Does Inoculation with Arbuscular Mycorrhizal Fungi Reduce Trunk Disease in Grapevine Rootstocks?
Abstract
1. Introduction
2. Methods
2.1. Experimental Design
2.2. Rootstock Preparation
2.3. Pathogen Inoculum Preparation
2.4. Application of Treatments, and Plant Culture
2.5. Data Collection
2.5.1. Vine Growth Response
2.5.2. Pathogen Isolation and Assessment of Necrosis
2.5.3. Digital Droplet PCR Quantification
2.5.4. Quantification of AM Fungal DNA Using ddPCR
2.5.5. Quantification of I. liriodendra DNA Using ddPCR
2.6. Statistical Analysis
3. Results
3.1. Plant Response
3.2. Necrosis
3.3. Molecular Quantification
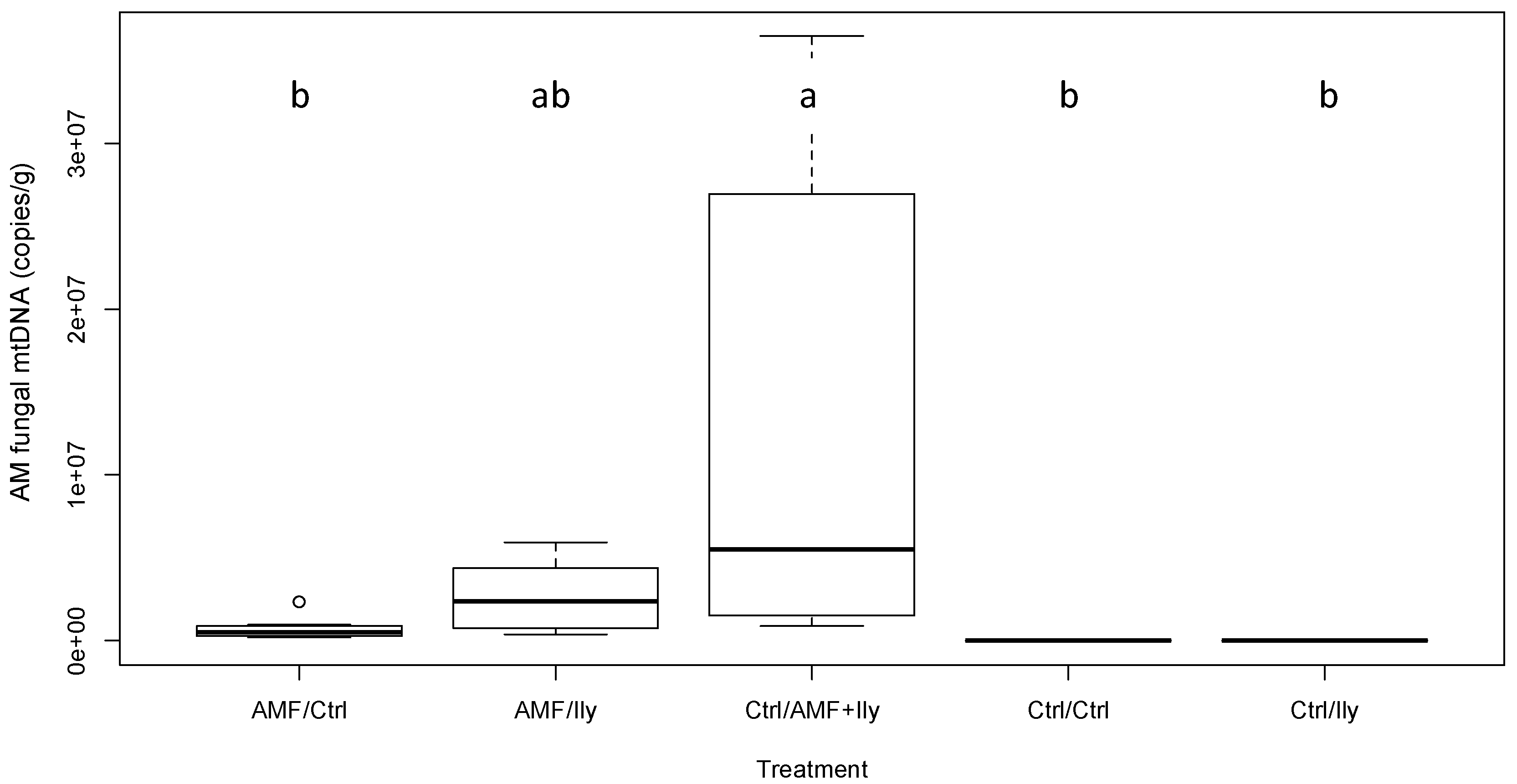
3.3.1. AMF
3.3.2. Ilyonectria
4. Discussion
4.1. Vine Growth
4.2. Pathogen Isolation and Necrosis
4.3. Ilyonectria and AM Fungal Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Úrbez-Torres, J.R.; Haag, P.; Bowen, P.; O’Gorman, D.T. Grapevine trunk diseases in British Columbia: Incidence and characterization of the fungal pathogens associated with black foot disease of grapevine. Plant Dis. 2014, 98, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Agusti-Brisach, C.; Armengol, J. Black-foot disease of grapevine: An update on taxonomy, epidemiology and management strategies. Phytopathol Meiterr. 2013, 52, 245–261. [Google Scholar]
- Halleen, F.; Fourie, P.H.; Crous, P.W. A review of black foot disease of grapevine. Phytophatol. Mediterr. 2006, 45, S55–S67. [Google Scholar]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Linderman, R.G.; Davis, A.E. Comparative response of selected grapevine rootstocks and cultivars to inoculation with different mycorrhizal fungi. Am. J. Enol. Vitic. 2001, 52, 8–11. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; van Tuinen, D.; Adrian, M.; Wipf, D. Arbuscular mycorrhiza symbiosis in viticulture: A review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef]
