Abstract
In many parts of the world, the agricultural sector is faced with a number of challenges including those arising from abiotic environmental stresses which are the key factors responsible for most reductions in agrifood production. Crude oil contamination, an abiotic stress factor and a common environmental contaminant, at toxic levels has negative impacts on plants. Although various attempts have been made to demonstrate the impact of abiotic stresses on crops, the underlying factors responsible for the effects of crude oil and its induced abiotic stresses on the composition of the stressed plants are poorly understood. Hence, this review provides an in-depth examination of the: (1) effect of petroleum hydrocarbons on plants; (2) impact of abiotic environmental stresses on crop quality; (3) mechanistic link between crude oil stress and its induced abiotic stresses; as well as (4) mode of action/plant response mechanism to these induced stresses. The paper clearly reveals the implications of crude oil-induced abiotic stresses arising from the soil-root-plant route and from direct application on plant leaves.
1. Introduction
Crude oil pollution, arising from exploration and processing operations, is a common environmental challenge []. This introduction of crude oil (via large or small spills) into the environment could arise from technical errors, deliberate human acts as well as transportation and storage faults [,,,,]. Schmidt-Etkin [] noted that although large spills sometimes occur with serious environmental and socioeconomic damage, small spills are more common. As explained in detail in a later section, the impacts and damages caused by these spills generally depend on the location, oil type, volume, closeness to sensitive resources, and season, among other factors []. Nonetheless, accidental large-scale oil spills make up a significant part of contaminants in the globe []. There are more cases of oil spills on land than those recorded in water [] in which plant life in agricultural fields becomes exposed to petroleum hydrocarbons (PH) [] with both acute and chronic effects on agricultural produce [].
Interestingly, the various technological/prevention measures coupled with better industry practices have helped in the global reduction in oil spillage but the risk involved in significant oil spills remain []. Table 1 provides a record of the largest oil spill cases in the world’s history.

Table 1.
The top 20 largest oil spills (>125,000 tonnes) in world’s history.
Although the effect of PH such as from crude oil spills in the environment has been studied for many years, even with respect to the impact on plants, there is still a gap in understanding how the composition of the affected plants is influenced. Some researchers, such as Levy and Gopalakrishnan [], Okpokwasili and Odokuma [], Njoku et al. [], and Ylitalo et al. [], have reported the effect of crude oil spills on the general environment while others like Venosa et al. [], Ebuehi et al. [], Couto et al. [], as well as Adekunle [], have indicated how the remediation of such polluted sites can be carried out. Various remediation approaches, including bioremediation, have been suggested.
Evaluation of the effect of crude oil on plant growth/yield has also gained attention and the outcome of such investigations have been documented in Kuhn et al. [], Adam and Duncan [], Adieze et al. [], Inckot et al. [], Baruah et al. [], as well as Odukoya et al. [,]. Nonetheless, the effects of crude oil on the composition and quality of crops still remain unclear. In most cases, such as in Baruah et al. [], Chupakhina and Maslennikov [], and Noori et al. [], the impact of crude oil on only a few crop quality parameters was investigated.
Considering the human dependence on agricultural produce for food, this review provides needed information on the impact of crude oil-induced abiotic stresses on plant composition. It discusses the general impact of abiotic environmental stresses on the composition of agricultural produce (particularly with respect to crop quality), and summarises the relationship between these stresses (i.e., crude oil stress and its induced abiotic stresses) via a mechanistic link to provide clarity on the response patterns observed in crude oil-stressed plants. A chemical classification of petroleum hydrocarbons is provided in Figure 1.
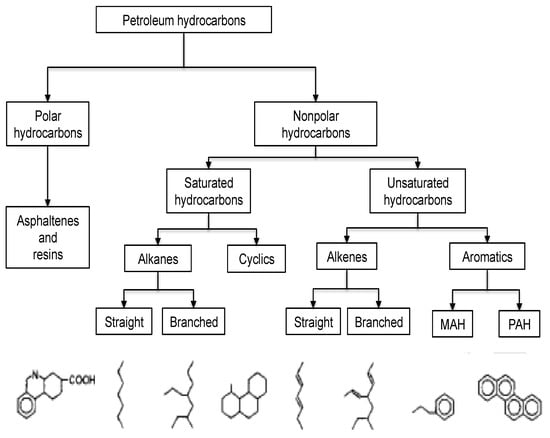
Figure 1.
Chemical classification of petroleum hydrocarbons. (MAH = Monoaromatic hydrocarbons; PAH = Polycyclic aromatic hydrocarbons). Source: Coulon and Wu [].
2. Effects of PH on the Growing Environment and Plants
The behaviour of PH in the environment determines where they are likely to be found, such as in air, water, soil, sediment, food, or other media that people might come in contact with []. In the view of Oghenejoboh et al. [], the factors governing the spread/migration of spilled oil in soil include the amount of oil spilled, physical properties of the spilled oil (density and viscosity), and physical properties of the soil medium itself, particularly its porosity. Although the introduction of PH affects the physical, chemical, and biological properties of soil [], these factors (i.e., the soil type, quantity, and composition of the spilled hydrocarbon) also determine the level of alteration of the soil properties [,].
On agricultural lands, Plice [] and also De Jong [], reported that oil spills can result in reduced plant growth for some time. PH contamination which is associated with an increase in carbon/nitrogen (C/N) ratio and nitrogen deficiency [], may also lead to a reduction in plant stem height, stem density, aboveground biomass, and include death of the plant [,,,,]. Meanwhile, the response of plants to the presence of PH in soil varies [,,] and is associated with the plant age [], species of the plant involved, type and concentration of the petroleum, time of exposure to the contaminant [,,] as well as the season [,], among other factors.
De Jong [], referring to the experiment of Carr [], indicated the possibility of a low concentration of crude oil in the environment supporting plant growth. Although this test was conducted in duplicate, Carr [] found out that at 0.75% w/w of crude oil addition to the soil, the growth of soybeans was improved. Adieze et al. [] also recorded an increase in shoot height and weight of two of the plant species examined at 1% w/w oil-in-soil. This stimulating effect according to Plice [] may be as a result of the bacterial breakdown of the hydrocarbons. Other possible reasons for plant growth-stimulation, as suggested by Baker [], although subject to further investigation, could be attributed to the release of nutrients from the oil, oil-killed vegetation, or hormonal influence. Be that as it may, there are claims that this occurrence in plants is a result of ‘petroleum auxin’ identified as naphthenic acids, which (i) improve the yield of different varieties of crops; (ii) stimulates photosynthesis; and (iii) increases protein nitrogen [].
Notwithstanding possible positive effects, most studies regarding the growth of plants in soil containing PH have reported negative effects [] including on the germination of seeds [,]. Reductions in the plant growth could be a result of (1) the direct toxic effect of oil on plants [,]; (2) absence of viable seeds leading to lack of germination; (3) reduced germination; (4) unfavourable soil conditions []; and (5) inhibition of bacterial decomposition of the soil organic matter and associated nutrient remineralization by the toxic components of oil []. The inhibition of germination may be linked to oil entering the seed and killing the embryo, or oil coating the seed and hindering the uptake of oxygen and water required for germination []. Merkl et al. [] added that reduced seedling emergence could be as a result of toxic effects of the oil or from the adverse soil moisture conditions. Other effects of oil pollution on plants as stated by Baker [] include yellowing and death of oiled leaves, varying sensitivity, and recovery rates of perennials, among others, while a complete elimination may occur at chronic levels. Heavier oils compared to lighter oils present less immediate toxic impact on plants and other organisms [].
