage [10]. The tapetum of 83121A was strikingly degraded at the uninucleate stage. The development of microspores in 83121A stopped at the uninucleate stage and was followed by breakdown. Moreover, microspores of 83121A did not form pollen exine after being released from the tetrad [10]. DGMS abortion occurred at the late tetrad stage. The important characteristic of DGMS was the abnormal development of the tapetum. Moreover, the pollen mother cell primary wall surrounding the developing microspores in DGMS remained intact until the very late pollen stage [23,24]. Nigra CMS abortion occurred at the sporogenesis cell stage with abnormal tapetal cell differentiation and development [24]. Ogu CMS abortion often occurred at the early tetrad stage, and its abnormal activities of tapetal cells were observed after meiosis. Most of the Ogu CMS microspores were released from tetrads and then were aborted after being squashed by hypertrophic tapetum cells [24].
In conclusion, although the abortion period and abortion characteristics of the various male sterility types in B. oleracea are different, almost all of them show abnormal development of the tapetum. The tapetum is the innermost cell of the anther wall. It transports various nutrients to the pollen mother cell and plays a key role in the development of the pollen mother cells and microspores [25,26]. Many studies have shown that abnormalities in the differentiation and development of tapetum cells can directly or indirectly lead to pollen abortion and male sterility [27].
4. Molecular Biological Study of Male Sterility in B. oleracea
4.1. Expression Analysis of Male Sterile Related Genes
The expression of DGMS related genes in cabbage was studied at the transcriptional level using the cDNA-AFLP differential display method. The results showed that the expression of a dominant male sterile gene (Ms-cd1) may hinder the normal release of microspores in tetrads and inhibit the expression of genes encoding pectin methylesterase, pectase lyase, thioredoxin, rapid alkalinization factor, and proline-rich protein [23]. Using an Arabidopsis whole-genome microarray, the genome-wide gene expression profiles during anther abortion of four types of B. oleracea male sterility (Nig CMS, RGMS, Ogu CMS, and DGMS) were comprehensively analyzed. In total, 105 candidate genes specifically expressed in the tapetum were identified [24]. Moreover, it was shown that the main reason for the designation of four types of male sterility was the disturbance of the abnormal tapetum during normal development of the microspores. Label-free quantitative mass spectrometry was used to analyze the differential protein levels of RGMS 83121A and its wild-type buds before the microspore binuclei stage. A total of 1245 protein types were identified to have significant differential abundances [28]. The identified proteins were mainly involved in pollen wall synthesis, fatty acid metabolism, amino acid synthesis, and protein processing modification, suggesting that these metabolic pathways play an important role in cabbage reproductive development.
4.2. Molecular Markers Associated with Male Sterility
Many studies have reported the molecular markers associated with dominant genic male sterile gene Ms-cd1 in cabbage [29,30]. For example, the SSR (Simple Sequence Repeats) and SRAP (Sequence-related Amplified Polymorphism) markers linked to Ms-cd1 were obtained by bulk segregant analysis. The genetic distance between SSR marker 8C0909 and Ms-cd1 was found to be 2.06 cM [30]. Three SRAP markers, ENA14F-CoEm7RSC, ENA20R-rem2SC, and CoEm17RE37SC, were converted into SCAR (Sequence Characterized Amplified Region) markers, and the genetic distances between the SCAR markers and Ms-cd1 were 0.18, 0.39, and 4.23 cM, respectively [30]. A KASP (Kompetitive Allele Specific PCR) molecular marker closely linked to DGMS was developed from resequencing data and Ms-cd1 gene mapping [31]. This marker can be used for rapid identification of the dominant male sterile gene locus.
Based on comparative genomic and transcriptomic analysis, BoCYP704B1 was identified as an important candidate gene linked to RGMS in the 83121A line [10]. Cloning and sequencing showed that a 5424-bp Ty3-gypsy type retrotransposon was inserted in the first exon of BoCYP704B1 in 83121A. The retrotransposon insertion in BoCYP704B1 not only blocked gene expression, but also changed the structure of the encoded protein. Molecular markers completely linked to the male sterile gene in 83121A were developed from the mutation of BoCYP704B1 [10]. Using map-based cloning technology, the RGMS gene ms3 of cabbage line 51S was fine mapped in a 187.5 kb region on chromosome C01, and BoTPD1 was identified as a candidate gene for male sterility [32]. It was found that a 182 bp fragment was inserted in the BoTPD1 gene of the male sterile mutant. The molecular marker designed according to this variant site is closely linked to male sterility and can be used for assisted screening of male sterile plants [32].
CMS in B. oleracea is typically regulated by mitochondrial-specific genes. For example, the male sterility of Ogu CMS and Pol CMS is controlled by the mitochondrial specific genes orf138 and orf224, respectively [33,34,35]. According to the sequence of orf138, several specific molecular markers were designed, which can be used for the identification of Ogu CMS plants [36,37,38,39].