- Nogales, A.; Aguirreolea, J.; Maria, E.S.; Camprubi, A. Response of mycorrhizal grapevine to Armillaria mellea inoculation: Disease development and polyamines. Plant Soil 2009, 317, 177–187. [Google Scholar] [CrossRef]
- Azcón-Aguilar, C.; Barea, J.M. Arbuscular mycorrhizas and biocontrol of soil-borne plant pathogens–An overview of the mechanisms involved. Mycorrhiza 1996, 6, 457–464. [Google Scholar] [CrossRef]
- Petit, E.; Gubler, W.G. Influence of Glomus intraradices on Black Foot Disease caused by Cylindrocarpon macrodidymum on Vitis rupestris under controlled conditions. Plant Dis. 2006, 90, 1481–1484. [Google Scholar] [CrossRef]
- Cordier, C.; Gianinazzi, S.; Gianinazzi-Pearson, V. Colonisation patterns of root tissues by Phytopthora nicotianae var parasitica related to reduced disease in mycorrhizal tomato. Plant Soil 1996, 185, 223–232. [Google Scholar] [CrossRef]
- Dehne, H.W. Interaction between vesicular-arbuscular mycorrhizal fungi and plant pathogens. Phytopathology 1982, 72, 1115–1119. [Google Scholar]
- Pozo, M.J.; Azcón-Aguilar, C. Unravelling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.M.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; Luque, J.; Estaun, V.; Camprubi, A.; Garcia-Figueres, F.; Calvet, C. Differential growth of mycorrhizal field-inoculated grapevine rootstocks in two replant soils. Am. J. Enol. Vitic. 2009, 60, 484–489. [Google Scholar]
- Holland, T.C.; Bowen, P.A.; Bogdanoff, C.; Hart, M. Arbuscular mycorrhizal fungal communities associated with Vitis vinifera vines under different frequencies of irrigation. Am. J. Enol. Vitic. 2014, 65, 222–229. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ.; U.S. National Institutes of Health: Bethesda, MD, USA, 1997. Available online: https://imagej.nih.gov/ij/ (accessed on 1 December 2009).