Baker [] indicated that photosynthetic rate is consistently reduced by oils, while the level of reduction in this rate depends on the kind and amount of oil as well as the plant species. For instance, the experiment of Odukoya [] involving selected green leafy vegetables (GLV) and a fruit vegetable (tomato (Solanum lycopersicum) indicated that the effect of crude oil contamination on the species differed. Generally, crude oil at the concentration used in this experiment {≤10,000 mg/kg total petroleum hydrocarbons (TPH)} altered stomatal conductance (a measure of the rate of diffusion of carbon dioxide (CO2) into leaves for photosynthesis, and water loss via transpiration []), growth, yield, and composition of the GLV. Figure 2 and Figure 3 show the impact of the crude oil contamination on the stomatal conductance (mmol/m2s) as well as growth of Brassica juncea, Brassica oleracea, and Lactuca sativa. The effect on the flower production date of B. juncea is also provided in Figure 4. The influence on yield and levels of phytochemicals in the selected GLV has been previously discussed (see Odukoya et al. []).
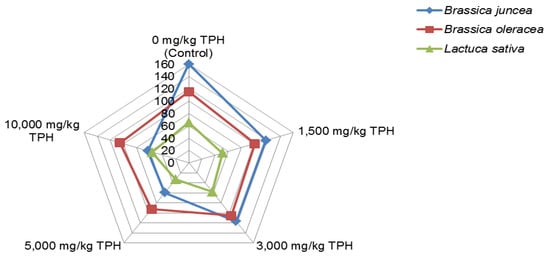
Figure 2.
Effect of crude oil contamination on stomatal conductance (mmol/m2s) of selected green leafy vegetables prior to harvest. Source: Odukoya [].

Figure 3.
The growth of three green leafy vegetables ((A) B. juncea, (B) B. oleracea and (C) L. sativa) in pots containing crude oil at different concentrations. Treatments T1, T2, T3, T4 and T5 are 0 mg/kg TPH (Control), 1500 mg/kg TPH, 3000 mg/kg TPH, 5000 mg/kg TPH, and 10,000 mg/kg TPH, respectively. Source: Odukoya [].
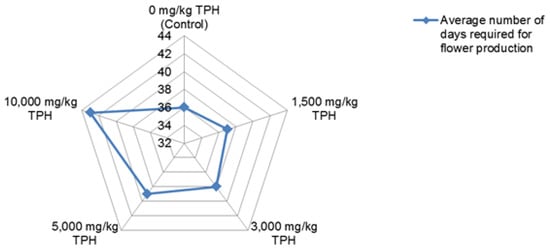
Figure 4.
The effect of crude oil contamination on flower production date of B. juncea. Source: Odukoya [].
Odukoya et al. [,] also recorded that crude oil contamination at concentration as low as 5000 mg/kg TPH affected the growth, yield, fruit production, and ripening of Micro-Tom tomato fruits (Figure 5).

Figure 5.
Growth of Micro-Tom tomatoes in pots containing crude oil at 0 mg/kg TPH (left plant) and 5000 mg/kg TPH (right plant), before fruiting (A) and during ripening (B). Source: Odukoya [].
Considering the effect of time of spillage on plant response, Baker [] reported that application of an emulsion of light oil on young plants in the light when the stomata are opened will lead to death of the plant while the same application at night when the stomata are closed would not harm them. Baker [] also indicated that there is an increased level of phytotoxic risk at high environmental temperature (such as during sunny or hot weather) compared to other times.
3. Abiotic Environmental Stresses and Crop Quality
Abiotic stresses, which include environmental contaminants [], have been identified as the key factors responsible for most of the reduction in agrifood production (Figure 6); they also play a major role in determining the nutritional value/quality of fruits and vegetables during their growth, harvesting, handling, storage, and transport to end users []. The impact made by these abiotic stresses have been found to depend on the (1) the part (tissue or organ) of the plants involved [,]; (2) crop species; (3) duration; as well as (4) intensity of the stress [] and could cause morphological, physiological, biochemical, and molecular alterations within the affected plants []. Retardation of plant growth may also occur as the plants make efforts to conserve and reallocate resources that can become limited under extreme stress conditions [].
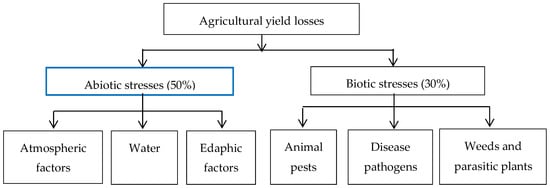
Figure 6.
Agricultural yield losses due to abiotic and biotic stress. Adapted from: Gaur and Sharma [].
Wang and Frei [] indicated that all quality traits of agricultural produce can be influenced by abiotic environmental stress factors which can be linked with the different physiological reactions/responses in the affected plants as a result of changes in gene expression and enzyme activity, oxidative stress arising from the accumulation of reactive oxygen species (ROS), accelerated senescence, which shortens the crop maturity period and alters the nutrient distribution processes within the stressed plants, reduced water content which leads to increase in nutrient content, changes in mineral uptake and translocation, as well as reduced biomass/yield which is the most obvious and identified effect of abiotic environmental stress on agricultural produce. Although some crops respond differently, in most cases, environmental stresses cause (1) a decrease in starch concentration, lipids {particularly the polyunsaturated fatty acids (PUFA)}, feed value, and physical/sensory (P/S) traits; (2) an increase in protein and antioxidant contents; and (3) no clear trend in sugar and mineral contents [].
On the other hand, one of the common mechanisms by which plants adapt to abiotic stresses encountered in the environment involves the accumulation of compatible solutes which are highly soluble and low molecular weight compounds with no toxic effect at high concentrations []. The different forms of these compounds accumulated may be species-specific and include amino acids (like proline), glycine betaine, sugars (such as sucrose, and trehalose), and sugar alcohols (like sorbitol and mannitol) [,,]. These compatible solutes do not interfere with normal cellular metabolism [,].
4. Mechanistic Link between Crude Oil Contamination and Induced Abiotic Stresses
4.1. Induced Physical Influence
The coating of plant leaves with oil may lead to temperature stress owing to the blockage of the transpiration pathways while the process of photosynthesis in the leaves would be negatively affected [,]. The penetration of oxygen into the soil could also be restrained by the layer of oil covering the soil surface resulting in anaerobic soil conditions for the plant roots [,] and contributes to oxygen stress on these roots [].
Nevertheless, the level of reduction in transpiration and photosynthesis arising from the physical blockage of the stomata depend on the extent of oil covering on the plant which is associated with the hydrologic conditions, amount, type, and dispersion ability of the spilled oil [].
4.2. Induced Chemical Influence
The chemical impact of oils on vegetation depends on the type of oil while the fouling of leaves by oil may have more immediate effects than fouling of the soil surface []. Pezeshki et al. [] reported that reduced stomatal conductance with no detectable photosynthetic activity was evident shortly after leaf fouling which suggests potential breakdown of the photosynthetic apparatus in the leaves directly subjected to oil application. In their view, this breakdown of leaf structure and/or the chlorophyll system may be associated with blocked stomata giving rise to reduced transpiration, thus increasing the leaf temperature with the possibility of an adverse effect of the oil on cellular integrity of the leaf tissue. They added that plants may recover from the initial, short-term (often dramatic) adverse effects of oil on leaves while refined products, compared to crude oils, present a different effect on leaves.