4.3. The Mechanism of Ogu CMS
Ogu CMS has been widely used in the breeding of B. oleracea. Several studies have shown that the Ogu CMS was controlled by orf138 gene, which was generated by the rearrangement of the mitochondrial genome [40,41]. Previous studies of orf138 revealed that at least nine variants of orf138 designated as A, B, …, I were identified; these variants included the F type characterized by a 39-bp deletion [42]. This type was also called Kosena according to the name of a radish variety from which the Kos CMS line was obtained [43]. It is well known that the F type variant of orf138 was also discovered in white-headed cabbage. Studies have shown that the protein encoded by the orf138 gene would accumulate on the mitochondrial membrane, which may interfere with the expression of some key genes, such as atp6, atp8, and cox I, in the electron-transport chain and inhibit the normal development of anthers [44,45,46,47,48]. The whole mitochondrial genome sequencing showed that the mitochondrial genome of Ogura CMS type was highly rearranged compared with the normal-type genome [49]. Four unique regions were generated from the rearrangement in Ogu CMS mitochondrial genome, and most of the unique regions are composed of known Brassicaceae mitochondrial sequences [49]. The results suggested that the unique regions of Ogu CMS mitochondrial genome were produced by integration and shuffling of pre-existing mitochondrial sequences during the evolution of Brassicaceae, and novel genes such as orf138 may have been generated from the shuffling process of the mitochondrial genome [49]. The conjoint analysis of transcriptome and proteome suggested that the tapetum programmed cell death was disturbed and the synthesis of sporopollenin was inhibited in Ogu CMS cabbage [50].
4.4. The Fertility-Restored Gene Rfo of Ogu CMS
Studies have shown that the Ogu CMS fertility-restored materials only exist in R. sativus [51,52,53]. At present, the Ogu restorer materials have been found in European radish, Japanese radish, and Chinese radish. The restorer nuclear gene in the Ogu CMS restorer line can disturb the stability of the protein ORF138, which would reduce the accumulation of ORF138, leading to the restoration of fertility [47,54]. The restorer locus Rfo has been obtained by map-based cloning [55,56]. Studies showed that the Rfo locus contains three genes (PPR-A, PPR-B, and PPR-C) organized in tandem, which are predicted to encode highly similar proteins [57]. PPR-B was genetically defined as the restorer gene and is predicted to encode a pentatricopeptide repeat (PPR) protein comprising 17 PPR repeats. Compared with PPR-B, the protein encoded by PPR-A has a longer C-terminal tail and a deletion of four amino acids in the third PPR repeat. The PPR-C gene contains a 17-bp deletion compared with PPR-A and PPR-B, which leads to a frameshift and a premature stop codon about in the middle of the gene. Genetic transformation experiments further showed that only PPR-B instead of PPR-A and PPR-C can restore the fertility of Ogu CMS [57]. Koizuka et al. (2003) also cloned the fertility-restored gene orf687 (Rfk) of radish Kos CMS, which was identified as the same gene as Rfo [58]. In addition to Rfo, some new restorer genes have been found in R. sativus, and most of them are homologous genes of Rfo. For example, a novel fertility-restored gene Rft controlling fertility restoration of Ogu CMS was identified in Japanese wild radish [59]. In addition, a number of new homologous genes of Rfo were found in Chinese radish materials. These genes, including Rfob, Rfoc, RsRf3-1/RsRf3-2, RsRf3-4, and RsRf3-5, were mainly produced by recombination during hybridization [60,61,62,63,64].
4.5. Other Male Sterile Related Genes in B. oleracea
Male sterile plants can be used as a useful tool to study the expression patterns and biological functions of anther development-related genes [65]. Many genes associated with male sterility have been cloned, such as BoBHLH1, BoMF1, BoMF2, and BoMYB1. The BoBHLH1 gene is downregulated in Ogu CMS and encodes the bHLH transcription factor, which is homologous to Arabidopsis AtBHLH151 and is induced by jasmonic acid signaling. BoBHLH1 is preferentially expressed in cabbage anthers and has two expression peaks in the early and late stages of anther development [66]. The promoter of Ogu CMS related gene BoMF1 from B. oleracea was cloned by genomic walking. The promoter was able to drive the GUS gene that is exclusively expressed in anther and pollen of Arabidopsis thaliana [67]. BoMF2 encodes the AT-hook DNA binding protein and was found to be up-regulated in Ogu CMS. BoMF2 was mainly expressed in wild-type cabbage stamens during the tetrad stage. However, BoMF2 expression continued into the mature pollen stage of Ogu CMS flowers [68]. Arabidopsis with overexpression of BoMF2 showed significantly shorter siliques than the wild type, as well as a decrease in pollen viability [68]. BoMYB1 encodes a MYB transcription factor and was downregulated in Ogu CMS. This gene was preferentially expressed in cabbage anthers and reached its expression peak in the late stage of anther development. The expression of BoMYB1 was induced by the plant hormones salicylic acid and methyl jasmonate and regulated the expression of anther development genes [69,70].