- Providencia, I.E.; Nadimi, M.; Beaudet, D.; Morales, G.R.; Hijri, M. Detection of a transient mitochondrial DNA heteroplasmy in the progeny of crossed genetically divergent isolates of arbuscular mycorrhizal fungi. New Phytol. 2013, 200, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Li, Y.; Hamel, C.; Hanson, K.; Hart, M. Site specificity in establishment of a commercial arbuscular mycorrhizal fungal inoculant. Sci. Total Environ. 2018, 660, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Camprubi, A.; Estuan, V.; Nogales, A.; Garcia-Figueres, F.; Pitet, M.; Calvet, C. Response of grapevine rootstock Richter 110 to inoculation with native and selected arbuscular mycorrhizal fungi and growth performance in a replant vineyard. Mycorrhiza 2008, 18, 211–216. [Google Scholar] [CrossRef]
- Belew, T.A.; Mokashi, M.N.; Getachew, Y.; Patil, C.P. Effects of salinity and mycorrhizal inoculation (Glomus fasciculatum) on growth responses of grape rootstocks (Vitis spp.). S. Afr. J. Enol. Vitic. 2010, 31, 82–88. [Google Scholar] [CrossRef][Green Version]
- Nicolás, E.; Maestre-Valero, J.F.; Alarcón, J.J.; Pedrero, F.; Vicentre-Sánchez, J.; Bernabé, A.; Gómez-Montiel, J.; Hernández, J.A.; Fernández, F. Effectiveness and persistence of arbuscular mycorrhizal fungi on the physiology, nutrient uptake and yield of Crimson seedless grapevine. J. Agric. Sci. 2015, 153, 1084–1096. [Google Scholar] [CrossRef]
- Holland, T.C.; Hart, M.M.; Bogdanoff, C.; Bowen, P.A. Response of grapevine rootstocks to soil inocula from different sources. Am. J. Enol. Vitic. 2018, 69, 94–100. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Corkidi, L.; Allen, E.B.; Merhaut, D.; Allen, M.F.; Downer, J.; Bohn, J.; Evans, M. Assessing the infectivity of commercial mycorrhizal inoculants in plant nursery conditions. J. Environ. Hortic. 2004, 22, 149–154. [Google Scholar]
- Thomsen, C. Establishment, Persistence, and Spread of a Commercially Available Arbuscular Mycorrhizal Fungal Inoculant in Viticulture. Master’s Thesis, University of British Columbia Okanagan, Kelowna, BC, Canada, 2018. [Google Scholar]
- Koide, R.T. The nature of growth depression in sunflower caused by vesicular-arbuscular mycorrhizal infection. New Phytol. 1985, 99, 449–462. [Google Scholar] [CrossRef]
- Graham, J.K.; Eissenstat, D.M. Field evidence for carbon cost of citrus mycorrhizas. New Phytol. 1998, 140, 103–110. [Google Scholar] [CrossRef]
- Holland, T.C.; Vukicevich, E.; Thomsen, C.; Pogiatzis, A.; Hart, M.M.; Bowen, P.A. Arbuscular mycorrhizal fungi in viticulture: Should we use biofertilizers? Catal. Discov. Pract. 2018, 2, 59–63. [Google Scholar] [CrossRef]
- Pongrácz, D.P. Rootstocks for Grapevines; David Philip: Cape Town, South Africa, 1983. [Google Scholar]
- Brown, D.S.; Jaspers, M.V.; Ridgway, C.J.; Barclay, C.J.; Jones, E.E. Susceptibility of four grapevine rootstocks to Cylindrocarpon parva. N. Z. Plant Prot. 2013, 66, 249–253. [Google Scholar]
- Pozo, M.J.; Cordier, C.; Dumas-Gaudot, E.; Gianinazzi, S.; Barea, J.M.; Azcón-Aguilar, C. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 2002, 53, 525–534. [Google Scholar] [CrossRef]
