Relative Salt Tolerance of Four Herbaceous Perennial Ornamentals
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture
2.2. Treatments and Irrigation
2.3. Greenhouse Environmental Conditions
2.4. Visual Quality and Leachate
2.5. Growth Measurements
2.6. Tissue Mineral Analysis
2.7. Experimental Design and Statistical Analysis
3. Results
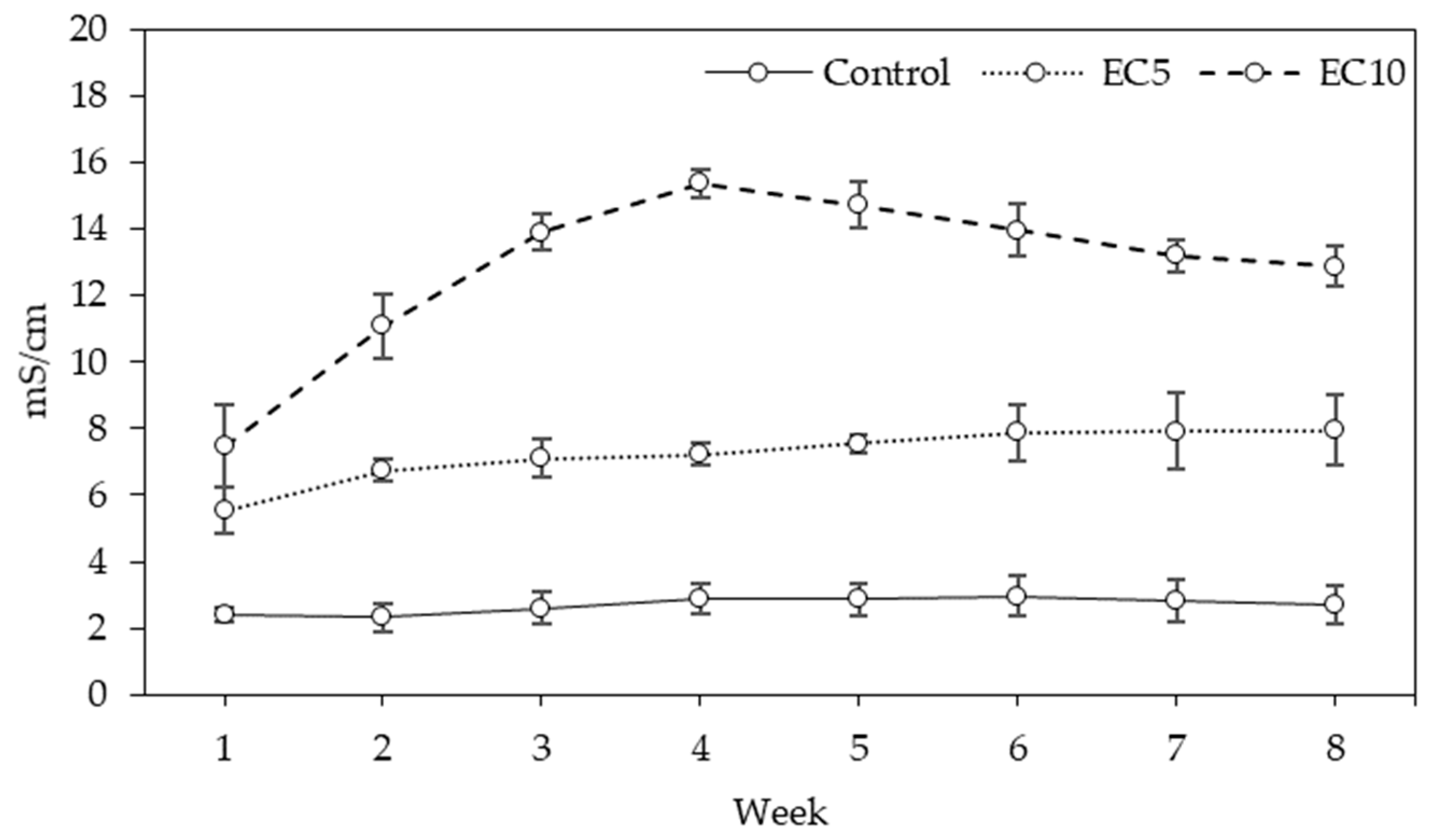
3.1. Leachate
3.2. Relative Tissue DW
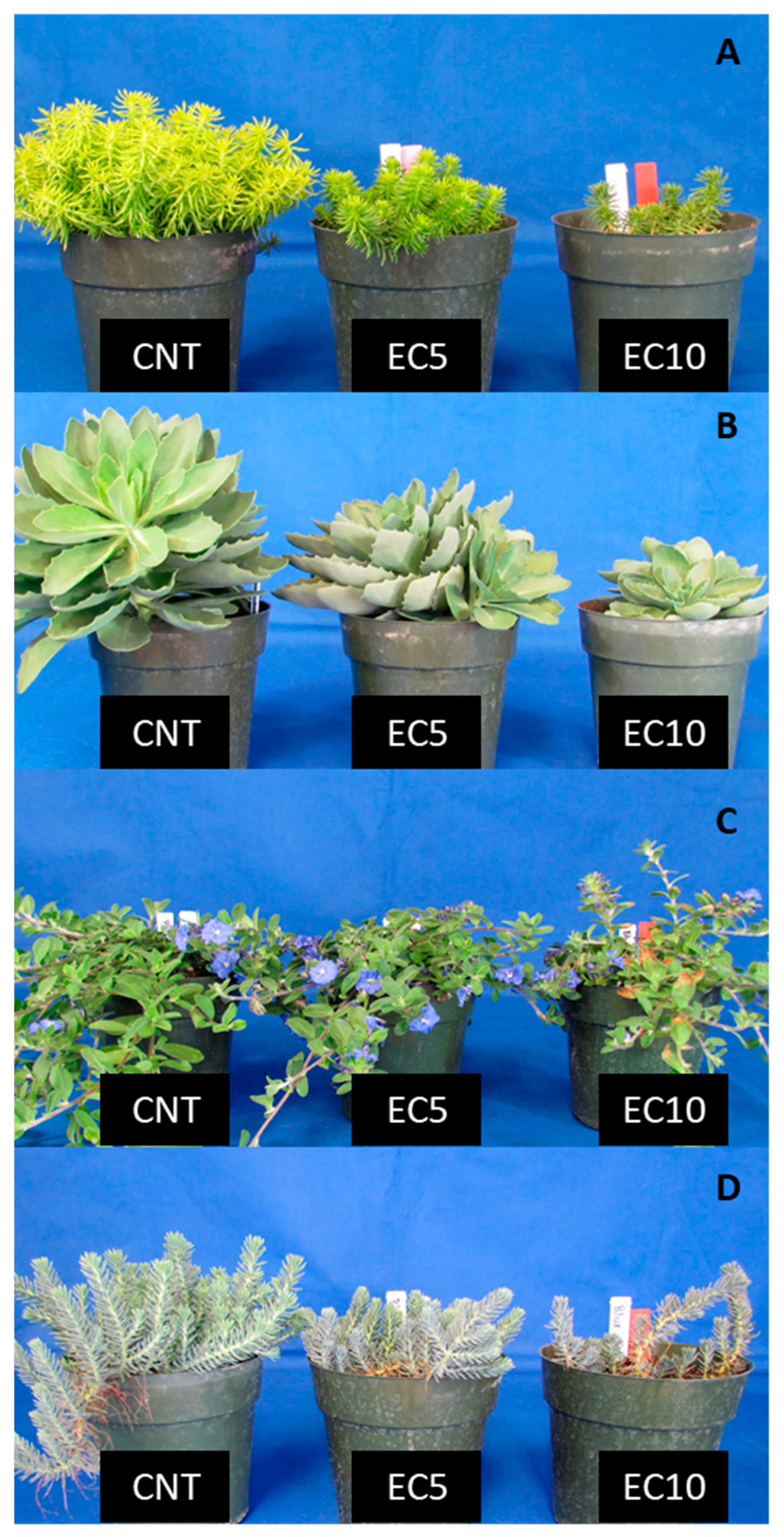
3.3. Visual Score
3.4. Ion Concentrations
4. Discussion
4.1. Leachate
4.2. Plant Growth
4.3. Visual Quality
4.4. Ion Concentrations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cook, C.; Bakker, K. Water Security: Debating an Emerging Paradigm. Glob. Environ. Chang. 2012, 22, 94–102. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Robinson, S.; Toulmin, C.; Muir, J.F.; Pretty, J.; Haddad, L.; Thomas, S.M.; Lawrence, D.; Beddington, J.R.; Crute, I.R. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- EPA. Guidelines for Water Reuse; EPA: Washington, DC, USA, 2012; EPA16251R-921004. [Google Scholar]
- Toze, S. Practicalities of Using Recycled Water. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3. [Google Scholar] [CrossRef]
- Carter, C.T.; Grieve, C.M.; Poss, J.A.; Suarez, D.L. Production and Ion Uptake of Celosia Argentea Irrigated with Saline Wastewaters. Sci. Hortic. 2005, 106, 381–394. [Google Scholar] [CrossRef]
- Cassaniti, C.; Leonardi, C.; Flowers, T.J. The Effects of Sodium Chloride on Ornamental Shrubs. Sci. Hortic. 2009, 122, 586–593. [Google Scholar] [CrossRef]
- Niu, G.; Cabrera, R.I. Growth and Physiological Responses of Landscape Plants to Saline Water Irrigation: A Review. HortScience 2010, 45, 1605–1609. [Google Scholar] [CrossRef]
- Wu, S.; Sun, Y.; Niu, G.; Lizette, G.; Pantoja, G.; Rocha, A.C. Responses of Six Lamiaceae Landscape Species to Saline Water Irrigation. J. Environ. Hortic. 2016, 34, 30–35. [Google Scholar]