Furthermore, as plants like other organisms produce ROS in response to abiotic and biotic stresses [], environmental contamination arising from PH reduce the availability of essential nutrients (like nitrogen and oxygen) required for plant growth [,], including water [] owing to the surface covering of the plant roots by the hydrophobic contaminant, thereby enhancing the production of ROS and hence, oxidative stress in plants [,].
Basically, PH may affect plants by (1) upsetting the plant-water relationships; (2) direct effect on plant metabolism such as nutrient uptake []; (3) their toxicity to living cells [,]; and (4) the reduction in oxygen exchange between the atmosphere and the soil which have negative effects on plants [,].
4.3. The Mechanistic Link
Crude oil/PH contamination, an abiotic factor [], leads to oxidative stress in plants [] and gives rise to the production of ROS. Figure 7 shows the mechanistic link between crude oil contamination/stress and identified crude oil-induced abiotic stresses.
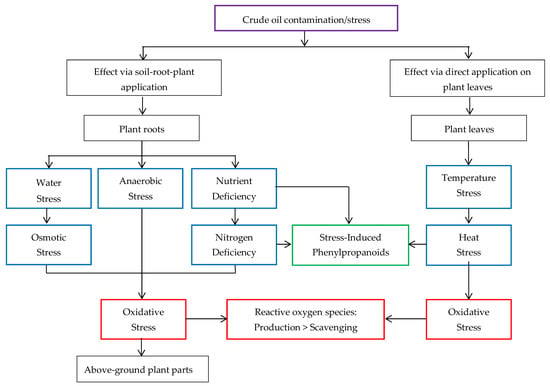
Figure 7.
Illustration of the mechanistic link between crude oil contamination/stress and the crude oil-induced abiotic stresses as it affects agrifood production.
5. The Mode of Action/Plant Response Mechanisms to the Crude Oil-Induced Abiotic Stresses
5.1. Water (Drought) and Osmotic Stresses
“Drought is a meteorological term for a scarcity of water” ([], p. 510) while water stress on plants occurs when there is a limitation of water supply to the roots, or when the transpiration rate exceeds the absorption rate [,]. Photosynthesis and growth are among the key processes affected by drought [,] whose effects can: (1) be direct, such as resulting from the alteration in the diffusion of CO2 in leaves via a decrease in stomatal and mesophyll conductances [,]; or (2) give rise to secondary effects-oxidative stress []. In Farooq et al. [], it was added that drought is also associated with accelerated leaf senescence and reduction in crop yield.
5.1.1. Plants’ Responses to Water Stress
Akinci and Losel [] indicated that plant adaptation to dry environments can be expressed at four different levels which are phenological or developmental, morphological, physiological, and metabolic while the latter (metabolic or biochemical adaptation) is the least understood. Whereas some responses of plants to water stress occur at the leaf level [], it is the general response at the whole-plant level, involving carbon assimilation and the distribution of photoassimilates to different plant parts and reproductive ability that eventually determine the survival of these plants and persistence under environmental stress []. Nonetheless, carbon assimilation at this whole-plant level always decreases as a result of (1) reduction in CO2 diffusion into the leaf; (2) diversion of carbon distribution to non-photosynthetic organs and defence molecules; or (3) biochemical changes in the leaf leading to the down-regulation of photosynthesis []. The various responses of plants to water stress are summarised in Table 2.

Table 2.
Summary of plants’ responses to water stress.
5.1.2. Osmotic Stress
In line with Zhu et al. [], osmotic stress may be used to refer to conditions in which there is a limitation on plant growth and development as a result of shortage of water availability. Although osmotic stress can result from drought, excessive salt in water, chilling, and freezing [], Xiong and Zhu [] identified drought and high salinity as the chief causes of stress to plants under natural conditions.
Plants respond to osmotic stress at the morphological, anatomical, and cellular levels [] which results in alterations in their development (such as the plant life cycle, limitation of shoot growth, and enhancement of root growth), regulation in ion transport as well as metabolic changes which may involve carbon metabolism and production of compatible solutes [,]. Some of these responses according to Xiong and Zhu [] are triggered by the primary osmotic stress signals while others can be linked to secondary stresses/signals resulting from the primary signals. Examples of the secondary signals are phytohormones [such as abscisic acid (ABA) and ethylene], ROS as well as intracellular second messengers (e.g., phospholipids) [].
Generally, the response of plants to osmotic stresses can be classified into three different forms involving (a) the maintenance of homeostasis; (b) detoxification of harmful elements; and (c) efforts towards growth recovery [].
5.2. Anaerobic Stress
Higher plants, being aerobes, need molecular oxygen from their environment for survival []. However, under certain environmental conditions, there is a possibility of shortage in the supply of O2 to plant tissues []. When this happens, that is, when there is restricted aeration of part or all of the plant, “the resulting tissue hypoxia or anoxia inevitably suppresses oxygen-dependent pathways especially the energy-generating system, disturbs functional relationships between organs such as roots and shoots, as well as suppresses both carbon assimilation and photosynthate utilization” [].
This shortage in the supply of oxygen has more direct effect on underground organs such as the roots and seeds; the shoot systems are then indirectly affected as a result of the negative impact of the stress on the root functions upon which the shoots depend []. The mitochondria have also been identified by Vartapetian et al. [] as suffering from oxygen deficiency before other cell organelles are affected. Most plants tissues can, however, withstand anoxia (lack of oxygen) only for a short period of time before irreversible damage occurs [].
Plants’ Responses to Anaerobic Stress
Depletion of oxygen affects cell physiology; it alters gene expression, energy consumption, cellular metabolism, growth, and development []. Based on findings by the authors, Blokhina et al. [] indicated that lack of oxygen primarily results in a decrease in adenylate energy charge, cytoplasmic acidification, anaerobic fermentation, increase in cytosolic Ca2+ concentration, alterations in the redox state, and a decline in the membrane barrier function. Low-oxygen (hypoxia) stress is also reported to induce significant changes in the transcriptome as well as a shift from aerobic to anaerobic respiration [].
- (a)
- Pasteur effect: Mustroph et al. [] identified the Pasteur effect as a common eukaryotic response to oxygen deficiency at the cellular level. According to Winkler et al. ([], p. 721), this definition “that the rate of fermentation rises when oxygen is excluded” for the Pasteur effect by Krebs [] has long been accepted in place of that involving “the inhibition of glycolysis by respiration”. Kennedy et al. [] added that most anaerobic-intolerant plants exhibit a pronounced Pasteur effect.
- (b)
- ROS production and oxidative stress: Like many other stress conditions, hypoxia is associated with the excess generation of ROS []. Along these lines, there are two models which suggest (1) a decrease in ROS under oxygen deprivation {low NADPH-nicotine adenine dinucleotide phosphate []—oxidase activity}; or (2) an increase in ROS due to inhibition of the mitochondrial electron transport chain.
- (c)
- Gene expression: As the synthesis of several proteins involved in glycolysis and fermentation processes is induced in plants under anaerobic conditions [], Agarwal and Grover [] noted that plants respond to low O2-stress condition via specific alterations in gene expression. Generally, anaerobiosis gives rise to the alteration of gene expression in plants which leads to the accumulation of anaerobic proteins (ANPs) [] many of which are metabolic pathway enzymes [].
5.3. Nutrient Deficiency
Plants need nutrient elements for their normal growth and development in which the deficiency in any required element will have a significant impact []. Clarkson et al. [] indicated that the level of availability of certain mineral nutrients can alter plant transpiration, stomatal conductance, and root hydraulic conductivity while the deficiency of any of these three plant nutrients—nitrate, phosphate, and sulphate—in the growth medium, would impact the stomatal conductance and root hydraulic conductivity in a similar way.
