Evaluation of Compatibility, Growth Characteristics, and Yield of Tomato Grafted on Potato (‘Pomato’)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Characteristics
2.2. Plant Materials
2.3. Production of Potato and Tomato Seedlings
2.4. Grafting of Potato and Tomato Seedlings
2.5. Pomato Plant Transplanting
2.6. Pomato Harvesting
2.7. Data Collection
2.8. Statistical Analysis
3. Results
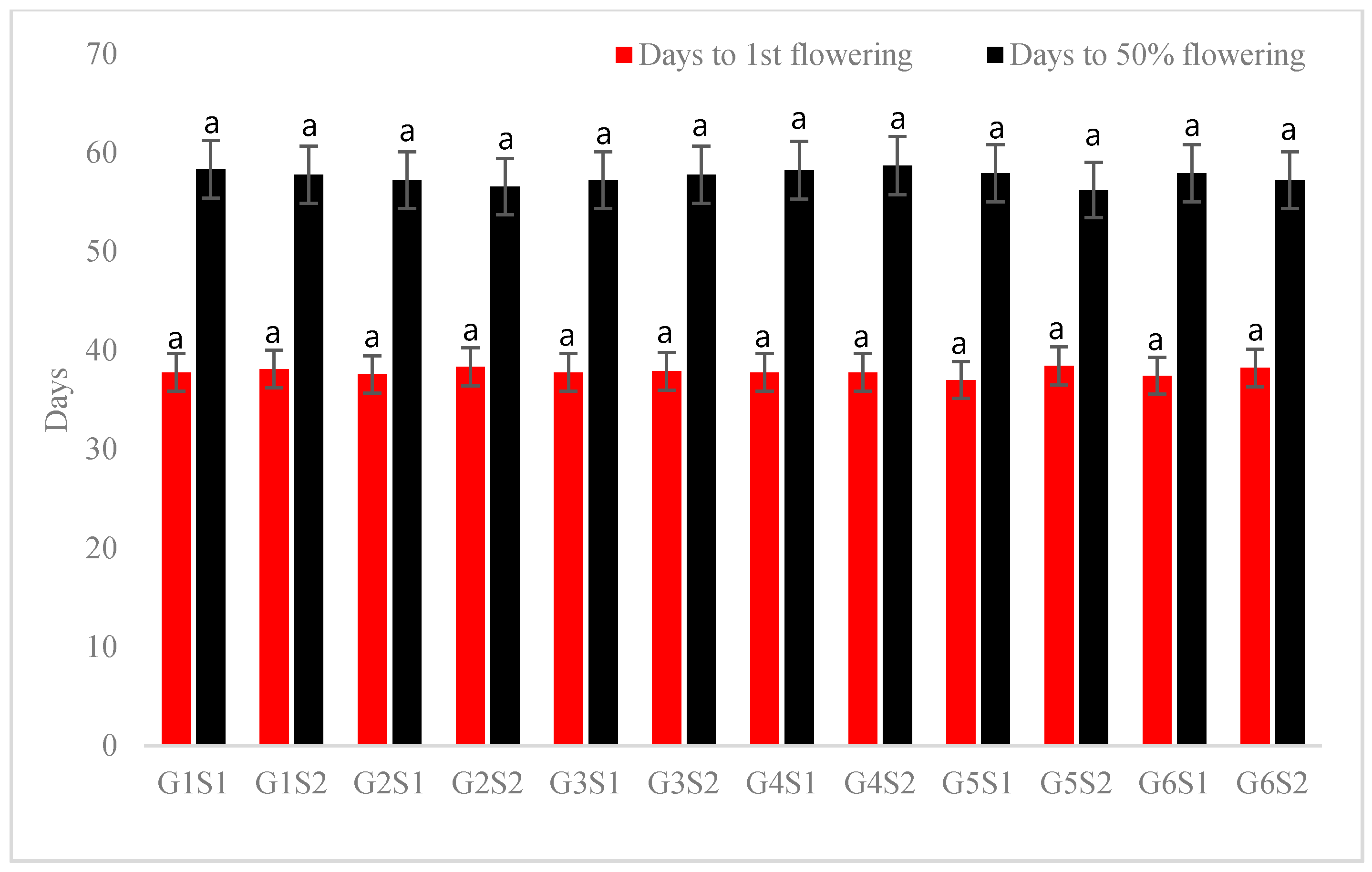
3.1. Growth and Flowering Characteristics
3.2. Tomato Fruit Yield of Pomato Plants
3.3. Potato Tuber Yield of Pomato Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hansord, P. TomTato Solanum lycopersicum, Solanum tuberosum. 2015. Available online: https://www.thompson-morgan.com/p/tomato-orkado-f1-hybrid/gww0050TM (accessed on 15 May 2019).