- Zollinger, N.; Koenig, R.; Cerny-Koenig, T.; Kjelgren, R. Relative Salinity Tolerance of Intermountain Western United States Native Herbaceous Perennials. HortScience 2007, 42, 529–534. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving Crop Salt Tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.; Carrión, C.; García-Agustín, P.; Noguera, V.; Fornes, F.; Belda, R.M. Pre-Conditioning Ornamental Plants to Drought by Means of Saline Water Irrigation as Related to Salinity Tolerance. Sci. Hortic. 2007, 113, 52–59. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium Chloride Toxicity and the Cellular Basis of Salt Tolerance in Halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.; Grattan, S.R. How Salinity Damages Citrus: Osmotic Effects and Specific Ion Toxicities. Horttechnology 2005, 15, 95–99. [Google Scholar] [CrossRef]
- Bernstein, L.; Francois, L.E.; Clark, R.A. Salt Tolerance of Ornamental Shrubs and Ground Covers. Am. Soc. Hort. Sci. J. 1972, 97, 550–556. [Google Scholar]
- Moran, R. Crassulaceae Stonecrop Family. J. Arizona-Nevada Acad. Sci. 1993, 27, 190–194. [Google Scholar]
- Monterusso, M.A.; Bradley Rowe, D.; Rugh, C.L. Establishment and Persistence of Sedum Spp. and Native Taxa for Green Roof Applications. HortScience 2005, 40, 391–396. [Google Scholar]
- Gravatt, D.A.; Martin, C.E.; Url, S. Comparative Ecophysiology of Five Species of Sedum (Crassulaceae) under Well-Watered and Drought-Stressed Conditions. Oecologia 1992, 92, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Ketjarun, K.; Staples, G.W.; Swangpol, S.C.; Traiperm, P. Micro-Morphological Study of Evolvulus Spp. (Convolvulaceae): The Old World Medicinal Plants. Bot. Stud. 2016, 57. [Google Scholar] [CrossRef] [PubMed]