In line with Kandlbinder et al. [], there are two contrasting developmental and metabolic effects that can be induced by nutrient deficiency. At the first instance, which shows the adaptive response, the growth of plants may decrease in an organised manner in which the number as well as size of each part of the plant (involving the roots, leaves, shoots, and regenerative organs) are reduced while the metabolic activity and ‘fitness’ are to a large extent unaffected []. In the second, the unbalanced response gives rise to a disturbed environment and dysfunction of the whole plant—the plant becomes stressed [].
5.3.1. Plants’ Responses to Nutrient Deficiency
(a) Biosynthesis of stress-induced phenylpropanoids: Environmental (biotic and abiotic) stresses like pathogen attack, wounding, nutrient deficiencies, and temperature, among others (Figure 8), are capable of enhancing the levels of phenylpropanoids in plants [,].
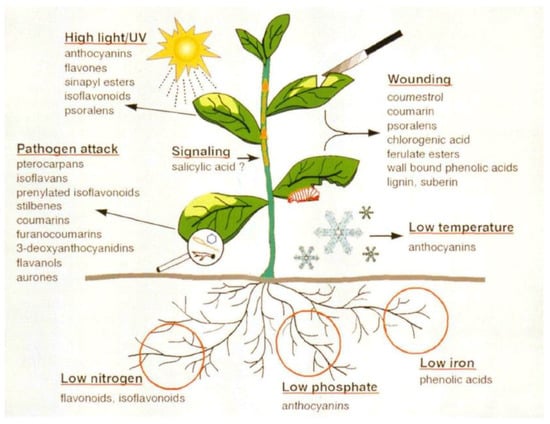
Figure 8.
Examples of stress-induced phenylpropanoids. Source: Dixon and Paiva [].
These phenylpropanoids are plant secondary metabolites [,,] derived from trans-cinnamic acid, produced from the deamination of L-phenylalanine via the action of the enzyme—phenylalanine ammonia-lyase (PAL) [,]. The other two enzymes involved in the first three steps that brings about the synthesis of these secondary metabolites (phenylpropanoid-derived compounds), which together are referred to as the general phenylpropanoid pathway (GPP) (Figure 9), are cinnamate 4-hydroxylase (C4H) and p-coumaroyl coenzyme A ligase (4CL) [].
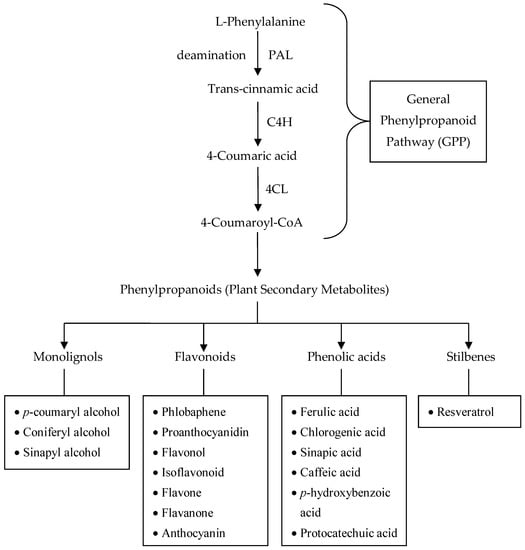
Figure 9.
Biosynthesis pathway of some plant phenylpropanoid compounds (PAL = phenylalanine ammonia lyase; C4H = cinnamate 4-hydroxylase; 4CL = p-coumaroyl coenzyme A ligase). Adapted from: [,].
In line with Lillo et al. [], the shikimate pathway is found in plants and provides phenylalanine for the synthesis of protein and secondary metabolites (like lignin and flavonoids). This shikimate pathway, with distinct patterns of organ-specific as well as tissue-specific activity, however, depends on developmental regulation and environmental stimuli []. The enzymes of this pathway also respond to nitrogen and amino acid starvation [].
On the other hand, Gershenzon [] indicated that nitrogen, phosphorus, potassium, and sulphur deficiencies most times lead to higher concentrations of phenolic compounds.
(b) Oxidative stress: Following the acknowledgment of the relationship between macronutrient deficiency and oxidative stress by Tewari et al. [], Kandlbinder et al. [] in their study found that N-, P-, and S-nutrient deprivation triggered redox changes and induced oxidative stress.
5.3.2. Nitrogen Deficiency/Stress
Aside from oxygen, carbon, and hydrogen, among other mineral nutrients, plants need greater amounts of nitrogen [] whose deficiency Kovacik et al. [] regarded as an abiotic stress factor based on the experimental results of Shin et al. [] in which H2O2 production occurred in nitrogen-deprived roots. Kovacik et al. [] also considered its absence as a form of abiotic stress.
Nitrogen deficiency is associated with increased phenolic concentration [,,] including flavonoids [,,] and coumarins [,]. It is, however, linked with reduced mass-based protein content []. In the view of Bongue-Bartelsman and Phillips [], the increased deamination of phenylalanine could be responsible for elevated flavonoid content under nitrogen limitation.
In addition, Kovacik et al. [] indicated that nitrogen deficiency will affect amino acids and carbon metabolism while Kovacik et al. [] as well as Shin et al. [] added that nitrogen limitation can encourage the generation of ROS. When the level of these ROS produced exceeds that of removal by the antioxidant defense mechanisms, oxidative stress occurs in the cell [].
The indicators of senescence, which is an important outcome of N or P deficiency [], is reported to be similar to those of oxidative stress which include net loss of chloroplastic pigments and proteins [,,,], lipid peroxidation, and membrane alterations, giving rise to a progressive decline in photosynthetic capacity [,].
5.4. Temperature (Heat) Stress
Although living organisms recognise temperature beyond the normal optimal as heat stress [], Wahid et al. [] referred the transient increase in temperature, usually 10–15 oC above ambient, as heat shock or heat stress. They also added that heat stress occurs when there is an increase in temperature above a threshold level for a period of time which can cause irreversible damage to plant growth as well as development. In other words, heat stress depends on the intensity (in terms of temperature in degrees), time of exposure, and rate of increase in temperature. For instance, very high temperatures may lead to severe cellular injury and cell death within minutes as a result of the damaging effect on cellular organization. However, at moderately high temperature, injuries, or death may still occur but after a long-term exposure [].
Plants’ Responses to Temperature (Heat) Stress
The direct adverse effects of high temperature include protein denaturation and aggregation, as well as increased fluidity of membrane lipids. Meanwhile, enzyme inactivation in chloroplast and mitochondria, inhibition of protein synthesis, protein degradation, and loss of membrane integrity are associated with indirect or slower heat injuries. The resultant effects of these injuries are starvation, growth inhibition, reduced ion flux as well as production of toxic compounds and ROS (Wahid et al. []). The established responses of plants to temperature/heat stress are highlighted in Table 3.

Table 3.
Summary of plants’ responses to temperature/heat stress.
5.5. Oxidative Stress
Oxidative stress is defined as a “state in which oxidation exceeds the antioxidant systems in the body secondary to a loss of balance between them” ([], p. 271) and according to Lushchak ([], p. 176) “its development is either the reason, or common event of many pathological states, including aging”. In line with Lushchak [], oxidative stress occurs in situations where the equilibrium between ROS generation and elimination is upset leading to their enhanced steady-state level. Birben et al. [] referred it to a shift in balance between oxidant/antioxidant which favours the oxidants as shown in Figure 10. This perturbation of the equilibrium between generation and scavenging of ROS may be caused by various biotic and abiotic stress factors which are known to reduce global crop production [].
