Approach to Yield Response of Young Almond Trees to Deficit Irrigation and Biostimulant Applications
Abstract
1. Introduction
2. Materials and Methods
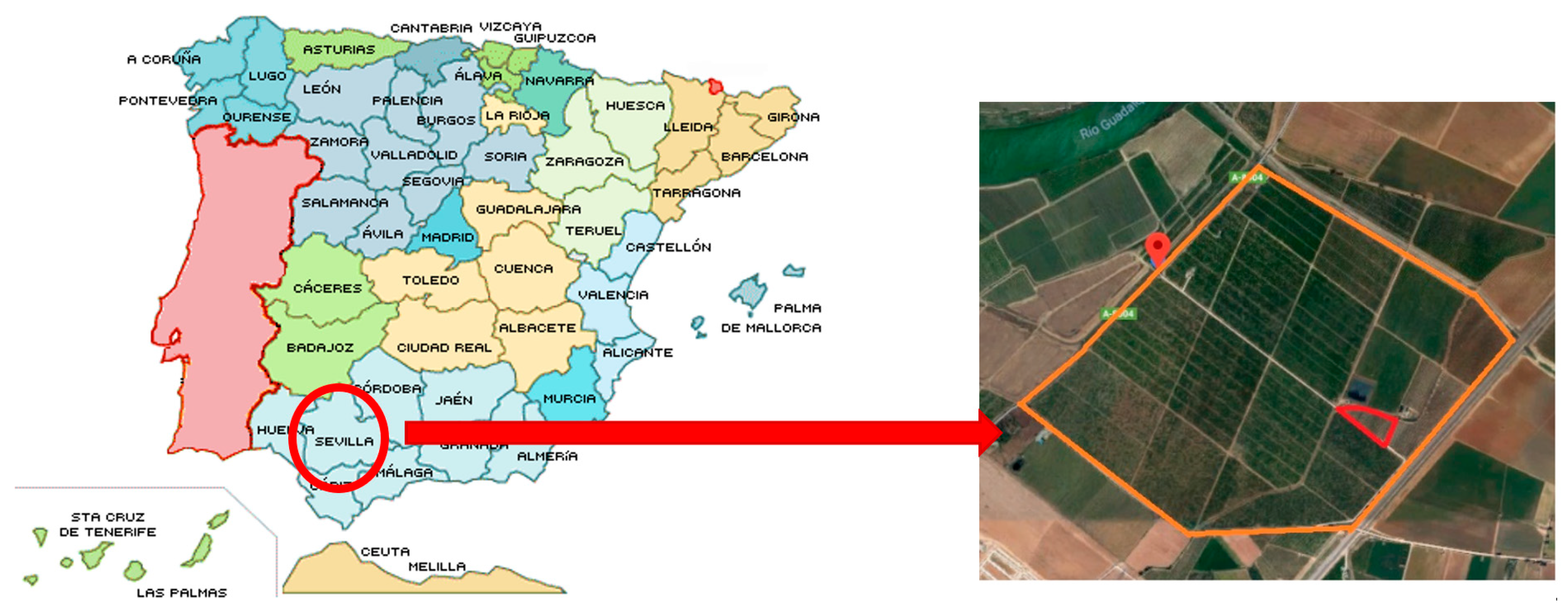
2.1. Experimental Location
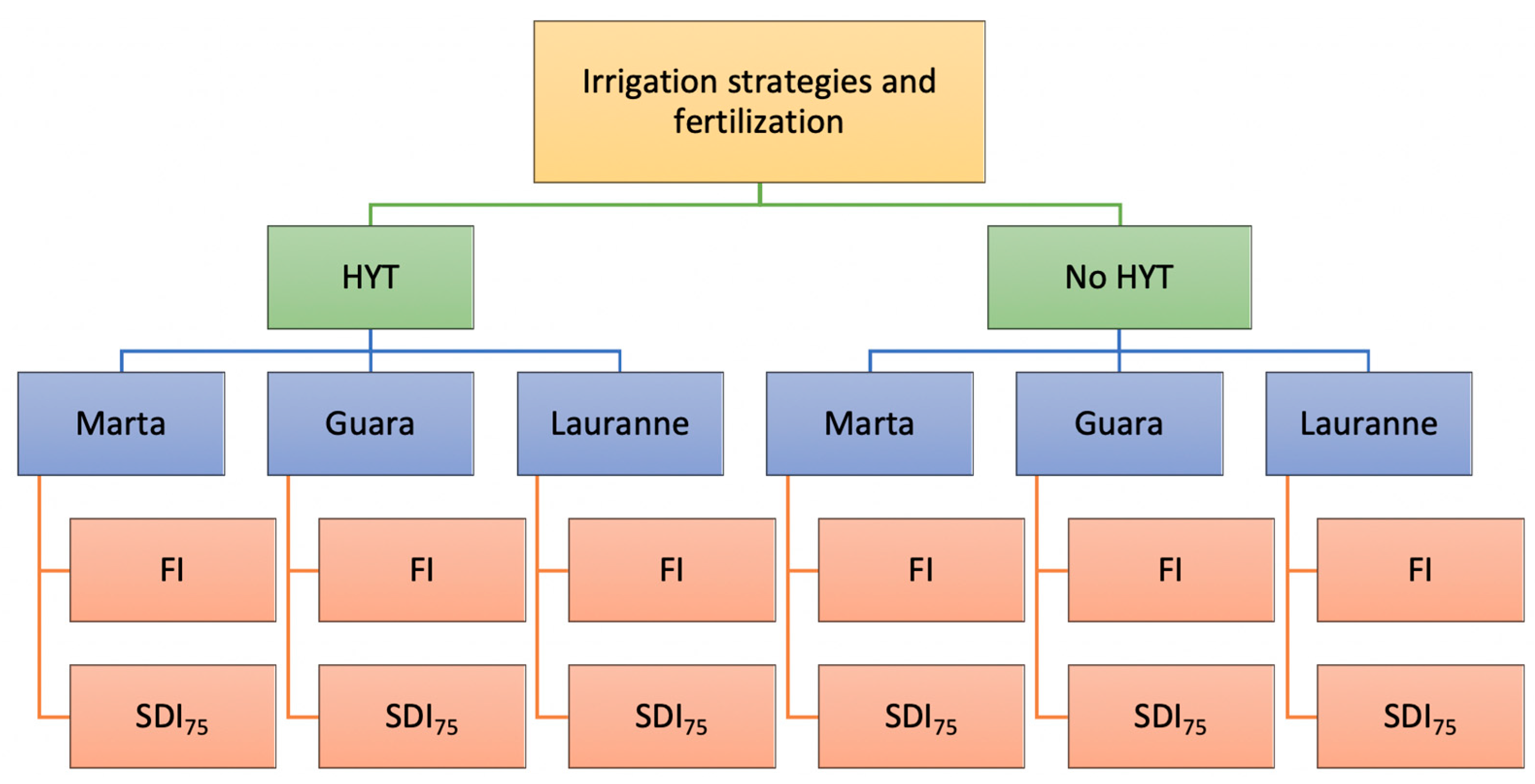
2.2. Irrigation Strategies and Fertilization
2.3. Plant Measurements
2.4. Experimental Details
3. Results and Discussion
3.1. Effects of DI and HYT Application Products in the Crop’s Physiological Status
3.2. Effects of HYT Products on the Nutritional Status of the Crop
3.3. Effects of DI and the Application of HYT Products in the Crop Yield Response
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 25 October 2018).
- Goldhamer, D.A.; Fereres, E. Establishing an almond water production function for California using long-term yield response to variable irrigation. Irrig. Sci. 2017, 35, 169–179. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration—Guidelines for computing crop water requirements—FAO Irrigation and drainage paper 56. Irrig. Drain. 1998, 1–15. [Google Scholar] [CrossRef]
- López-López, M.; Espadador, M.; Testi, L.; Lorite, I.J.; Orgaz, F.; Fereres, E. Water use of irrigated almond trees when subjected to water deficits. Agric. Water Manag. 2018, 195, 84–93. [Google Scholar] [CrossRef]
- Spinelli, G.M.; Snyder, R.L.; Sanden, B.L.; Shackel, K.A. Water stress causes stomatal closure but does not reduce canopy evapotranspiration in almond. Agric. Water Manag. 2016, 168, 11–22. [Google Scholar] [CrossRef]
- Romero, P.; Botia, P.; Garcia, F. Effects of regulated deficit irrigation under subsurface drip irrigation conditions on vegetative development and yield of mature almond trees. Plant Soil 2004, 260, 169–181. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M.; Salinas, M. Regulated deficit irrigation in almonds: Effects of variations in applied water and stress timing on yield and yield components. Irrig. Sci. 2006, 24, 101–114. [Google Scholar] [CrossRef]