- Kanechi, M.; Fujiwara, S.; Shintani, N.; Suzuki, T.; Uno, Y. Performance of Herbaceous Evolvulus Pilosus on Urban Green Roof in Relation to Substrate and Irrigation. Urban For. Urban Green. 2014, 13, 184–191. [Google Scholar] [CrossRef]
- Tanji, K.K.; Kielen, N.C. Agricultural Drainage Water Management in Arid and Semi-Arid Areas; FAO Irrigation and Drainage Paper 61; FAO: Rome, Italy, 2002. [Google Scholar]
- Torres, A.P.; Mickelbart, M.V.; Lopez, R.G. Leachate Volume Effects on PH and Electrical Conductivity Measurements in Containers Obtained Using the Pour-through Method. Horttechnology 2010, 20, 608–611. [Google Scholar] [CrossRef]
- Havlin, J.L.; Soltanpour, P.N. A Nitric Acid Plant Tissue Digest Method for Use with Inductively Coupled Plasma Spectrometry. Commun. Soil Sci. Plant Anal. 1980, 11, 969–980. [Google Scholar] [CrossRef]
- Isaac, R.A.; Johnson, W.C. Collaborative Study of Wet and Dry Ashing Techniques for the Elemental Analysis of Plant Tissue by Atomic Absorption Spectrophotometry. J. Assoc. Off. Anal. Chem. 1975, 58, 436–440. [Google Scholar]
- Gavlak, R.G.; Horneck, D.A.; Miller, R. Plant, Soil, and Water Reference Methods for the Western Region; Western Rural Development Center: Logan, UT, USA, 1994. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; 29 Rev. 1; FAO: Rome, Italy, 1985; ISBN 92-5-102263-1. [Google Scholar]
- Tester, M.; Davenport, R. Na+ Tolerance and Na+ Transport in Higher Plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Zhang, J.L.; Flowers, T.J.; Wang, S.M. Mechanisms of Sodium Uptake by Roots of Higher Plants. Plant Soil 2010, 326, 45–60. [Google Scholar] [CrossRef]
- White, P.; Broadley, M.R. Chloride in Soils and Its Uptake and Movement within the Plant: A Review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef]
- Essah, P.; Davenport, R.; Tester, M. Sodium Influx and Accumulation in Arabidopsis. Plant Physiol. 2003, 133, 307–318. [Google Scholar] [CrossRef]