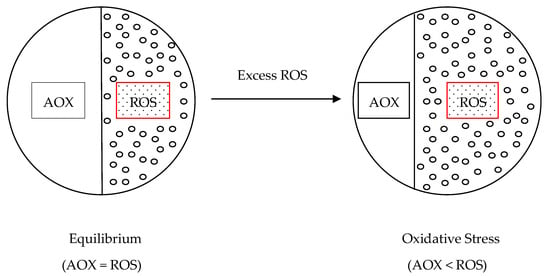
Figure 10.
Relationship between oxidants (ROS) and antioxidants (AOX) leading to oxidative stress.
The ROS consists of free radicals, reactive molecules, as well as ions that are derived from O2, which in plants, depending on their concentrations, can either be harmful or beneficial []. The most common ROS are singlet oxygen (1O2), superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•) []. At elevated concentrations, ROS bring about damage to biomolecules while at low/moderate concentration, they function as second messengers in intracellular signaling cascades that mediate several responses in plant cells [] such as stomatal closure [,,], programmed cell death [,,], gravitropism [,], and acquisition of tolerance to both biotic and abiotic stresses []. Mittler [] added that ROS levels that are too low (cytostatic level) or too high (cytotoxic level) have a negative effect on plant growth and development while ROS level within the right range (basal level) support plant health.
As noted by Sharma et al. [], in plants, ROS are generated in both unstressed and stressed cells at different locations in chloroplasts, mitochondria, plasma membranes, peroxisomes, apoplast, endoplasmic reticulum, and cell walls. These authors added that the ROS are usually produced as a result of the leakage of electrons onto O2 from the electron transport activities of chloroplasts, mitochondria, and plasma membranes or as byproducts of various metabolic pathways in different cellular compartments.
Generally, under ideal growth conditions, the generation of ROS in organelles is low [] but are formed excessively during adverse/stressful conditions [,], which can bring about damage to biomolecules like lipids, proteins, and deoxyribonucleic acid (DNA) []. These reactions according to Sharma et al. [] can affect intrinsic membrane properties such as fluidity, ion transport, loss of enzyme activity, protein cross-linking, inhibition of protein synthesis, and DNA damage, which eventually lead to cell death. Gill and Tuteja [] pointed out that in addition to the ability of ROS to damage cells, they can also initiate responses such as new gene expression.
Plants’ Responses to ROS and Oxidative Stress (Antioxidant Systems)
In line with Gill and Tuteja [], the balance between generation of ROS and scavenging at the proper site as well as time, determines whether ROS will act as damaging, protective or signaling factors. Hence, as a result of the multifunctional roles of these ROS, cells must control their levels to prevent any oxidative injury but not eliminate them completely []. The scavenging or detoxification of excess ROS is carried out by the antioxidant system comprising the non-enzymatic antioxidants and enzymatic antioxidants []. The former class of non-enzymatic antioxidants within cells is comprised of ascorbic acid (AA), glutathione (GSH), carotenoids, α-tocopherol, phenolics and amino acids like proline while the latter class (enzymatic antioxidants) include superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), and guaiacol peroxidase (GPX) [,].
Basically, the ability of plants to maintain a high antioxidant capacity to scavenge toxic ROS (i.e., reduce oxidative stress) implies increased plant tolerance level to environmental stresses [,]. Figure 11 provides a connection between the impact of the various levels of ROS on plants and the antioxidant system.
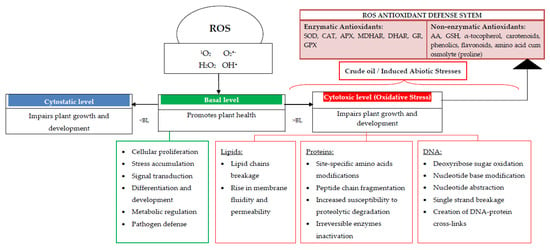
Figure 11.
Connection between the impact of the various levels of ROS on plants and the antioxidant system. ROS = reactive oxygen species; BL = basal level; DNA = deoxyribonucleic acid; SOD = superoxide dismutase, CAT = catalase, APX = ascorbate peroxidase, MDHAR = monodehydroascorbate reductase, DHAR = dehydroascorbate reductase, GR = glutathione reductase, GPX = guaiacol peroxidase; AA = ascorbic acid, GSH = glutathione. Adapted from: Reference [,,].
6. Conclusions
Although a number of studies have recorded the negative impacts of crude oil contamination at toxic levels on plants, this review elucidates the underlying factors responsible for the observed responses in the crude oil-stressed plants. It highlights the various ways in which crude oil and its induced abiotic stresses may affect the composition of agricultural produce. It is believed that a clear understanding on the influence of crude oil contamination/induced abiotic stresses on crop yield, quality, and agrifood production, in general, would assist the government, agronomists, environmental as well as food scientists in proffering solutions to the problem of food security in regions of the world prone to/affected by crude oil spills.
Author Contributions
J.O., R.L., and R.S. provided the concept. J.O. and R.S. wrote, reviewed and edited the manuscript.
Funding
This research was funded by the Petroleum Technology Development Fund (PTDF), Nigeria, PhD scholarship award grant number PTDF/E/OSS/PHD/OJO/625/12.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Athar, H.; Ambreen, S.; Javed, M.; Hina, M.; Rasul, S.; Zafar, Z.U.; Manzoor, H.; Ogbaga, C.C.; Afzal, M.; Al-Qurainy, F.; et al. Influence of sub-lethal crude oil concentration on growth, water relations and photosynthetic capacity of maize (Zea mays L.) plants. Environ. Sci. Pollut. Res. 2015, 23, 18320–18331. [Google Scholar] [CrossRef] [PubMed]
- Khamehchiyan, M.; Hossein Charkhabi, A.; Tajik, M. Effects of crude oil contamination on geotechnical properties of clayey and sandy soils. Eng. Geol. 2007, 89, 220–229. [Google Scholar] [CrossRef]
- Ajagbe, W.O.; Omokehinde, O.S.; Alade, G.A.; Agbede, O.A. Effect of crude oil impacted sand on compressive strength of concrete. Constr. Build. Mater. 2012, 26, 9–12. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Elkin, A.A.; Makarov, S.O.; Cunningham, C.J.; Peshkur, T.A.; Atlas, R.M.; Philp, J.C. Oil spill problems and sustainable response strategies through new technologies. Environ. Sci. Process. Impacts 2015, 17, 1201–1219. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, J.O. Influence of Bioremediation on the Chemical and Nutritional Composition of Produce from Crude Oil-Polluted Sites. Ph.D. Thesis, Cranfield University, Cranfield, UK, 2016. [Google Scholar]
- Ndimele, P.E.; Saba, A.O.; Ojo, D.O.; Ndimele, C.C.; Anetekhai, M.A.; Erondu, E.S. Remediation of crude oil spillage. In The Political Ecology of Oil and Gas Activities in the Nigerian Aquatic Ecosystem; Ndimele, P.E., Ed.; Academic Press: London, UK, 2018; pp. 369–384. [Google Scholar]
- Schmidt-Etkin. Spill occurences: A world overview. In Oil Spill science and Technology: Prevention, Response, and Cleanup, 1st ed.; Fingas, M., Ed.; Gulf Professional Publishing: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Yavari, S.; Malakahamad, A.; Sapari, N.B. A review on phytoremediation of crude oil spills. Water Air Soil Pollut. 2015, 226, 226. [Google Scholar] [CrossRef]