- Egea, G.; Nortes, P.A.; González-Real, M.M.; Baille, A.; Domingo, R. Agronomic response and water productivity of almond trees under contrasted deficit irrigation regimes. Agric. Water Manag. 2010, 97, 171–181. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. (Amsterdam) 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R.E. The impact of growth regulators on the a-tocopherol status in water-stressed Poapratensis L. Int. Turfgrass Soc. Res. J. 1997, 8, 1364–1373. [Google Scholar]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Paradiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Spann, T.M.; Little, H.A. Effect of Stimplex® Crop Biostimulant on Drought Tolerance of ‘Hamlin’ Sweet Orange. Proc. Fla. State Hort. Soc. 2010, 123, 100–104. [Google Scholar]
- Zaghloul, A.E.; Moursi, E.A. Effect of Irrigation Scheduling under some Biostimulants Foliar Application for Navel Orange Trees on some Water Relations, Productivity, Fruit Quality and Storability in the North Nile Delta Region. Alexandria Sci. Exch. J. 2017, 38, 671–685. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Hernandez, A.; Rodriguez, V.M.; Ponce, J.R.; Ramos, V.; Muriel, J.L.; Zuazo, D.V.H.D. Estimating Almond Crop Coefficients and Physiological Response to Water Stress in Semiarid Environments (SW Spain). J. Agric. Sci. Technol. 2015, 17, 1255–1266. [Google Scholar]
- Shackel, K. A plant-based approach to deficit irrigation in trees and vines. HortScience 2011, 46, 173–177. [Google Scholar] [CrossRef]
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of plant growth and drought tolerance by native microorganisms (AM Fungi and bacteria) from dry environments: Mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef]
- Creus, C.M.; Sueldo, R.J.; Barassi, C.A. Water relations in Azospirillum-inoculated wheat seedlings under osmotic stress. Can. J. Bot. 1998, 76, 238–244. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Parađiković, N.; Zeljković, S.; Tkalec, M.; Vinković, T.; Maksimović, I.; Haramija, J. Influence of biostimulant application on growth, nutrient status and proline concentration of begonia transplants. Biol. Agric. Hortic. 2017, 33, 89–96. [Google Scholar] [CrossRef]
- Casanovas, E.M.; Barassi, C.A.; Sueldo, R.J. Azospirillum inoculation mitigates water stress effects in maize seedlings. Cereal Res. Commun. 2002, 30, 343–350. [Google Scholar]