5. Application of Male Sterility in B. oleracea
5.1. Application of DGMS
In order to develop DGMS lines, DGMS79-399-3 was used to backcross with excellent inbred lines for more than five generations [5,11,71]. Sensitive male sterile plants in the backcross progeny were able to produce trace pollen under low temperature induction. Then, sensitive male sterile plants were selfed to produce homologous male sterile plants, which were screened by test crosses and molecular markers [5,11]. The homozygous DGMS lines, which were propagated by tissue culture, crossed with a male fertile sister line. The seeds from the male sterile plants were DGMS lines which can be used to cross with a common inbred line to produce F1 hybrids (Figure 1A). To date, many excellent varieties of cabbage, have been bred using DGMS lines, such as Zhonggan 16, Zhonggan 17, Zhonggan 18, Zhonggan 19, and Zhonggan 21.
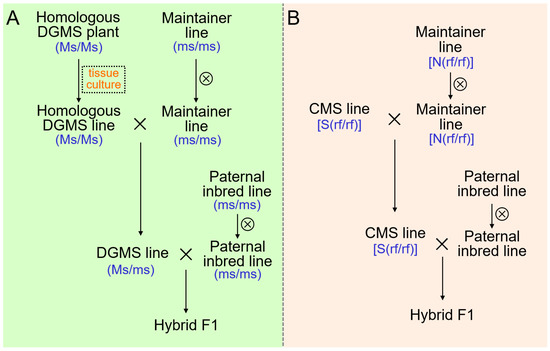
Figure 1.
Two strategies of hybrids’ production with male sterility in B. oleracea crops. (A) Hybrids production strategy with dominant genic male sterility (DGMS) (Ms, dominant male sterile gene; ms, male fertile gene that is the allele of Ms). (B) Hybrids production strategy with Ogura CMS (S, mitochondrial sterile gene; N, mitochondrial sterile gene; rf, recessive nuclear sterile gene of Ogura CMS). The symbol × represents hybridization and ⊗ represents selfing.
5.2. Application of Ogu CMS and Its Fertility-Restored Gene Rfo
Ogu CMS is the most widely used male sterile type in B. oleracea breeding (Figure 1B). The Ogu CMS source has been used as the female parent for backcrossing with excellent self-compatible lines for more than five generations, producing many Ogu CMS lines, such as CMS02-6, CMS87-534, and CMS8180 [5,38]. The first cabbage F1 cultivar using the Ogu-INRA CMS was registered in the official French seed catalog in 1993. In 1999, the French catalog listed 65 F1 cultivars of different cabbage types that have been produced using the Ogu-INRA CMS system [16]. Currently, many excellent varieties, such as Zhonggan 628, Zhonggan 56, Jinggan 4, Xiyuanqiufeng, Sugan 27, and Chunqiutingmei have been bred from Ogu CMS lines in China.
The Ogu CMS in B. oleracea is derived from radish. There is no Ogu CMS restorer gene in B. oleracea. All offspring produced by Ogu CMS lines are male sterile. Therefore, we cannot isolate new breeding materials from some excellent Ogu CMS germplasms by selfing. Development of Ogu CMS restorer lines is of great importance for the innovation and utilization of Ogu CMS germplasm in B.oleracea. Using distant hybridization and embryo rescue techniques, the Ogu CMS restorer gene in B. napus has been successfully introduced into Chinese broccoli. Through multi-generation backcrosses combined with marker screening, a Chinese broccoli Ogu CMS restorer line with a normal number of chromosomes was developed [72]. The fertility of Ogu CMS germplasm with resistance to clubroot was restored using the restorer line. Then, plants containing the clubroot resistance site CRb were acquired by selfing [73]. This work laid the foundation for the cultivation of clubroot-resistant varieties.
In addition to distant hybridization, we may also be able to introduce the Rfo gene into B. oleracea through genetic transformation. The transgenic and non-transgenic plants in the selfing or backcross offsprings of Ogu CMS materials with fertility restoration can be further distinguished by markers.
6. Perspectives
In recent years, important research progress has been made on the male sterility of B. oleracea, and a large number of new B. oleracea varieties have been bred using these male sterile lines. The breeding technology system of DGMS and Ogu CMS lines in B. oleracea has previously been established (Figure 1). However, there are some defects in DGMS and Ogu CMS lines that must be addressed. The low temperature sensitivity of DGMS is closely related to its genetic background [5]. Only the low temperature sensitive genotype can be transformed into the male sterile line, while DGMS plants lacking any low temperature sensitivity cannot be used. Except for the Chinese Academy of Agricultural Sciences, most B. oleracea breeding units around the world use Ogu CMS. Maternal genetic characteristics of the Ogu CMS may harbor certain negative effects. For example, the cytoplasm of the offspring of Ogu CMS lines is always consistent with the CMS source. The presence of a single cytoplasm raises the risk of resistance loss, such as the one that was observed during the corn spot disease pandemic caused by T-type sterile cytoplasm in the USA [74]. In order to solve these problems, new cytoplasmic sterile resources should be explored to induce cytoplasmic diversification. Additionally, new male sterile lines could also be created through genetic engineering.