- Levy, J.K.; Gopalakrishnan, C. Promoting ecological sustainability and community resilience in the US Gulf Coast after the 2010 Deepwater Horizon oil spill. J. Nat. Resour. Policy Res. 2010, 2, 297–315. [Google Scholar] [CrossRef]
- Okpokwasili, G.; Odokuma, L. Effect of salinity on biodegradation of oil spill dispersants. Waste Manag. 1990, 10, 141–146. [Google Scholar] [CrossRef]
- Njoku, K.L.; Akinola, M.O.; Oboh, B.O. Phytoremediation of crude oil contaminated soil: The effect of growth of Glycine max on the physico-chemistry and crude oil contents of soil. Nat. Sci. 2009, 7, 22–30. [Google Scholar]
- Ylitalo, G.M.; Krahn, M.M.; Dickhoff, W.W.; Stein, J.E.; Walker, C.C.; Lassitter, C.L.; Garrett, E.S.; Desfosse, L.L.; Mitchell, K.M.; Noble, B.T.; et al. Federal seafood safety response to the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 20274–20279. [Google Scholar] [CrossRef]
- Venosa, A.D.; Suidan, M.T.; Wrenn, B.A.; Strohmeier, K.L.; Haines, J.R.; Eberhart, B.L.; King, D.; Holder, E. Bioremediation of an experimental oil spill on the shoreline of Delaware Bay. Environ. Sci. Technol. 1996, 30, 1764–1775. [Google Scholar] [CrossRef]
- Ebuehi, O.; Abibo, I.; Shekwolo, P.; Sigismund, K.; Adoki, A.; Okoro, I. Remediation of crude oil contaminated soil by enhanced natural attenuation technique. J. Appl. Sci. Environ. Manag. 2005, 9, 103–106. [Google Scholar]
- Couto, M.N.P.F.S.; Monteiro, E.; Vasconcelos, M.T.S.D. Mesocosm trials of bioremediation of contaminated soil of a petroleum refinery: Comparison of natural attenuation, biostimulation and bioaugmentation. Environ. Sci. Pollut. Res. 2010, 17, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, I.M. Bioremediation of soils contaminated with Nigerian petroleum products using composted municipal wastes. Bioremediat. J. 2011, 15, 230–241. [Google Scholar] [CrossRef]
- Kuhn, W.; Gambino, R.; Al-Awadhi, N.; Balba, M.T.; Dragun, J. Growth of tomato plants in soil contaminated with Kuwait crude oil. J. Soil Contam. 1998, 7, 801–806. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Influence of diesel fuel on seed germination. Environ. Pollut. 2002, 120, 363–370. [Google Scholar] [CrossRef]
- Adieze, I.E.; Orji, J.C.; Nwabueze, R.N.; Onyeze, G.O.C. Hydrocarbon stress response of four tropical plants in weathered crude oil contaminated soil in microcosms. Int. J. Environ. Stud. 2012, 69, 490–500. [Google Scholar] [CrossRef]
- Inckot, R.C.; Santos, G.D.O.; de Souza, L.A.; Bona, C. Germination and development of Mimosa pilulifera in petroleum-contaminated soil and bioremediated soil. Flora 2011, 206, 261–266. [Google Scholar] [CrossRef]
- Baruah, P.; Saikia, R.R.; Baruah, P.P.; Deka, S. Effect of crude oil contamination on the chlorophyll content and morpho-anatomy of Cyperus brevifolius (Rottb.) Hassk. Environ. Sci. Pollut. Res. 2014, 21, 12530–12538. [Google Scholar] [CrossRef]
- Odukoya, J.; Lambert, R.; Sakrabani, R. Influence of sub-lethal concentrations of crude oil on tomato yield and quality. SAJFTE 2018, 4, 722–733. [Google Scholar]
- Odukoya, J.; Lambert, R.; Sakrabani, R. Impact of crude oil on yield and phytochemical composition of selected green leafy vegetables. Int. J. Veg. Sci. 2019. [Google Scholar] [CrossRef]
- Chupakhina, G.N.; Maslennikov, P.V. Plant adaptation to oil stress. Russ. J. Ecol. 2004, 35, 290–295. [Google Scholar] [CrossRef]
- Noori, A.S.; Maivan, H.Z.; Alaie, E. Changes in total phenol and flavonoid contents in Chrysanthemum leucanthemum under crude oil contamination. Adv. Environ. Biol. 2012, 6, 3057–3064. [Google Scholar]
- Coulon, F.; Wu, G. Determination of Petroleum Hydrocarbon Compounds from Soils and Sediments Using Ultrasonic Extraction. In Hydrocarbon and Lipid Microbiology Protocols: Petroleum, Hydrocarbon and Lipid Analysis; McGenity, T.J., Timmis, K.N., Nogales, B., Eds.; Springer Protocols Handbooks: Berlin, Heidelberg, 2014; pp. 31–46. [Google Scholar]
- United Nations Environment Programme, UNEP. Environmental Assessment of Ogoniland; 978-92-807-3130-9; United Nations Environment Programme: Nairobi, Kenya, 2011. [Google Scholar]
- Oghenejoboh, K.M.; Puyate, Y.T.; Abowei, M.F.N. Concentration distribution of spilled crude petroleum in different soils 2: Effects of volume of oil on spatial spread. Pollut. Res. 2008, 27, 605–610. [Google Scholar]
- Pezeshki, S.R.; DeLaune, R.D. United States Gulf of Mexico coastal marsh vegetation response and sensitivities to oil spill: A review. Environments 2015, 2, 586–607. [Google Scholar] [CrossRef]
- Plice, M.J. Some effects of crude petroleum on soil fertility. Soil Sci. Soc. Proc. 1948, 413–416. [Google Scholar] [CrossRef]
- De Jong, E. The effect of a crude oil spill on cereals. Environ. Pollut. 1980, 22, 187–196. [Google Scholar] [CrossRef]
- Merkl, N.; Schultze-Kraft, R.; Infante, C. Phytoremediation in the tropics—the effect of crude oil on the growth of tropical plants. Bioremediat. J. 2004, 8, 177–184. [Google Scholar] [CrossRef]
- Hershner, C.; Lake, J. Effects of chronic oil pollution on salt-marsh grass community. Mar. Biol. 1980, 56, 163–173. [Google Scholar] [CrossRef]
- Ferrell, R.E.; Seneca, E.D.; Linthurst, R.A. The effects of crude oil on the growth of Spartina alterniflora Liosel. and Spartina cynosuroides (L.) Roth. J. Exp. Mar. Biol. Ecol. 1984, 83, 27–39. [Google Scholar] [CrossRef]
- Alexander, S.K.; Webb, J.W. Relationship of Spartina alterniflora growth to sediment oil content following an oil spill. In 1987 Oil Spill Conference Proceedings; American Petroleum Institute: Washington, DC, USA, 1987; Volume 1, pp. 445–449. [Google Scholar]
- Lin, Q.; Mendelssohn, I.A. A comparative investigation of the effects of South Louisiana crude oil on the vegetation of fresh, brackish and salt marshes. Mar. Pollut. Bull. 1996, 32, 202–209. [Google Scholar] [CrossRef]
- Lin, Q.; Mendelssohn, I.A. The combined effects of phytoremediation and biostimulation in enhancing habitat restoration and oil degradation of petroleum contaminated wetlands. Ecol. Eng. 1998, 10, 263–274. [Google Scholar] [CrossRef]
- Baker, J. The effects of oils on plants. Environ. Pollut. 1970, 1, 27–44. [Google Scholar] [CrossRef]
- Baek, K.; Kim, H.; Oh, H.; Yoon, B.; Kim, J.; Lee, I. Effects of crude oil, oil components, and bioremediation on plant growth. J. Environ. Sci. Health A 2004, 39, 2465–2472. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; Hester, M.W.; Lin, Q.; Nyman, J.A. The effects of oil spill and clean-up on dominant US Gulf coast marsh macrophytes: A review. Environ. Pollut. 2000, 108, 129–139. [Google Scholar] [CrossRef]
- Alexander, S.K.; Webb, J.W. Seasonal response of Spartina alterniflora to oil. In 1985 Oil Spill Conference Proceedings; American Petroleum Institute: Washington, DC, USA, 1985; Volume 1, pp. 355–357. [Google Scholar]
- Carr, R. Vegetative growth in soils containing crude petroleum. Soil Sci. 1919, 8, 67–68. [Google Scholar] [CrossRef][Green Version]