- Yasinok, A.E.; Sahin, F.I.; Eyidogan, F.; Mustafa Kuru, M.; Haberal, M. Grafting tomato plant on tobacco plant and its effect on tomato plant yield and nicotine content. J. Sci. Food Agric. 2009, 89, 1122–1128. [Google Scholar] [CrossRef]
- Haberal, M.; Korpe, D.A.; Iseri, O.D.; Sahin, F.I. Grafting tomato onto tobacco rootstocks is a practical and feasible application for higher growth and leafing in different tobacco–tomato unions. Biol. Agric. Hortic. 2016, 32, 248–257. [Google Scholar] [CrossRef]
- Haywood, V.; Kragler, F.; Lucas, W.J. Plasmodesmata: Pathways for protein and ribonucleoprotein signaling. Plant Cell 2002, 14, S303–S325. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, J.P. Existence d’un stimulus photope´riodique nonspe ´cifique capable de provoquer la tube´risation chez Helianthus tuberosus. L. Bull. Soc. Bot. Fr. 1965, 112, 333–340. [Google Scholar] [CrossRef]
- Gisbert, C.; Prohens, J.; Raigon, M.D.; Stommel, J.R.; Nuez, F. Eggplant relatives as sources of variation for developing new rootstocks: effects of grafting on eggplant yield and fruit apparent quality and composition. Sci. Hortic. 2011, 128, 14–22. [Google Scholar] [CrossRef]
- Chailakhyan, M.K.; Yanina, L.I.; Devedzhyan, A.G.; Lotova, G.N. Photoperiodism and tuber formation in grafting of tobacco onto potato. Dokl. Akad. Nauk SSSR 1981, 257, 1276–1280. [Google Scholar]
- Kudo, H.; Harada, T. Graft-transmissible RNA from Tomato Rootstock Changes Leaf Morphology of Potato Scion. HortScience 2007, 42, 225–226. [Google Scholar] [CrossRef]
- Reinhard, R. Biotechnology for Beginners; Elsevier: Amsterdam, The Netherlands, 2008; p. 210. [Google Scholar]
- Nusrat, M.F. Cell Compatibility Analysis of Pomato (Solanum tuberosum L. and Solanum lycopersicum L.) Using Local Varieties of Potato. Master’s Thesis, SAU, Dhaka, Bangladesh, 2014. [Google Scholar]
- Jude, G. Potato tom opens fresh doors. 2013. [Google Scholar]
- Jabr, F. The Science of Pomato Plants and Fruit Salad Trees. Scientific American, 2013. Available online: http://blogs.scientificamerican.com/brainwaves/the-science-ofpomato-plants-and-fruit-salad-trees (accessed on 15 May 2019).
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits—A developmental perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Sergeeva, L.I.; Romanov, G.A. Hormonal regulation of tuber formation in potato plants. Russi. J. Plant Physiol. 2012, 59, 451–466. [Google Scholar] [CrossRef]
- Wang, H.; Schauer, N.; Usadal, B.; Frasse, P.; Zouine, M.; Hernould, M.; Latche, A.; Pech, J.C.; Fernie, A.R.; Bouzayen, M. Regulatory features underlying pollination-dependent and independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 2009, 21, 1428–1452. [Google Scholar] [CrossRef] [PubMed]
- Matsu, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Ribak, E.T.; Kaneh, S.A.; Maor, C.H.; Amsellem, Z.; Eshed, Y.; Lifschitz, E. A cytokinin-activating enzyme promotes tuber formation. Curr. Biol. 2013, 23, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Fleishon, S.; Eilon, S.; Naomi, O.; David, W. Negative reciprocal interactions between gibberellin and cytokinin in tomato. New Phytol. 2011, 190, 609–617. [Google Scholar] [CrossRef]
- Smith, H.; Whitelam, G.C. Phytochrome, a family of photoreceptors with multiple physiological roles. Plant Cell Environ. 1990, 13, 695–707. [Google Scholar] [CrossRef]