The breeding of male sterile lines by conventional breeding methods requires a large time investment and is costly. This method cannot meet the needs of rapid agricultural development. Genetic engineering technology is highly efficient for the directional improvement of crops. So far, genetic engineering has been used to successfully create male sterility in a variety of plants. The hybrids of maize, rapeseed, and lettuce bred by these male sterile lines have been successfully commercialized [75]. A common method for creating male sterility by genetic engineering is to use the cytotoxic protein Barnase gene to destroy tapetum cells under the regulation of a tapetum-specific promoter, leading to microspore abortion. This method has been used to create male sterility in tobacco, cabbage, rapeseed, and lettuce [76,77,78,79,80]. Other methods, such as the introduction of genes that cause abnormal mitochondrial development and knockout of important genes associated with pollen development through gene editing, can also be used to create male sterility [81,82,83]. However, the stability and field application of genetically engineered male sterile lines need to be further investigated, and the safety of transgenic crops also needs to be considered.
The recessive male sterile mutants in B. oleracea are abundant. RGMS lines with stable sterility usually have good economic characteristics but are restricted in production and application because of their maintenance and reproduction difficulty. To solve this problem, genetically modified methods can be used to create a male sterile maintainer line to produce 100% male sterile individuals [84,85]. A fertility restorer gene and reporter gene can be constructed into tightly linked elements and then introduced into the male sterile plant to create a maintainer line. This maintainer line has the same genetic background as the male sterile line except for the transgenic site of interest. The restorer gene contained in the maintainer transgenic site can cause fertility in the maintainer plant, and the reporter gene can be the green fluorescent protein (GFP) gene. The GFP gene expression is driven by a seed coat-specific promoter. The pollen produced by the maintainer line after meiosis all carry the sterility gene, and 50% of the pollen also carries the transgenic site (restoring gene + GFP gene). When the maintainer line is crossed with the male sterile line, 50% sterile seeds (without the transgenic site) and 50% fertile seeds (with the transgenic site) can be obtained. Since fertile seeds contain the GFP gene specifically expressed in the seed coat, the two types of seeds can be easily distinguished by fluorescence sorting equipment. Sterile seeds can be used directly for hybrid production, while fertile seeds continue to be kept as maintainer plants (Figure 2). This technology is expected to make effective use of RGMS and create broader use for the application of heterosis in B. oleracea.
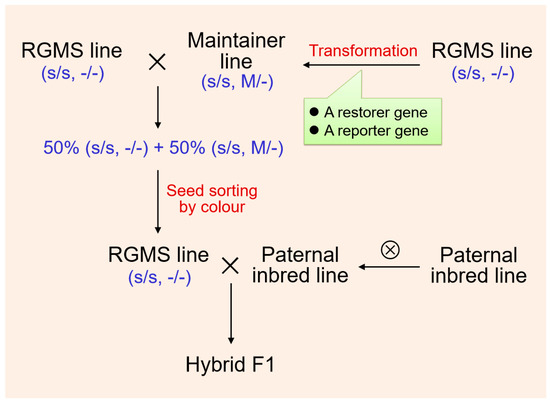
Figure 2.
Hybrids’ production strategy with recessive genic male sterility (RGMS) (s, recessive male sterile gene; M, transgenic locus comprising a fertility restorer gene and a reporter gene).
Author Contributions
Conceptualization, J.J. and L.Y.; writing—original draft preparation, J.J., J.H., and L.Y.; writing—review and editing, J.J., Z.F., Y.Z., M.Z., H.L., Y.W., Y.L., Z.L., and F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (32002034), National Key Research and Development Program (2017YFD0101804), the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2013-IVFCAAS), the Modern Agro-Industry Technology Research System (CARS-25-B-01).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, L.A.P.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef]
- Cheng, F.; Sun, R.; Hou, X.; Zheng, H.; Zhang, F.; Zhang, Y.; Liu, B.; Liang, J.; Zhuang, M.; Liu, Y.; et al. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016, 48, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Tanaka, N.; Niikura, S. Genetic analysis of the developmental characteristics related to the earliness of head formation in cabbage (Brassica oleracea L.). Breed. Sci. 2006, 56, 147–153. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, Y.; Yang, L.; Wang, X.; Zhuang, M.; Zhang, Y.; Sun, P. Breeding and seed production technique of dominant genic male sterile (DGMS) line and cytoplasmic male sterile (CMS) line in cabbage. Sci. Agric. Sin. 2004, 37, 717–723. (In Chinese) [Google Scholar]