- Rebetzke, G.; Read, J.; Barbour, M.; Condon, A.; Rawson, H. A hand-held porometer for rapid assessment of leaf conductance in wheat. Crop Sci. 2000, 40, 277–280. [Google Scholar] [CrossRef]
- Collier, R.J.; Renquist, B.J.; Xiao, Y. A 100-year review: Stress physiology including heat stress. J. Dairy Sci. 2017, 100, 10367–10389. [Google Scholar] [CrossRef]
- Kumar, S.P.; Minhas, P.S.; Govindasamy, V.; Choudhary, R.L. Influence of moisture stress on growth, development, physiological process and quality of fruits and vegetables and its management strategies. In Approaches to Plant Stress and Their Management; Gaur, R.K., Sharma, P., Eds.; Springer: New Delhi, India, 2014; pp. 125–148. [Google Scholar]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BioMed. Cent. Plant Biol. 2011, 11, 1–4. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Stressed food—the impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotech. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Gaur, R.K.; Sharma, P. Approaches to Plant Stress and Their Management; Springer: New Delhi, India, 2014. [Google Scholar]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. India 2004, 86, 407–421. [Google Scholar]
- Pezeshki, S.R.; DeLaune, R.D.; Nyman, J.A.; Lessard, R.R.; Canevari, G.P. Removing oil and saving oiled marsh grass using a shoreline cleaner. In 1995 International Oil Spill Conference Proceedings; American Petroleum Institute: Washington, DC, USA, 1995; pp. 203–209. [Google Scholar]
- Noori, A.; Maivan, H.Z.; Alaie, E.; Newman, L.A. Leucanthemum vulgare Lam. crude oil phytoremediation. Int. J. Phytoremed. 2015, 20, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Ogbo, E.M.; Zibigha, M.; Odogu, G. The effect of crude oil on growth of the weed (Paspalum scrobiculatum L.)-phytoremediation potential of the plant. Afr. J. Environ. Sci. Technol. 2009, 3, 229–233. [Google Scholar]
- Noori, A.S.; Maivan, H.Z.; Alaie, E. Leucanthemum vulgare Lam. germination, growth and mycorrhizal symbiosis under crude oil contamination. Int. J. Phytoremed. 2014, 16, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhou, Q. Oxidative stress biomarkers of the polychaete Nereis diversicolor exposed to cadmium and petroleum hydrocarbons. Ecotox. Environ. Saf. 2008, 70, 106–114. [Google Scholar] [CrossRef]
- Prendeville, G.N.; Warren, G.F. Effect of four herbicides and two oils on leaf-cell membrane permeability. Weed Res. 1977, 30, 251–258. [Google Scholar] [CrossRef]
- Stebbings, R.E. Recovery of salt marsh in Britanny sixteen months after heavy pollution by oil. Environ. Pollut. 1970, 1, 163–167. [Google Scholar] [CrossRef]
- Nie, M.; Xian, N.; Fu, X.; Chen, X.; Li, B. The interactive effects of petroleum-hydrocarbon spillage and plant rhizosphere on concentrations and distribution of heavy metals in sediments in the Yellow River Delta, China. J. Hazard. Mater. 2010, 174, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Lisar, S.Y.S.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I.M.M. Water stress in plants: Causes, effects and responses. In Water Stress; Rahman, I.M., Hasegawa, H., Eds.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Chaves, M.M. Effects of water deficits on carbon assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot-Lond. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Akinci, S.; Losel, D.M. Plant water-stress response mechanisms. In Water Stress; Rahman, I.M., Hasegawa, H., Eds.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field. Photosynthesis and growth. Ann. Bot-Lond. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Timpa, J.D.; Burke, J.J.; Quisenberry, J.E.; Wendt, C.W. Effect of water stress on the organic acid and carbohydrate compositions of cotton plants. Plant Physiol. 1986, 82, 724–728. [Google Scholar] [CrossRef]
- Sharp, R.E. Interaction with ethylene: Changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ. 2002, 25, 211–222. [Google Scholar] [CrossRef]
- Sharp, R.E.; Davies, W.J. Regulation of growth and development of plants growing with a restricted supply of water. In Plants under Stress; Jones, H.G., Flowers, T.J., Jones, M.B., Eds.; Seminar Series 39; Society for Experimental Biology, Cambridge University Press: Cambridge, UK, 1989; pp. 71–93. [Google Scholar]
- Alam, S.M. Nutrient uptake by plants under stress conditions. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 285–313. [Google Scholar]
- Cornic, G.; Le Gouallec, J.L.; Briantais, J.M.; Hodges, M. Effect of dehydration and high light on photosynthesis of two C3 plants (Phaseolus vulgaris L. and Elatostema repens (Lour.) Hall f.). Planta 1989, 177, 84–90. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Cleland, R.E. Water stress and protein synthesis II. Interaction between water stress, hydrostatic pressure, and abscisic acid on the pattern of protein synthesis in Avena coleoptiles. Plant Physiol. 1975, 55, 782–785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ben-Zioni, A.; Itai, C.; Vaadia, Y. Water and salt stresses, kinetin and protein synthesis in tobacco leaves. Plant Physiol. 1967, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with water stress: Evolution of osmolyte systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Pham Thi, A.T.; Borrel-Flood, C.; Da Silva, J.V.; Justin, A.M.; Mazliak, P. Effects of water stress on lipid metabolism in cotton leaves. Phytochemistry 1985, 24, 723–727. [Google Scholar]
- Teotia, S.; Singh, D. Oxidative stress in plants and its management. In Approaches to Plant Stress and Their Management; Gaur, R.K., Sharma, P., Eds.; Springer: New Delhi, India, 2014; pp. 227–253. [Google Scholar]
- Zhu, J.; Hasegawa, P.M.; Bressan, R.A. Molecular aspects of osmotic stress in plants. Crit. Rev. Plant Sci. 1997, 16, 253–277. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Vartapetian, B.B.; Jackson, M.B. Plant adaptations to anaerobic stress. Ann. Bot-Lond. 1997, 79 (Suppl. A), 3–20. [Google Scholar] [CrossRef]
- Ellis, M.H.; Dennis, E.S.; Peacock, W.J. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 1999, 119, 57–64. [Google Scholar] [CrossRef]
- Vartapetian, B.B.; Andreeva, I.N.; Generozova, I.P.; Polyakova, L.I.; Maslova, I.P.; Dolgikh, Y.I.; Stepanova, A.Y. Functional electron microscopy in studies of plant response and adaptation to anaerobic stress. Ann. Bot-Lond. 2003, 91, 155–172. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Rumpho, M.E.; Fox, T.C. Anaerobic metabolism in plants. Plant Physiol. 1992, 100, 1–6. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Chang, R. Sensing and Signalling in response to oxygen deprivation in plants and other organisms. Ann. Bot-Lond. 2005, 96, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot-Lond. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R. MicroRNAs with macro-effects on plant stress responses. Semin. Cell. Dev. Biol. 2010, 21, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, A.; Lee, S.C.; Oosumi, T.; Zanetti, M.E.; Yang, H.; Ma, K.; Yaghoubi-Masihi, A.; Fukao, T.; Bailey-Serres, J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010, 152, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Sauer, M.W.; Starnes, C.A. Modulation of the Pasteur effect in retinal cells: Implications for understanding compensatory metabolic mechanisms. Exp. Eye Res. 2003, 76, 715–723. [Google Scholar] [CrossRef]