- Kerckhoffs, L.H.J.; Kelmenson, P.M.; Schreuder, M.E.L.; Kendrick, C.I.; Kendrick, R.E.; Hanhart, C.J.; Koornneef, M.; Pratt, L.H.; Cordonnier- Pratt, M.M. Characterization of the gene encoding the apoprotein of phytochrome B2 in tomato, and identification of molecular lesions in two mutant alleles. Mol. Gen. Genet. 1999, 261, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.E.P.; Carvalho, R.F.; Zsogon, A.; Bermudez, O.D.; Zambrano, B.; Robles, W.G.R.; Tavares, S. Grafting of tomato mutants onto potato rootstocks: An approach to study leaf derived signaling on tuberization. Plant Sci. 2005, 169, 680–688. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Yen, C.R. Rootstock age and grafting season affect graft success and plant growth of papaya (Carica papaya L.) in greenhouse. Chil. J. Agric. Res. 2018, 78, 59–67. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann and Kester’s Plant Propagation: Principles and Practices, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Kilany, O.A.; Abd El-Zaher, M.H.; Hamed, H.H. The relationship between the histological features in the grafting areas and the compatibility degrees of some mango cultivars onto nuclear seedlings. J. Hortic. Sci. Ornament. Plants 2012, 4, 58–65. [Google Scholar]
- Ewing, E.E.; Wareing, P.F. Shoot, stolon, and tuber formation on potato (Solanum tuberosum) cuttings in response to photoperiod. Plant Physiol. 1978, 61, 348–353. [Google Scholar] [CrossRef]
- Van den Berg, J.H.; Simko, I.; Davies, P.J.; Ewing, E.E. Halinska, A. Morphology and (14C) gibberellin A12 aldehyde metabolism in wildtype and dwarf Solanum tuberosum ssp. andigena grown under long and short photoperiods. J. Plant Physiol. 1995, 146, 467–473. [Google Scholar] [CrossRef]
- Palmer, C.E.; Smith, O.E. Cytokinins and tuber initiation in the potato Solanum tuberosum L. Nature 1999, 221, 279–280. [Google Scholar] [CrossRef]
- Galis, I.; Macas, J.; Viasak, J.; Ondrej, M.; Onckelen, H.A.V. The effect of an elevated cytokinin level using the ipt gene and N-6 benzyladenine on a single node and intact potato plant tuberization in vitro. J. Plant Growth Regul. 1995, 14, 143–150. [Google Scholar] [CrossRef]
- Fernie, A.R.; Willmitzer, L. Molecualr and biochemical triggeres of potato tuber development. Plant Physiol. 2001, 127, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.D.; James, P.; Prat, S.; Thomas, B. Phytochrome B affects the levels of a graft-transmissible signal involved in tuberization. Plant Physiol. 1998, 117, 29–32. [Google Scholar] [CrossRef] [PubMed]

| Soil Characters | Value |
|---|---|
| Organic matter | 1.44% |
| Potassium | 0.15 meq/100 g soil |
| Calcium | 3.60 meq/100 g soil |
| Magnesium | 1.00 meq/100 g soil |
| Total nitrogen | 0.072 |
| Phosphorus | 22.08 µg/g soil |
| Sulphur | 25.98 µg/g soil |
| Boron | 0.48 µg/g soil |
| Copper | 3.54 µg/g soil |
| Iron | 262.6 µg/g soil |
| Manganese | 164 µg/g soil |
| Zinc | 3.32 µg/g soil |
| Pomato Combinations | Combination Designation | Scion Age | Final Combinations |
|---|---|---|---|
| T1P1 | G1 | S1/S2 | G1S1/G1S2 |
| T1P2 | G2 | S1/S2 | G2S1/G2S2 |
| T1P3 | G3 | S1/S2 | G3S1/G3S2 |
| T2P1 | G4 | S1/S2 | G4S1/G4S2 |
| T2P2 | G5 | S1/S2 | G5S1/G5S2 |
| T2P3 | G6 | S1/S2 | G6S1/G6S2 |
| Treatment (Grafting Combination) | Plant Height (cm) | Leaf Number/Plant | Branch Number/Plant |
|---|---|---|---|
| G1S1 | 94.53 c | 26.89 b | 4.22 b |