- Filion, M.; St_Arnaud, M.; Jabaji-Hare, S.H. Quanitification of Fusarium solani f. sp phaseoli in mycorrhizal bean plants and surrounding mycorrhizosphere soil using real-time polymerase chain reaction and direct isolation on selective media. Phytopathology 2003, 93, 229–235. [Google Scholar] [CrossRef]
- Kjoller, R.; Rosendahl, S. The presence of the arbuscular mycorrhizal fungus Glomus intraradices influences enzymatic activities of the root pathogen Aphanomyces euteiches in pea roots. Mycorrhiza 1996, 6, 487–491. [Google Scholar] [CrossRef]
- Sukhada, M.; Manjula, R.; Rawal, R.D. Evaluation of arbuscular mycorrhiza and other biocontrol agentes against Phytopthora parasitica var. nicotianae infecting papaya (Carica papaya sv. Surya) and enumeration of pathogen population using immunotechniques. Biol. Control 2011, 58, 22–29. [Google Scholar]
- Mohandas, S.; Manjula, R.; Rawal, R.D.; Lakshmikantha, H.C.; Chakraborty, S.; Ramachandra, Y.L. Evaluation of arbuscular mycorrhiza and other biocontrol agents in managing Fusarium oxysporum f. sp Cubense infection in banana cv. Neypoovan. Biocontrol Sci. Technol. 2010, 20, 165–181. [Google Scholar] [CrossRef]
- Kapoor, R.; Sharma, D.; Bhatnagar, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.; Mrosk, C.; Isayenkov, S.; Strack, D. Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 2009, 68, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bedini, A.; Mercy, L.; Scheider, C.; Franken, P.; Lucic-Mercy, E. Unraveling the initial plant hormone signalling, metabolic mechanisms and plant defense triggering the endomycorrhizal symbiosis behavior. Front. Plant Sci. 2018, 17, 1800. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 762412. [Google Scholar] [CrossRef]
- De Román, M.; Fernández, I.; Wyatt, T.; Sahrawy, M.; Heil, M.; Pozo, M.J. Elicitation of foliar resistance mechanisms transiently impairs root association with arbuscular mycorrhizal fungi. J. Ecol. 2011, 99, 36–45. [Google Scholar] [CrossRef]
- Delaney, T.P.; Uknes, S.; Vernooij, B.; Friedrich, L.; Weymann, K.; Negrotto, D.; Gaffney, T.; Gut-Rella, M.; Kessmann, H.; Ward, E.; et al. A central role of salicylic acid in plant disease resistance. Science 1994, 266, 1247–1250. [Google Scholar] [CrossRef]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J.N. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in disease complexes: A growing trend. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host-multi-pathogen warfare: Pathogen interactions in co-infected plants. Front. Plant Sci. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Staddon, P.L.; Fitter, A.H.; Robinson, D. Effects of mycorrhizal colonization and elevate atmospheric carbon dioxide on carbon fixation and below-ground carbon partitioning in Plantago lanceolate. J. Exp. Bot. 1999, 50, 853–860. [Google Scholar] [CrossRef]
- Wright, D.P.; Read, D.J.; Scholes, J.D. Myorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ. 1998, 21, 881–891. [Google Scholar] [CrossRef]