- Krebs, H.A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972, 8, 1–35. [Google Scholar] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Umeda, M.; Uchimiya, H. Differential transcript levels of genes associated with glycolysis and alcohol fermentation in rice plants (Oryza sativa L.) under submergence stress. Plant Physiol. 1994, 106, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Grover, A. Isolation and transcription profiling of low-O2 stress-associated cDNA clones from the flooding-stress-tolerant FR13A rice genotype. Ann. Bot-Lond. 2005, 96, 831–844. [Google Scholar] [CrossRef]
- Sachs, M.M.; Subbaiah, C.C.; Saab, I.N. Anaerobic gene expression and flooding tolerance in maize. J. Exp. Bot. 1996, 47, 1–15. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta 2012, 1819, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, D.T.; Carvajal, M.; Henzler, T.; Waterhouse, R.N.; Smyth, A.J.; Cooke, D.T.; Steudle, E. Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress. J. Exp. Bot. 2000, 51, 342. [Google Scholar] [CrossRef]
- Kandlbinder, A.; Finkemeier, I.; Wormuth, D.; Hanitzsch, M.; Dietz, K. The antioxidant status of photosynthesizing leaves under nutrient deficiency: Redox regulation, gene expression and antioxidant activity in Arabidopsis thaliana. Physiol. Plant. 2004, 120, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Solecka, D. Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol. Plant. 1997, 19, 257–268. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, S. Biosynthesis and regulation of phenylpropanoids in plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Weaver, L.M.; Herrmann, K.M. Dynamics of the shikimate pathway in plants. Trends Plant Sci. 1997, 2, 346–351. [Google Scholar] [CrossRef]
- Gershenzon, J. Changes in the levels of plant secondary metabolites under water and nutrient stress. In Phytochemical Adaptations to Stress; Timmermann, B.N., Steelink, C., Loewus, F.A., Eds.; Springer Science+Business Media: New York, NY, USA, 1984. [Google Scholar]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative stress and antioxidant responses in young leaves of mulberry plants grown under nitrogen, phosphorus or potassium deficiency. J. Integr. Plant Biol. 2007, 49, 313–322. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Kovacik, J.; Klejdus, B.; Backor, M.; Repcak, M. Phenylalanine ammonia-lyase activity and phenolic compounds accumulation in nitrogen-deficient Matricaria chamomilla leaf rosettes. Plant Sci. 2007, 172, 393–399. [Google Scholar] [CrossRef]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Kovacik, J.; Repcak, M.; Kron, I. Nitrogen deficiency induced changes of free amino acids and coumarin contents in the leaves of Matricaria chamomilla. Acta Physiol. Plant. 2006, 28, 159–164. [Google Scholar] [CrossRef]
- Chishaki, N.; Horiguchi, T. Responses of secondary metabolism in plants to nutrient deficiency. In Plant Nutrition for Sustainable Food Production and Environment; Ando, T., Fujita, K., Mae, T., Matsumoto, H., Mori, S., Sekiya, J., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 341–345. [Google Scholar]
- Bongue-Bartelsman, M.; Phillips, D.A. Nitrogen stress regulates gene expression of enzymes in the flavonoid biosynthetic pathway of tomato. Plant Physiol. Biochem. 1995, 33, 539–546. [Google Scholar]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001, 24, 1189–1197. [Google Scholar] [CrossRef]
- Meyer, S.; Cerovic, Z.G.; Goulas, Y.; Montpied, P.; Demotes-Mainard, S.; Bidel, L.P.R.; Moya, I.; Dreyer, E. Relationships between optically assessed polyphenols and chlorophyll contents, and leaf mass per area ratio in woody plants: A signature of the carbon-nitrogen balance within leaves? Plant Cell Environ. 2006, 29, 1338–1348. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Trippi, V.S.; Gidrol, X.; Pradet, A. Effects of oxidative stress caused by oxygen and hydrogen peroxide on energy metabolism and senescence in oat leaves. Plant Cell Physiol. 1989, 30, 157–162. [Google Scholar] [CrossRef]
- Casano, L.M.; Gomez, L.D.; Trippi, V.S. Oxygen- and light-induced proteolysis in isolated oat chloroplasts. Plant Cell Physiol. 1990, 31, 377–382. [Google Scholar]
- Casano, L.M.; Martin, M.; Sabater, B. Sensitivity of superoxide dismutase transcript levels and activities to oxidative stress is lower in mature-senescent than in young barley leaves. Plant Physiol. 1994, 106, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-DÖring, P.; Vierling, E.; Scharf, K. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; InTech: Rijeka, Croatia, 2013; pp. 169–205. [Google Scholar]
- Guilioni, L.; Wery, J.; Tardieu, F. Heat stress-induced abortion of buds and flowers in pea: Is sensitivity linked to organ age or to relations between reproductive organs? Ann. Bot-Lond. 1997, 80, 159–168. [Google Scholar] [CrossRef]
- Wollenweber, B.; Porter, J.R.; Schellberg, J. Lack of interaction between extreme high-temperature events at vegetative and reproductive growth stages in wheat. J. Agron. Crop Sci. 2003, 189, 142–150. [Google Scholar] [CrossRef]
- Simões-Araújo, J.L.; Rumjanek, N.G.; Margis-Pinheiro, M. Small heat shock proteins genes are differentially expressed in distinct varieties of common bean. Braz. J. Plant Physiol. 2003, 15, 33–41. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Yoshikawa, T.; Naito, Y. What is oxidative stress? Jpn. Med. Assoc. J. 2002, 124, 1549–1553. [Google Scholar]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Phys. C 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Yan, J.; Tsuichihara, N.; Etoh, T.; Iwai, S. Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant Cell Environ. 2007, 30, 1320–1325. [Google Scholar] [CrossRef]
- Jabs, T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 1999, 57, 231–245. [Google Scholar] [CrossRef]
- Bethke, P.C.; Jones, R.L. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J. 2001, 25, 19–29. [Google Scholar] [CrossRef]
- Joo, J.H.; Bae, Y.S.; Lee, J.S. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001, 126, 1055–1060. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Sun, B.; Jing, Y.; Chen, K.; Song, L.; Chen, F.; Zhang, L. Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). J Plant Physiol. 2007, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Zaefyzadeh, M.; Quliyev, R.A.; Babayeva, S.M.; Abbasov, M.A. The effect of the interaction between genotypes and drought stress on the superoxide dismutase and chlorophyll content in durum wheat landraces. Turk. J. Biol. 2009, 33, 1–7. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).