- Kaul, M.L.H. Male Sterility in Height Plants; Springer: Berlin/Heidelberg, Germany, 1988; pp. 193–220. [Google Scholar]
- Wise, R.P.; Pring, D.R. Nuclear-mediated mitochondrial gene regulation and male fertility in higher plants: Light at the end of the tunnel? Proc. Natl. Acad. Sci. USA 2002, 99, 10240–10242. [Google Scholar] [CrossRef] [PubMed]
- Vedel, F.; Pla, M.; Vitart, V.; Gutierres, S.; Chetrit, P.; Paepe, R.D. Molecular basis of nuclear and cytoplasmic male sterility in higher plants. Plant Physiol. Biochem. 1994, 32, 601–608. [Google Scholar]
- Fang, Z.; Sun, P.; Liu, Y.; Yang, L.; Wang, X.; Zhuang, M. Investigation of different types of male sterility and application of dominant male sterility in cabbage. China Veg. 2001, 1, 6–10. (In Chinese) [Google Scholar]
- Ji, J.; Yang, L.; Fang, Z.; Zhuang, M.; Zhang, Y.; Lv, H.; Liu, Y.; Li, Z. Recessive male sterility in cabbage (Brassica oleracea var. capitata) caused by loss of function of BoCYP704B1 due to the insertion of a LTR-retrotransposon. Theor. Appl. Genet. 2017, 130, 1441–1451. [Google Scholar] [CrossRef]
- Fang, Z.; Sun, P.; Liu, Y.; Yang, L.; Wang, X.; Hou, A.; Bian, C. A male sterile line with dominant gene (MS) in cabbage (Brassica oleracea var. capitata) and its utilization for hybrid seed production. Euphytica 1997, 97, 265–268. [Google Scholar]
- Ogura, H. Studies on the new male sterility in Japanese radish with special reference to the utilization of this sterility towards the practical raising of hybrid seeds. Mem. Fac. Agric. Kagoshima Univ. 1968, 6, 39–78. [Google Scholar]
- Bannerot, H.; Boulidard, L.; Cauderon, Y.; Tempe, J. Transfer of cytoplasmatic male sterility from Raphanus sativus to Brassica oleracea. Proc. Eucarpia Meet Crucif. Crop Sect. 1974, 25, 52–54. [Google Scholar]
- Walters, W.T.; Mustschler, A.M.; Eaele, D.E. Protoplast fusion-derived Ogura male-sterile cauliflower with cold tolerance. Plant Cell Rep. 1992, 10, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Fang, Z.; Liu, Y.; Yang, L.; Zhuang, M. Chloroplast and mitochondrial SSR help to distinguish allo-cytoplasmic male sterile types in cabbage (Brassica oleracea L. var. capitata). Mol. Breed. 2012, 30, 709–716. [Google Scholar] [CrossRef]
- Pelletier, G.; Budar, F. Brassica Ogu-INRA cytoplasmic male sterility: An example of successful plant somatic fusion for hybrid seed production. In Somatic Genome Manipulation; Springer: New York, NY, USA, 2015; pp. 199–216. [Google Scholar] [CrossRef]
- Fu, T. Production and research on rapeseed in the People’s Republic of China. Crucif. Newslett. 1981, 6, 6–7. [Google Scholar]
- Fu, T.; Yang, X.; Yang, G. Development and studies on polima cytoplasmic male sterile “three lines” in Brassica napus L. J. Huazhong Agric. Univ. 1989, 8, 201–207. (In Chinese) [Google Scholar]
- Yang, G.; Fu, T. The inheritance of polima cytoplasmic male sterility in Brassica napus L. Plant Breed. 1990, 104, 121–124. [Google Scholar]
- Yarrow, S.; Burnett, L.; Wildeman, R.; Kemble, R.J. The transfer of polima cytoplasmic male sterility from oil seed rape (B. napus) to broccoli (B. oleracea) by protoplast fusion. Plant Cell Rep. 1990, 9, 185–188. [Google Scholar] [CrossRef]
- Yang, G.; Fu, T.; Yang, X. Studies on the ecotypical male sterile line of Brassica napus L. Acta Agron. Sin. 1995, 21, 129–135. (In Chinese) [Google Scholar]
- Pearson, O.H. Cytoplasmically inherited make sterility characters and flavor components from the species cross B.nigra Koch×B.oleracea L. J. Am. Soc. Hortic. Sci. 1972, 97, 397–402. [Google Scholar]
- Lou, P.; Kang, J.; Zhang, G.; Bonnema, G.; Fang, Z.; Wang, X. Transcript profiling of a dominant male sterile mutant (Ms-cd1) in cabbage during flower bud development. Plant Sci. 2007, 172, 111–119. [Google Scholar] [CrossRef]
- Ma, Y.; Kang, J.; Wu, J.; Zhu, Y.; Wang, X. Identification of tapetum-specific genes by comparing global gene expression of four different male sterile lines in Brassica oleracea. Plant Mol. Biol. 2015, 87, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E. Tapetum character states: Analytical keys for tapetum types and activities. Can. J. Bot. 1997, 75, 1448–1459. [Google Scholar] [CrossRef]
- Scott, R.J.; Spielman, M.; Dickinson, H.G. Stamen structure and function. Plant Cell 2004, 16, S46–S60. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.D.; Hsing, Y.I.; Huang, A.H. Transcriptomes of the anther sporophyte: Availability and use. Plant Cell Physiol. 2011, 52, 1459–1466. [Google Scholar] [CrossRef]
