Efficacy of Orange Essential Oil and Citral after Exposure to UV-C Irradiation to Inhibit Penicillium digitatum in Navel Oranges
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Penicillium Cultures and Inoculum Preparation
2.3. Essential Oils and Chemicals
2.4. Irradiation of Essential Oil and Citral
2.5. Gas Chromatograph/Mass Spectrometry (GC-MS) Analysis
2.6. Infection and Postharvest Treatment of Oranges
2.7. Statistical Analysis
3. Results and discussion
3.1. Composition of Orange EO and Citral
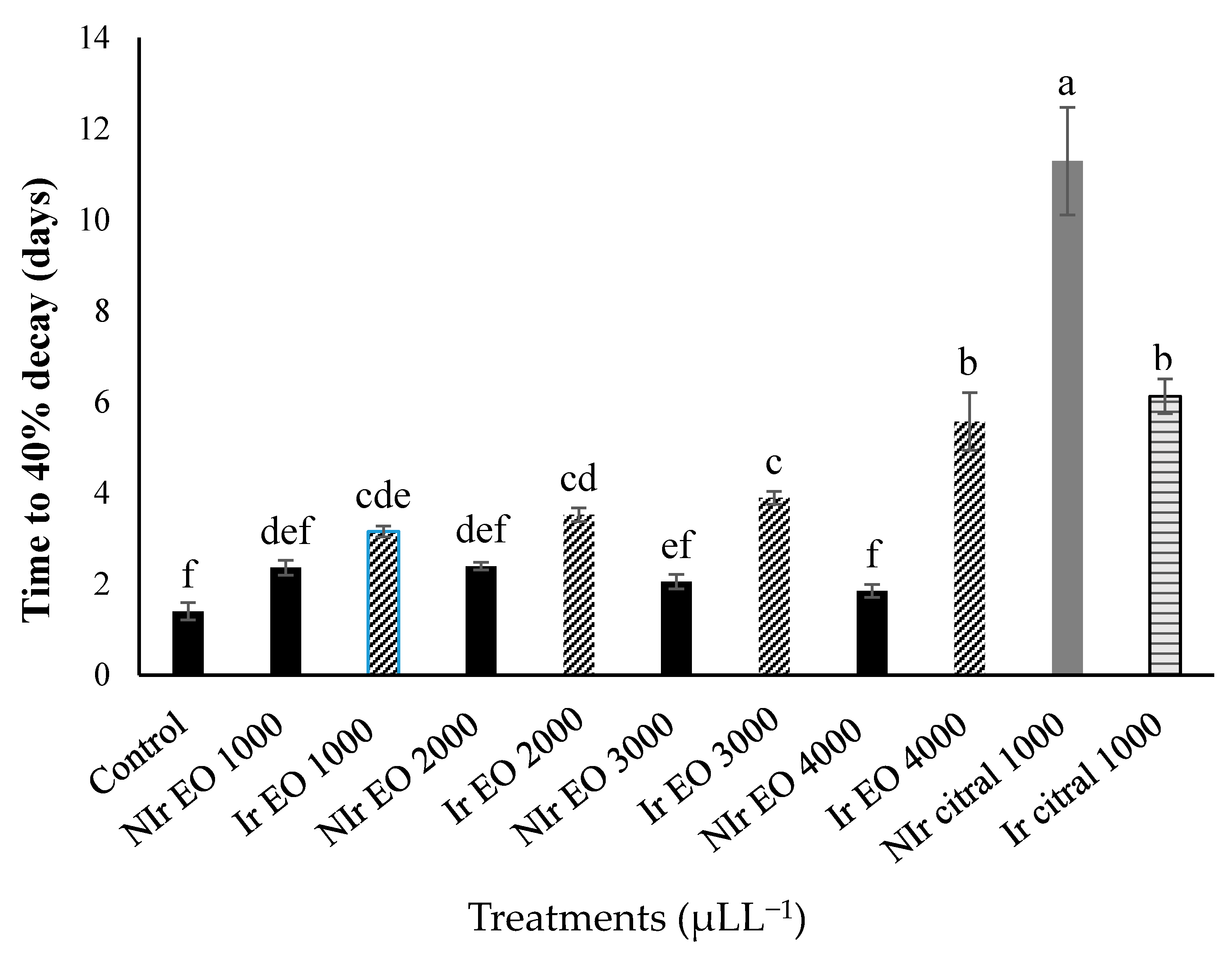
3.2. Effects of Orange EO and Citral on Decay in Inoculated Navel Oranges
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Golding, J.; Archer, J. Advances in postharvest handling of citrus fruit. In Achieving Sustainable Cultivation of Tropical Fruits; Yahia, E.M., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 65–90. [Google Scholar]
- Palou, L.; Smilanick, J.L.; Droby, S. Alternatives to conventional fungicides for the control of citrus postharvest green and blue moulds. Stewart Postharvest Rev. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Ismail, M.; Zhang, J. Post-harvest Citrus Diseases and their control. In Outlooks on Pest Management; Research Information: London, UK, 2004; Volume 15, pp. 29–35. [Google Scholar] [CrossRef]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef]
- Eckert, J.W.; Sievert, J.R.; Ratnayake, M. Reduction of imazalil effectiveness against citrus green mold in California packinghouses by resistant biotypes of Penicillium digitatum. Plant Dis. 1994, 78, 971–973. [Google Scholar] [CrossRef]
- Torres-Alvarez, C.; Núñez González, A.; Rodríguez, J.; Castillo, S.; Leos-Rivas, C.; Báez-González, J.G. Chemical composition, antimicrobial, and antioxidant activities of orange essential oil and its concentrated oils. J. Food 2017, 15, 129–135. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Bustamante, J.; Van Stempvoort, S.; García-Gallarreta, M.; Houghton, J.A.; Briers, H.K.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Clean. Prod. 2016, 137, 598–605. [Google Scholar] [CrossRef]
- Svoboda, K.P.; Greenaway, R.I. Lemon scented plants. Internat. J. Aromather. 2003, 13, 23–32. [Google Scholar] [CrossRef]
- Droby, S.; Eick, A.; Macarisin, D.; Cohen, L.; Rafael, G.; Stange, R.; McColum, G.; Dudai, N.; Nasser, A.; Wisniewski, M.; et al. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Technol. 2008, 49, 386–396. [Google Scholar] [CrossRef]
- Simas, D.L.R.; De Amorim, S.H.B.M.; Goulart, F.R.V.; Alviano, C.S.; Alviano, D.S.; Da Silva, A.J.R. Citrus species essential oils and their components can inhibit or stimulate fungal growth in fruit. Indust. Crops Products 2017, 98, 108–115. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Du Plooy, W.; Regnier, T.; Combrinck, S. Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol. Technol. 2009, 53, 117–122. [Google Scholar] [CrossRef]
- Wang, H.; Tao, N.; Huang, S.; Liu, Y. Effect of Shatangju (Citrus reticulata Blanco) Essential Oil on Spore Germination and Mycelium Growth of Penicillium digitatum and P. italicum. J. Essent. Oil Bear. Plants 2012, 15, 715–723. [Google Scholar] [CrossRef]