| G1S2 | 93.35 c | 23.89 c | 4.33 b |
| G2S1 | 94.41 c | 22.56 c | 3.44 c |
| G2S2 | 95.67 c | 25.89 c | 4.59 b |
| G3S1 | 93.31 c | 23.35 c | 3.55 c |
| G3S2 | 96.21 c | 26.56 b | 4.36 b |
| G4S1 | 110.3 b | 27.33 b | 4.37 b |
| G4S2 | 112.7 b | 24.47 c | 4.22 b |
| G5S1 | 115.13 a | 33.67 a | 6.66 a |
| G5S2 | 111.23 b | 26.33 b | 5.11 ab |
| G6S1 | 112.3 b | 27.86 b | 5.22 ab |
| G6S2 | 111.3 b | 28.44 b | 4.22 b |
| Graft Combination | Number of Clusters per Plant | Number of Fruit per Cluster | Number of Fruit per Plant | Fruit Length (cm) | Fruit Diameter (cm) | Single Fruit Weight (g) | Tomato Yield per Plant (kg) |
|---|---|---|---|---|---|---|---|
| G1S1 | 15.22 c | 14.00 b | 215.78 c | 3.54 c | 2.52 b | 5.11 c | 1.34 b |
| G1S2 | 14.88 c | 13.00 c | 215.56 c | 3.53 c | 2.62 b | 5.33 c | 1.36 b |
| G2S1 | 15.11 c | 14.33 b | 216.78 c | 3.06 c | 2.92 b | 6.78 b | 1.53 b |
| G2S2 | 15.22 c | 13.66 c | 216.78 c | 2.01 d | 2.07 b | 5.00 c | 1.55 b |
| G3S1 | 14.88 c | 14.66 b | 219.33 c | 2.18 d | 2.00 b | 5.78 c | 1.76 b |
| G3S2 | 15.11 c | 14.33 b | 216.89 c | 2.86 cd | 2.51 b | 5.11 c | 1.53 b |
| G4S1 | 16.56 b | 15.11 b | 249.90 b | 4.35 b | 3.18 b | 5.11 c | 1.27 b |
| G4S2 | 15.89 b | 13.56 c | 215.70 c | 4.31 b | 3.00 b | 5.77 c | 1.24 b |
| G5S1 | 18.00 a | 17.00 a | 272.00 a | 5.18a | 3.94 a | 7.66 a | 2.25 a |
| G5S2 | 15.00 b | 14.44 c | 217.30 c | 4.60 b | 3.14 a | 6.11 b | 1.90 b |
| G6S1 | 15.33 b | 14.67 b | 225.10 c | 3.14 c | 2.99 b | 6.33 b | 1.58 b |
| G6S2 | 16.22 b | 14.22 b | 230.90 c | 4.37 b | 2.12 b | 6.44 b | 1.24 b |
| Graft Combination | Number of Tubers /Plant | Single Tuber Weight (g) | Tuber Yield/Plant (kg) |
|---|---|---|---|
| G1S1 | 3.11 c | 64.00 b | 0.23 c |
| G1S2 | 4.22 ab | 62.67 c | 0.30 b |
| G2S1 | 4.00 ab | 60.89 c | 0.28 b |
| G2S2 | 4.44 b | 60.89 c | 0.31 a |
| G3S1 | 4.11 ab | 64.17 b | 0.27 b |
| G3S2 | 4.11 ab | 63.78 b | 0.26 bc |
| G4S1 | 3.66 c | 72.89 a | 0.27 b |
| G4S2 | 4.44 ab | 72.67 a | 0.32 a |
| G5S1 | 4.22 ab | 68.44 a | 0.28 b |
| G5S2 | 4.87 a | 70.00 a | 0.33 a |
| G6S1 | 5.00 a | 78.50 a | 0.36 a |
| G6S2 | 4.22 ab | 62.83 c | 0.27 bc |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arefin, S.M.A.; Zeba, N.; Solaiman, A.H.; Naznin, M.T.; Azad, M.O.K.; Tabassum, M.; Park, C.H. Evaluation of Compatibility, Growth Characteristics, and Yield of Tomato Grafted on Potato (‘Pomato’). Horticulturae 2019, 5, 37. https://doi.org/10.3390/horticulturae5020037
Arefin SMA, Zeba N, Solaiman AH, Naznin MT, Azad MOK, Tabassum M, Park CH. Evaluation of Compatibility, Growth Characteristics, and Yield of Tomato Grafted on Potato (‘Pomato’). Horticulturae. 2019; 5(2):37. https://doi.org/10.3390/horticulturae5020037
Chicago/Turabian StyleArefin, S. M. Anamul, Naheed Zeba, Abul Hasnat Solaiman, Most Tahera Naznin, Md Obyedul Kalam Azad, Mourita Tabassum, and Cheol Ho Park. 2019. "Evaluation of Compatibility, Growth Characteristics, and Yield of Tomato Grafted on Potato (‘Pomato’)" Horticulturae 5, no. 2: 37. https://doi.org/10.3390/horticulturae5020037
APA StyleArefin, S. M. A., Zeba, N., Solaiman, A. H., Naznin, M. T., Azad, M. O. K., Tabassum, M., & Park, C. H. (2019). Evaluation of Compatibility, Growth Characteristics, and Yield of Tomato Grafted on Potato (‘Pomato’). Horticulturae, 5(2), 37. https://doi.org/10.3390/horticulturae5020037