- Ji, J.; Yang, L.; Fang, Z.; Zhuang, M.; Zhang, Y.; Lv, H.; Liu, Y.; Li, Z. Complementary transcriptome and proteome profiling in cabbage buds of a recessive male sterile mutant provides new insights into male reproductive development. J. Proteom. 2018, 179, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lou, P.; Bonnema, G.; Yang, B.; He, H.; Zhang, Y.; Fang, Z. Linkage mapping of a dominant male sterility gene DGMS in Barssica oleracea. Genome 2005, 48, 848–854. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Zhang, H.; Ma, Y.; Guo, A.; Wang, X. Fine mapping of a male sterility gene MS-cd1 in Brassica oleracea. Theor. Appl. Genet. 2011, 123, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, X.; Yuan, K.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Wang, Y.; Liu, Y.; Li, Z.; et al. A user-friendly KASP molecular marker developed for the DGMS-based breeding system in Brassica oleracea species. Mol. Breed. 2019, 39, 90–96. [Google Scholar] [CrossRef]
- Han, F.; Yuan, K.; Kong, C.; Zhang, X.; Yang, L.; Zhuang, M.; Zhang, Y.; Li, Z.; Wang, Y.; Fang, Z.; et al. Fine mapping and candidate gene identifcation of the genic male sterile gene ms3 in cabbage 51S. Theor. Appl. Genet. 2018, 131, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Rown, G.G. Haracterization of expression of a mitochondrial gene associated with the Brassica polima CMS developmental influences. Curr. Genet. 1993, 24, 316–322. [Google Scholar] [CrossRef]
- Brown, G.G.; Domaj, M.; Dupauw, M.; Jean, M.; Li, X.Q.; Landry, B.S. Molecular analysis of Brassica CMS and its application to hybrid seed production. Acta Hortic. 1998, 459, 265–274. [Google Scholar] [CrossRef]
- Hanson, M.; Bentolila, S. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 2004, 16 (Suppl. 1), S154–S169. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Yu, X.; Wang, S.; Cao, J. Molecular identification of the cytoplasmic male sterile (CMS) type in common head cabbage. Mol. Plant Breed. 2009, 7, 1149–1153. (In Chinese) [Google Scholar]
- Zhang, Y.; Wang, X.; Li, C.; Song, H.; Ren, X.; Si, J. Molecular identification of Brassica oleracea CMS and the morphology response of flower to nuclear background. Acta Hortic. Sin. 2010, 37, 915–922. (In Chinese) [Google Scholar]
- Zhang, Y.; Fang, Z.; Wang, Q.; Liu, Y.; Yang, L.; Zhuang, M.; Sun, P. Molecular distinction of two Ogura CMS sources in Brassica oleracea var. capitata L. Sci. Agric. Sin. 2011, 44, 2959–2965. (In Chinese) [Google Scholar]
- Zhu, Q.; Kang, Z.; Jian, Y.; Ding, Y.; Kang, J. The molecular characteristics of Ogura cytoplasmic male sterility related gene orf138 in cabbage (Brassica oleracea var. capitata). Chin. Agric. Sci. Bull. 2012, 28, 104–109. (In Chinese) [Google Scholar]
- Fujii, S.; Toriyam, K. Genome barriers between nuclei and mitochondria exemplified by cytoplasmic male sterility. Plant Cell Physiol. 2008, 49, 1484–1494. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y. Male sterility and fertility restoration in crops. Ann. Rev. Plant Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef]
- Domblides, E.A.; Domblides, A.S.; Zayachkovskaya, T.V.; Bondareva, L.L. Identification of cytoplasm types in accessions of the family Brassicaceae (Brassicaceae Burnett) with DNA markers. Vavilov J. Genet. Breed. 2015, 19, 529. [Google Scholar] [CrossRef]
- Yamagishi, H.; Terachi, T. Intra-and inter-specifc variations in the mitochondrial gene orf138 of Ogura-type male sterile cytoplasm from Raphanus sativus and Raphanus raphanistrum. Theor. Appl. Genet. 2001, 103, 725–732. [Google Scholar] [CrossRef]
- Bonhomme, S.; Budar, F.; Ferault, M.; Pelletier, G. A 2.5kb Nco I fragment of Ogura radish mitochondrial DNA is correlated with cytoplasmic male-sterility in Brassica cybrids. Curr. Genet. 1991, 19, 121–127. [Google Scholar] [CrossRef]
- Bonhomme, S.; Budar, F.; Lancelin, D.; Small, I.; Defrance, M.C.; Pelletier, G. Sequence and transcript analysis of the Nco2.5 Ogura-specific fragment correlated with cytoplasmic male sterility in Brassica cybrids. Mol. Gen. Genet. 1992, 235, 340–348. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Makaroff, C.A. Characterization of the radish mitochondrial orfB locus: Possible relationship with male sterility in Ogura radish. Curr. Genet. 1993, 24, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, S.; Makaroff, C.A. Organ-specific reduction in the abundance of a mitochondrial protein accompanies fertility restoration in cytoplasmic male-sterile radish. Plant Mol. Biol. 1994, 26, 935–946. [Google Scholar] [CrossRef]