- Afek, U. Accumulation of Scoparone, a Phytoalexin Associated with Resistance of Citrus to Phytophthora citrophthora. Am. Phytopathol. Soc. 1988, 78, 1678–1682. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S. Microbiocidal Formulation Comprising Essential Oils or Their Derivatives. US Patent 7,465,469 B2, 16 December 2008. [Google Scholar]
- Khayyat, S.A.; Sameeh, M.Y. Bioactive epoxides and hydroperoxides derived from naturally monoterpene geranyl acetate. Saudi Pharm. J. 2018, 26, 14–19. [Google Scholar] [CrossRef]
- Khayyat, S. Thermal, photo-oxidation and antimicrobial studies of linalyl acetate as a major ingredient of lavender essential oil. Arab. J. Chem. 2020, 13, 1575–1581. [Google Scholar] [CrossRef]
- Wilson, N.D.; Ivanova, M.S.; Watt, R.A.; Moffat, A.C. The quantification of citral in lemongrass and lemon oils by near-infrared spectroscopy. J. Pharm. Pharmacol. 2002, 54, 1257–1263. [Google Scholar] [CrossRef]
- Leite, M.C.A.; Bezerra, A.P.D.B.; Sousa, J.P.D.; Guerra, F.Q.S.; Lima, E.D.O. Evaluation of Antifungal Activity and Mechanism of Action of Citral against Candida albicans. Evid. Based Complement Alternat. Med. 2014, 2014, 378280. [Google Scholar] [CrossRef]
- Rodov, V.; Ben-Yehoshua, S.; Fang, D.Q.; Kim, J.J.; Ashkenazi, R. Preformed antifungal compounds of lemon fruit: Citral and its relation to disease resistance. J. Agric. Food Chem. 1995, 43, 1057–1061. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Rodov, V. Developing a novel environmentally friendly microbiocidal formulation from peel of citrus fruit. Acta Hortic. 2006, 275–284. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Rodov, V.; Kim, J.J.; Carmeli, S. Preformed and induced antifungal materials of citrus fruits in relation to the enhancement of decay resistance by heat and ultraviolet treatments. J. Agric. Food Chem. 1992, 40, 1217–1221. [Google Scholar] [CrossRef]
- Knight, T.G. Investigation of the Physiological Basis of the Rind Disorder Oleocellosis in Washington Navel Oranges (Citrus Sinensis [L.] Osbeck). Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2002. [Google Scholar]
- Wuryatmo, E.; Klieber, A.; Scott, E.S. Inhibition of Citrus Postharvest Pathogens by Vapor of Citral and Related Compounds in Culture. J. Agric. Food Chem. 2003, 51, 2637–2640. [Google Scholar] [CrossRef]
- Rodov, V.; Nafussi, B.; Ben-Yehoshua, S. Essential oil components as potential means to control Penicillium digitatum pers. (Sacc.) and other postharvest pathogens of citrus fruit. Fresh Prod. 2011, 5, 43–50. [Google Scholar]
- Elgendy, E.M. Photooxygenation of Natural Limonene. J. Chin. Pharm. Sci. 1998, 50, 225–231. [Google Scholar]
- Li, L.J.; Hong, P.; Jiang, Z.D.; Yang, Y.F.; Du, X.P.; Sun, H.; Wu, L.M.; Ni, H.; Chen, F. Water accelerated transformation of d-limonene induced by ultraviolet irradiation and air exposure. Food Chem. 2018, 239, 434–441. [Google Scholar] [CrossRef]