- Cohen, A.C.; Bottini, R.; Piccoli, P.N. Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in arabidopsis plants. Plant Growth Regul. 2008, 54, 97–103. [Google Scholar] [CrossRef]
| DOY z | Tair (°C) | RH (%) | Rainfall (mm) | ET0 | KC | ETC | FI (m3 ha−1) | SDI75 (m3 ha−1) |
|---|---|---|---|---|---|---|---|---|
| 115–123 | 24.25 | 59.9 | 20 | 26.57 | 0.6 | 23.91 | 63.52 | 41.13 |
| 124–127 | 21.6 | 54 | 12 | 26.12 | 0.9 | 23.51 | 122.31 | 86.73 |
| 128–134 | 23.9 | 43.63 | 2.3 | 27.6 | 0.9 | 24.84 | 272.58 | 174.98 |
| 135–148 | 24.6 | 39.8 | 5.6 | 23.71 | 0.9 | 21.34 | 330.07 | 240.52 |
| 149–162 | 25.5 | 44.1 | 4 | 27.32 | 1.1 | 33.81 | 702.64 | 468.72 |
| 163–166 | 27.8 | 53.95 | 0 | 38.21 | 1.1 | 47.28 | 824.18 | 566.53 |
| 167–183 | 26.6 | 48.1 | 1 | 32.52 | 1.2 | 43.9 | 1427.18 | 952.71 |
| 184–190 | 33.5 | 34.9 | 0 | 43.79 | 1.2 | 59.12 | 1717.42 | 1184.72 |
| 191–197 | 26.25 | 53.45 | 0 | 35.51 | 1.2 | 42.61 | 2055.53 | 1401.9 |
| 198–206 | 28.45 | 49.5 | 0 | 47.14 | 1.2 | 49.5 | 2472.89 | 1647.32 |
| 207–211 | 26.05 | 52.25 | 0 | 39.61 | 1.1 | 49.02 | 2718.33 | 1858.22 |
| 212–218 | 33.2 | 41.2 | 0 | 55.36 | 1.1 | 68.51 | 3037.43 | 2089.4 |
| 219–225 | 32.5 | 42.6 | 0 | 38.16 | 1.1 | 47.22 | 3277.26 | 2392.38 |
| 226–232 | 32.6 | 36.3 | 0 | 36.65 | 1.1 | 45.35 | 3512.22 | 2687.63 |
| 233–240 | 20.18 | 42.6 | 0 | 45.97 | 0.8 | 36.78 | 3772.32 | 2744.94 |
| 241–283 | 25.16 | 58.1 | 2.3 | 39.37 | 0.8 | 29.52 | 4848.31 | 3626.59 |
| 284–293 | 18.3 | 82.56 | 21.9 | 15.05 | 0.7 | 9.22 | 5073.89 | 3787.92 |
| DOY z | HYT A® (L·ha−1) | HYT B® (L·ha−1) | Phenological Stage |
|---|---|---|---|
| 72 | 4 | 4 | Fruit setting |
| 93 | 2 | 2 | Fruit growth |
| 114 | 2 | 2 | Fruit growth |
| 142 | 1 | 1 | Fruit growth |
| 173 | 1 | 1 | Kernel filling |
| 199 | 1 | 1 | Kernel filling |
| 296 | 1 | 1 | Post-harvest |
| Phenological Stage | FI (HYT) | SDI75 (HYT) | FI | SDI75 |
|---|---|---|---|---|
| ‘Guara’ | ||||
| Vegetative growth | −0.68 ± 0.15 ns z | −0.64 ± 0.18 | −0.72 ± 0.13 | −0.75 ± 0.15 |
| Kernel filling | −0.66 ± 0.10 a | −0.74 ± 0.1 a | −1.01 ± 0.11 b | −0.95 ± 0.16 b |
| Harvest | −1.59 ± 0.11 a | −1.75 ± 0.16 b | −1.76 ± 0.13 b | −2.28 ± 0.21 c |
| ‘Marta’ | ||||
| Vegetative growth | −0.49 ± 0.1 a | −0.6 ± 0.12 b | −0.43 ± 0.11 a | −0.55 ± 0.13 ab |