- Duroc, Y.; Gaillard, C.; Hiard, S.; Defrance, M.C.; Pelletier, G.; Budar, F. Biochemical and functional characterization of orf138, a mitochondrial protein responsible for Ogura cytoplasmic male sterility in Brassiceae. Biochimie 2005, 87, 1089. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsuda, M.; Yasumoto, K.; Yamagishi, H.; Terachi, T. A complete mitochondrial genome sequence of Ogura-type male-sterile cytoplasm and its comparative analysis with that of normal cytoplasm in radish (Raphanus sativus L.). BMC Genom. 2012, 13, 352. [Google Scholar] [CrossRef]
- Xing, M.; Sun, C.; Li, H.; Hu, S.; Lei, L.; Kang, J. Integrated analysis of transcriptome and proteome changes related to the Ogura cytoplasmic male sterility in cabbage. PLoS ONE 2018, 13, e0193462. [Google Scholar] [CrossRef]
- Nieuwhof, M. Cytoplasmic-genetic male sterility in radish (Raphanus sativus L.). Identification of maintainers, inheritance of male sterility and effect of environmental factors. Euphytica 1990, 47, 171–177. [Google Scholar]
- Yamagishi, H. Distribution and allelism of restorer genes for Ogura cytoplasmic male sterility in wild and cultivated radishes. Genes Genet. Syst. 1998, 73, 79–83. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Shen, X.; Zhao, G. Inheritance of male sterility in spring-summer radish. Acta Hortic. Sin. 1999, 26, 238–243. (In Chinese) [Google Scholar]
- Bellaoui, M.; Pelletier, G.; Budar, F. The steady-level of mRNA from the Ogura cytoplasmic male sterility locus in Brassica cybrids is determined post-transcriptionally by its 3′ region. EMBO J. 1997, 16, 5057–5068. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.G.; Formanova, N.; Jin, H.; Wargachuk, R.; Dendy, C.; Pati, P.; Lafores, M.; Zhang, J.; Cheung, W.Y.; Landry, B.S. The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J. 2003, 35, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Desloire, S.; Gherbi, H.; Laloui, W.; Marhadour, S.; Clouet, V.; Cattolico, L.; Falentin, C.; Giancola, S.; Renard, M.; Budar, F.; et al. Identification of the fertility restoration locus Rfo in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep. 2003, 4, 588–594. [Google Scholar] [CrossRef]
- Uyttewaal, M.; Arnal, N.; Quadrado, M.; Martin-Canadell, A.; Vrielynck, N.; Hiard, S.; Gherbi, H.; Bendahmane, A.; Budar, F.; Mireau, H. Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by the fertility restorer locus for Ogura cytoplasmic male sterility. Plant Cell 2008, 20, 3331–3345. [Google Scholar] [CrossRef]
- Koizuka, N.; Imai, R.; Fujimoto, H.; Hayakawa, T.; Kimura, Y.; Kohno-Murase, J.; Sakai, T.; Kawasaki, S.; Imamura, J. Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J. 2003, 34, 407–415. [Google Scholar] [CrossRef]
- Yasumoto, K.; Terachi, T.; Yamagishi, H. A novel Rf gene controlling fertility restoration of Ogura male sterility by RNA processing of orf138 found in Japanese wild radish and its STS markers. Genome 2009, 52, 495–504. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Xiang, C.; Mei, S.; Zhou, Y.; Chen, G.; Wang, T. A new fertility restorer locus linked closely to the Rfo locus for cytoplasmic male sterility in radish. Theor. Appl. Genet. 2008, 117, 313–320. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Chen, J.; Xiang, C.; Mei, S.; Zhou, Y.; Wang, T. A chimeric Rfo gene generated by intergenic recombination cosegregates with the fertility restorer phenotype for cytoplasmic male sterility in radish. Mol. Breed. 2010, 25, 339–349. [Google Scholar] [CrossRef]
- Wang, Z.; De, W.; Gao, L.; Mei, S.; Zhou, Y.; Xiang, C.; Wang, T. Heterozygous alleles restore male fertility to cytoplasmic male-sterile radish (Raphanus sativus L.): A case of overdominance. J. Exp. Bot. 2013, 64, 2041. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Mei, S.; Gao, L.; Zhou, Y.; Wang, T. An insertion-deletion at a pentatricopeptide repeat locus linked to fertility transition to cytoplasmic male sterility in radish (Raphanus sativus L.). Mol. Breed. 2015, 35, 108. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Cai, Q.; Mei, S.; Gao, L.; Zhou, Y. Identification of promoter exchange at a male fertility restorer locus for cytoplasmic male sterility in radish (Raphanus sativus L.). Mol. Breed. 2017, 37, 82. [Google Scholar] [CrossRef]
- Goldberg, R.B.; Beals, T.P.; Snaders, P.M. Anhter development: Basic principles and practical applications. Plant Cell 1993, 5, 1217–1229. [Google Scholar]