- Rudbäck, J.; Ramzy, A.; Karlberg, A.-T.; Nilsson, U. Determination of allergenic hydroperoxides in essential oils using gas chromatography with electron ionization mass spectrometry. J. Sep. Sci. 2014, 37, 982–989. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Calandra, M.J.; Impellizzeri, J.; Wang, Y. An HPLC method for hydroperoxides derived from limonene and linalool in citrus oils, using post-column luminol-mediated chemiluminescence detection. Flavour Fragr. J. 2015, 30, 121–130. [Google Scholar] [CrossRef]
- Schieberle, P.; Maier, W.; Firl, J.; Grosch, W. HRGC separation of hydroperoxides formed during the photosensitized oxidation of (R)—(+)-Limonene. J. High Resolut. Chromat. 1987, 10, 588–593. [Google Scholar] [CrossRef]
- Price, C.C.; Dickman, M.L. Kinetics of the Acid-Catalyzed Cyclization of Citral and Citronellal. Indust. Eng. Chem. 1948, 40, 257–261. [Google Scholar] [CrossRef]
| Non-irradiated Orange EO | Irradiated Orange EO | |||
|---|---|---|---|---|
| Compound | Peak Area (%) a | Compound | Peak Area (%) a | |
| D-Limonene | 88.8 | Group 1b: | D-Limonene | 60.1 |
| β-Myrcene | 1.5 | Benzaldehyde | 3.8 | |
| Benzaldehyde | 1.2 | Ethylbenzene | 1.5 | |
| Ethylbenzene | 0.8 | Linalool | 1.3 | |
| α-Pinene | 0.5 | α-Pinene | 0.4 | |
| Octanal | 0.5 | β-Myrcene | 0.3 | |
| Linalool | 0.5 | Group 2b: | l-Carvone | 3.3 |
| 3-Carene | 0.4 | trans-Limonene oxide | 2.5 | |
| Decanal | 0.2 | trans-Carveol | 2.1 | |
| cis-Limonene oxide | 1.8 | |||
| (2S,4R)-p-Mentha-6,8-diene 2 hydroperoxide | 1.8 | |||
| (1S,4R)-p-Mentha-2,8-diene, 1-hydroperoxide | 1.4 | |||
| cis-Carveol | 1.4 | |||
| (1R,4R)-p-Mentha-2,8-diene, 1-hydroperoxide | 1.4 | |||
| trans-p-Mentha-2,8-dienol | 1.3 | |||
| p-Mentha-1,8-dien-7-ol | 0.8 | |||
| p-Mentha-1(7),8(10)-dien-9-ol | 0.5 | |||
| p-Mentha-1(7),8-dien-2-ol | 0.4 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B.; Kirkman, T.; Pristijono, P. Efficacy of Orange Essential Oil and Citral after Exposure to UV-C Irradiation to Inhibit Penicillium digitatum in Navel Oranges. Horticulturae 2020, 6, 102. https://doi.org/10.3390/horticulturae6040102
Rahman MM, Wills RBH, Bowyer MC, Golding JB, Kirkman T, Pristijono P. Efficacy of Orange Essential Oil and Citral after Exposure to UV-C Irradiation to Inhibit Penicillium digitatum in Navel Oranges. Horticulturae. 2020; 6(4):102. https://doi.org/10.3390/horticulturae6040102
Chicago/Turabian StyleRahman, Mohammad M., Ron B. H. Wills, Michael C. Bowyer, John B. Golding, Timothy Kirkman, and Penta Pristijono. 2020. "Efficacy of Orange Essential Oil and Citral after Exposure to UV-C Irradiation to Inhibit Penicillium digitatum in Navel Oranges" Horticulturae 6, no. 4: 102. https://doi.org/10.3390/horticulturae6040102
APA StyleRahman, M. M., Wills, R. B. H., Bowyer, M. C., Golding, J. B., Kirkman, T., & Pristijono, P. (2020). Efficacy of Orange Essential Oil and Citral after Exposure to UV-C Irradiation to Inhibit Penicillium digitatum in Navel Oranges. Horticulturae, 6(4), 102. https://doi.org/10.3390/horticulturae6040102