- Hart, M.M.; Forsythe, J.A.; Oshowski, B.; Buckling, H.; Jansa, J.; Kiers, E.T. Hiding in a crowd–Does diversity facilitate persistence of a low-quality fungal partner in the mycorrhizal symbiosis? Symbiosis 2013, 59, 47–56. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Graham, J.H.; Duncan, L.W.; Eissenstat, D.M. Carbohydrate allocation patterns in citrus genotypes as affected by phosphorus nutrition, mycorrhizal colonization and mycorrhizal dependency. New Phytol. 1997, 135, 335–343. [Google Scholar] [CrossRef][Green Version]
- Schreiner, R.P. Effects of native and nonnative arbuscular mycorrhizal fungi on growth and nutrient uptake of ‘Pinot noir’ (Vitis vinifera L.) in two soils with contrasting levels of phosphorus. Appl. Soil Ecol. 2007, 36, 205–215. [Google Scholar] [CrossRef]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
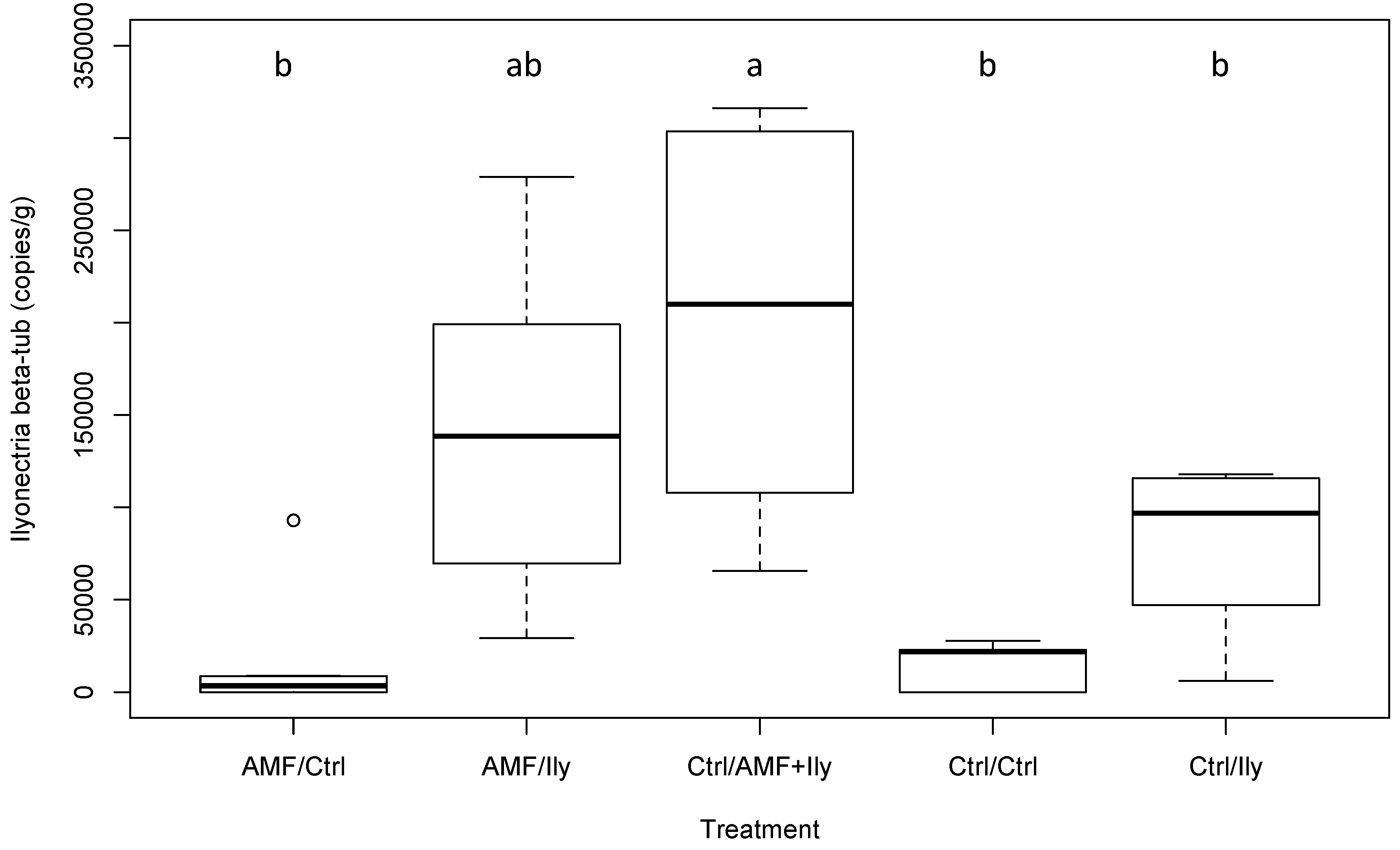
| Treatment | May 2018 | August 2018 | Description |
|---|---|---|---|
| Ctrl/Ctrl | No addition | No addition | Non-microbial control, receiving neither AM fungi nor Ilyonectria |
| Ctrl/Ily | No addition | Pathogen | Pathogen positive control, receiving only Ilyonectria in August |
| AMF/Ctrl | AM fungi | No addition | Mycorrhizal pre-inoculation control, receiving AM fungi at the first time point, but no pathogen |
| AMF/Ily | AM fungi | Pathogen | Pre-inoculated pathogen treatment, first receiving AM fungi in May, then exposed to Ilyonectria in August. |
| Ctrl/AMF + Ily | No addition | Pathogen + AM fungi | Co-inoculation treatment, applying both AM fungi and Ilyonectria concurrently in August. |
| Measure | F-Value | p | Average |
|---|---|---|---|
| Dry Stem Mass | 1.24 | 0.308 | 11.6 g |
| Dry Leaf Mass | 0.651 | 0.662 | 12.2 g |
| Fresh Root Mass | 1.53 | 0.201 | 333.5 g |
| Internode Width | 1.52 | 0.206 | 223 mm |
| Internode Length | 0.188 | 0.965 | 2.3 mm |
| Leaf Greenness | 0.594 | 0.705 | 23.6 spad units |
| % Stem Necrosis | 0.577 | 0.717 | 25% |
| AM fungal copy number | 5.15 | <0.001 * | See Figure 1 |
| Ilyonectria copy number | 5.59 | <0.001 * | See Figure 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holland, T.; Bowen, P.; Kokkoris, V.; Urbez-Torres, J.R.; Hart, M. Does Inoculation with Arbuscular Mycorrhizal Fungi Reduce Trunk Disease in Grapevine Rootstocks? Horticulturae 2019, 5, 61. https://doi.org/10.3390/horticulturae5030061
Holland T, Bowen P, Kokkoris V, Urbez-Torres JR, Hart M. Does Inoculation with Arbuscular Mycorrhizal Fungi Reduce Trunk Disease in Grapevine Rootstocks? Horticulturae. 2019; 5(3):61. https://doi.org/10.3390/horticulturae5030061
Chicago/Turabian StyleHolland, Taylor, Patricia Bowen, Vasilis Kokkoris, Jose Ramon Urbez-Torres, and Miranda Hart. 2019. "Does Inoculation with Arbuscular Mycorrhizal Fungi Reduce Trunk Disease in Grapevine Rootstocks?" Horticulturae 5, no. 3: 61. https://doi.org/10.3390/horticulturae5030061
APA StyleHolland, T., Bowen, P., Kokkoris, V., Urbez-Torres, J. R., & Hart, M. (2019). Does Inoculation with Arbuscular Mycorrhizal Fungi Reduce Trunk Disease in Grapevine Rootstocks? Horticulturae, 5(3), 61. https://doi.org/10.3390/horticulturae5030061