- Liu, H.; Kang, J.; Xie, J.; Jian, Y.; Ding, Y. Cloning an expression of an Ogu CMS-related anther-preferential transcription factor in Brassica oleracea. Acta Hortic. Sin. 2010, 37, 1953–1960. (In Chinese) [Google Scholar]
- Guo, Y.; Xie, J.; Jian, Y.; Yu, J.; Kang, J. Cloning and functional analysis of Ogu CMS-related gene BoMF1 Promoter in Brassica oleracea. Acta Hortic. Sin. 2013, 40, 887–895. (In Chinese) [Google Scholar]
- Kang, J.; Guo, Y.; Chen, Y.; Li, H.; Zhang, L.; Liu, H. Upregulation of the AT-hook DNA binding gene BoMF2 in OguCMS anthers of Brassica oleracea suggests that it encodes a transcriptional regulatory factor for anther development. Mol. Biol. Rep. 2014, 41, 512–527. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Z.; Liu, H.; Kang, J. Cloning and expression of an Ogura cytoplasmic male sterile (Ogu CMS) related MYB transcription factor in Brassica oleracea var. capitata. J. Agric. Biotech. 2012, 20, 627–635. (In Chinese) [Google Scholar]
- Chen, Y.; Xie, J.; Guo, Y.; Kang, J. Transcriptional activation analysis of an OguCMS-related gene BoMYB1 in Brassica oleracea. Acta Agric. Boreal. Occident. Sin. 2014, 23, 120–126. (In Chinese) [Google Scholar]
- Yan, H.; Fang, Z.; Liu, Y.; Yang, L.; Zhuang, M.; Zhang, Y. In vitro conservation technique for the dominant genic male sterile materials in cabbage. Chin. J. Trop. Agric. 2013, 33, 35–39. (In Chinese) [Google Scholar]
- Yu, H.; Fang, Z.; Liu, Y.; Yang, L.; Zhuang, M.; Lv, H.; Li, Z.; Han, F.; Liu, X.; Zhang, Y. Development of a novel allele-specific Rfo marker and creation of Ogura CMS fertility-restored interspecific hybrids in Brassica oleracea. Theor. Appl. Genet. 2016, 129, 1625–1637. [Google Scholar] [CrossRef]
- Ren, W.; Li, Z.; Han, F.; Zhang, B.; Li, X.; Fang, Z.; Yang, L.; Zhuang, M.; Lv, H.; Liu, Y.; et al. Utilization of Ogura CMS germplasm with the clubroot resistance gene by fertility restoration and cytoplasm replacement in Brassica oleracea L. Hortic. Res. 2020, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Ullstrup, A.J. The impact of the southern corn leaf blight of epidemics 1970–1971. Annu. Rev. Phytopathol. 1972, 10, 37–50. [Google Scholar] [CrossRef]
- Kempken, F. Engineered Male Sterility. In Genetic Modification of Plants. Biotechnology in Agriculture and Forestry; Kempken, F., Jung, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 64. [Google Scholar] [CrossRef]
- Mariani, C.; Beuckeleer, M.D.; Truettner, J.; Leemans, J.; Goldberg, R. Induction of male sterility in plants by a chimeric ribonuclease gene. Nature 1990, 347, 737–741. [Google Scholar] [CrossRef]
- Reynaerts, A.; van de Wiele, H.; de Sutter, G.; Janssens, J. Engineered genes for fertility control and their application in hybrid seed production. Sci. Hortic. 1993, 55, 125. [Google Scholar] [CrossRef]
- Shen, G.; Wang, X.; Zhu, Y.; Yang, H.; Lu, G.; Wang, J.; Wan, X.; Zhang, J. Male sterile transgenic cabbage plants with TA29-barnase gene. Acta Phytophysiol. Sin. 2001, 27, 43–48. (In Chinese) [Google Scholar]
- He, Y.; Xiong, X.; Guan, C.; Li, X.; Lin, L.; Chen, S.; Liu, Z.; Li, W.; Zhong, J.; Liu, C.; et al. The pTA29-Barnase chimeric gene transformation of Brassica napus mediated by agrobacterium. Acta Agron. Sin. 2003, 29, 615–620. (In Chinese) [Google Scholar]
- Li, C.; He, S.; Lan, C.; Ren, X.; Si, J.; Li, C.; Song, H. Transgenic male sterile cabbage plants induced by BcA9-Barnase transformation. J. SW Univ. 2015, 37, 52–58. (In Chinese) [Google Scholar]
- Curtis, I.S.; He, C.; Scott, R.; Power, J.B.; Davey, M.R. Genomic male sterility in lettuce, a base line for the production of F1 hybrids. Plant Sci. 1996, 113, 113–119. [Google Scholar] [CrossRef]
- He, S.; Abad, A.R.; Gelvin, S.B.; Mackenzie, S.A. A cytoplasmic male sterility-associated mitochondrial protein causes pollen disruption in transgenic tobacco. Proc. Natl. Acad. Sci. USA 1996, 93, 11763–11768. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Shewry, P.H.; Barcelo, P.; Lazzeri, P.A.; Halfold, N.G. Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen development and male sterility in transgenic barley. Plant J. 2001, 28, 431–441. [Google Scholar] [CrossRef]
- Perez-Prat, E.; van Lookeren Campagne, M. Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci. 2002, 7, 199–203. [Google Scholar] [CrossRef]
- Brink, K.; Crowgey, E.; Dietrich, N.; Hondred, D.; Young, J.K.; Zhong, C.X. Plant Genome DNA Flanking SPT Event and Methods for Identifying SPT Event. U.S. Patent No. 8,257,930, 4 September 2012. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