| Kernel filling | −0.86 ± 0.16 ns | 0.83 ± 0.18 | −0.92 ± 0.16 | −0.94 ± 0.13 |
| Harvest | −1.65 ± 0.15 ns | −1.7 ± 0.14 | −1.74 ± 0.16 | −1.78 ± 0.16 |
| ‘Lauranne’ | ||||
| Vegetative growth | −0.66 ± 0.11 ns | −0.63 ± 0.10 | −0.66 ± 0.14 | −0.76 ± 0.12 |
| Kernel filling | −0.88 ± 0.14 ns | −0.89 ± 0.12 | −0.88 ± 0.12 | −0.92 ± 0.13 |
| Harvest | −1.71 ± 0.14 a | −1.8 ± 0.16 a | −1.88 ± 0.14 a | −2.1 ± 0.13 b |
| Phenological Stage | FI (HYT) | SDI75 (HYT) | FI | SDI75 |
|---|---|---|---|---|
| ‘Guara’ | ||||
| Vegetative growth | 122.8 ± 11.6 ns z | 116.1 ± 14.9 | 106.3 ± 11.7 | 103.5 ± 12.1 |
| Kernel filling | 219.8 ± 13.8 ns | 211.3 ± 13.4 | 214.8 ± 19.1 | 217.7 ± 18.3 |
| Harvest | 198.3 ± 21.5 ns | 188.5 ± 21.1 | 204.5 ± 21.9 | 197.4 ± 16.6 |
| ‘Marta’ | ||||
| Vegetative growth | 159.8 ± 16.3 a | 148.7 ± 14.9 a | 113.1 ± 11.2 b | 112.3 ± 16.8 b |
| Kernel filling | 204.5 ± 17.3 ns | 198.4 ± 20.1 | 204.4 ± 19.6 | 199.3 ± 18.8 |
| Harvest | 215.7 ± 16.3 ns | 213.6 ± 11.7 | 198.5 ± 19.9 | 204.5 ± 14.5 |
| ‘Lauranne’ | ||||
| Vegetative growth | 185.8 ± 16.7 a | 198.1 ± 19.2 a | 137.7 ± 19.3 b | 137.2 ± 14.5 b |
| Kernel filling | 257.9 ± 34.3 a | 263.1 ± 27.9 a | 207.7 ± 21.6 b | 225.5 ± 22.2 b |
| Harvest | 173.6 ± 21.4 ns | 167.4 ± 22.3 | 169.1 ± 37.9 | 173.2 ± 31.2 |
| Nutrients | ‘Guara’ | ‘Marta’ | ‘Lauranne’ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FI z (HYT) | SDI75 (HYT) | FI | SDI75 | FI (HYT) | SDI75 (HYT) | FI | SDI75 | FI (HYT) | SDI75 (HYT) | FI | SDI75 | |
| Ca (%) | 4.19 a y | 3.49 a | 3.03 b | 4.19 a | 4.24 | 4.67 | 3.76 | 3.78 | 4.19 a | 2.81 b | 3.16 b | 4.12 a |
| P (%) | 0.154 | 0.163 | 0.158 | 0.148 | 0.15 | 0.135 | 0.16 | 0.144 | 0.167 a | 0.187 a | 0.181 a | 0.15 b |
| Mg (%) | 0.542 | 0.559 | 0.523 | 0.608 | 0.548 | 0.635 | 0.541 | 0.524 | 0.586 | 0.46 | 0.536 | 0.609 |
| K (%) | 1.94 | 1.79 | 1.9 | 2.04 | 1.93 | 1.84 | 1.97 | 2.01 | 2.0 | 1.88 | 1.93 | 1.88 |
| N-Kjeldhal (%) | 2.66 | 2.55 | 2.67 | 2.61 | 2.57 | 2.4 | 2.64 | 2.49 | 2.72 | 2.77 | 2.8 | 2.79 |
| Cu (mg·kg−1) | 5.66 | 4.88 | 5.21 | 5.73 | 5.75 | 5.16 | 5.73 | 6.03 | 6.66 | 5.79 | 6.1 | 6.34 |
| Fe (mg·kg−1) | 70.3 a | 50.8 b | 46.5 b | 68.9 a | 58.9 b | 76.9 a | 57.1 b | 78.7 a | 90.4 a | 51.1 b | 57.4 b | 76.1 a |
| Mn (mg·kg−1) | 77.6 a | 54.5 b | 42.5 b | 83 a | 68.6 a | 72.1a | 48.2 b | 90 a | 93.1 a | 47.5 b | 54.4 b | 87.2 a |
| Zn (mg·kg−1) | 27.6 | 24.6 | 23.9 | 26.4 | 25.8 | 20.3 | 28.1 | 23.7 | 27.7 | 23.2 | 22.0 | 22.5 |
| Yields Parameters | FI (HYT) z | SDI75 (HYT) | FI | SDI75 |
|---|---|---|---|---|
| ‘Guara’ | ||||
| Kernel yield (kg·ha−1) | 2221 a y | 1980 ab | 1928 b | 1659 c |
| Unit weight (g) | 1.57 a | 1.56 a | 1.40 b | 1.40 b |
| Almonds number per tree | 6790 a | 6092 a | 6610 a | 5688 b |
| ‘Marta’ | ||||
| Kernel yield (kg·ha−1) | 1947 a | 1962 a | 1933 a | 1677 b |
| Unit weight (g) | 1.33 ns | 1.40 | 1.33 | 1.39 |
| Almonds number per tree | 7026 a | 6727 a | 6976 a | 5791 b |
| ‘Lauranne’ | ||||
| Kernel yield (kg·ha−1) | 2486 ns | 2578 | 2349 | 2343 |
| Unit weight (g) | 1.09 b | 1.28 a | 1.12 b | 1.13 b |
| Almonds number per tree | 10,948 a | 9668 b | 10,067 a | 9953 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Gordillo, S.; García-Tejero, I.F.; García-Escalera, A.; Galindo, P.; Arco, M.d.C.; Durán Zuazo, V.H. Approach to Yield Response of Young Almond Trees to Deficit Irrigation and Biostimulant Applications. Horticulturae 2019, 5, 38. https://doi.org/10.3390/horticulturae5020038
Gutiérrez-Gordillo S, García-Tejero IF, García-Escalera A, Galindo P, Arco MdC, Durán Zuazo VH. Approach to Yield Response of Young Almond Trees to Deficit Irrigation and Biostimulant Applications. Horticulturae. 2019; 5(2):38. https://doi.org/10.3390/horticulturae5020038
Chicago/Turabian StyleGutiérrez-Gordillo, Saray, Iván Francisco García-Tejero, Amelia García-Escalera, Pedro Galindo, María del Carmen Arco, and Víctor Hugo Durán Zuazo. 2019. "Approach to Yield Response of Young Almond Trees to Deficit Irrigation and Biostimulant Applications" Horticulturae 5, no. 2: 38. https://doi.org/10.3390/horticulturae5020038
APA StyleGutiérrez-Gordillo, S., García-Tejero, I. F., García-Escalera, A., Galindo, P., Arco, M. d. C., & Durán Zuazo, V. H. (2019). Approach to Yield Response of Young Almond Trees to Deficit Irrigation and Biostimulant Applications. Horticulturae, 5(2), 38. https://doi.org/10.3390/horticulturae5020038