| Source | Relative Shoot DW | Relative Root DW | Relative Total DW | Visual Score | Na+ | Cl− | Ca2+ | K+ | K+/Na+ Ratio |
|---|---|---|---|---|---|---|---|---|---|
| Model | <0.0001 * | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Variety | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment x Variety | 0.2455 | 0.8780 | 0.2191 | <0.0001 | <0.0001 | <0.0001 | 00.0057 | <0.0001 | <0.0001 |
| Varieties | EC5 | EC10 | ||
|---|---|---|---|---|
| Relative shoot DW (%) | ||||
| Angelina | 37.29 | B a z | 19.85 | B b |
| Autumn Joy | 53.28 | AB a | 15.70 | B b |
| Blue Daze | 72.47 | A a | 42.42 | A b |
| Blue Spruce | 43.93 | B a | 20.30 | B b |
| Relative root DW (%) | ||||
| Angelina | 57.94 | B a | 19.19 | B b |
| Autumn Joy | 53.78 | B a | 14.69 | B b |
| Blue Daze | 91.46 | A a | 57.93 | A b |
| Blue Spruce | 47.89 | B a | 14.06 | B b |
| Relative total DW (%) | ||||
| Angelina | 38.44 | B a | 19.81 | B b |
| Autumn Joy | 53.42 | AB a | 15.40 | B b |
| Blue Daze | 74.92 | A a | 44.42 | A b |
| Blue Spruce | 44.12 | B a | 19.88 | B b |
| Varieties | Control | EC5 | EC10 | |||
|---|---|---|---|---|---|---|
| Na+ (mg/g) | ||||||
| Angelina | 0.19 | B c * | 11.25 | AB b | 35.78 | A a |
| Autumn Joy | 0.19 | B c | 7.96 | C b | 16.35 | C a |
| Blue Daze | 0.46 | A c | 13.53 | A b | 26.38 | B a |
| Blue Spruce | 0.16 | B c | 9.23 | BC b | 32.15 | AB a |
| Cl− (mg/g) | ||||||
| Angelina | 2.70 | A c | 34.49 | A b | 56.57 | A a |
| Autumn Joy | 1.56 | B c | 24.60 | B b | 46.49 | A a |
| Blue Daze | 0.86 | C c | 10.58 | C b | 26.85 | B a |
| Blue Spruce | 2.71 | A c | 24.79 | B b | 52.01 | A a |
| Ca2+ (mg/g) | ||||||
| Angelina | 49.90 | A a | 41.29 | A b | 42.91 | A b |
| Autumn Joy | 45.94 | A a | 43.87 | A ab | 38.05 | B b |
| Blue Daze | 14.01 | C a | 10.78 | C b | 8.82 | D b |
| Blue Spruce | 33.29 | B a | 32.18 | B a | 32.19 | C a |
| K+ (mg/g) | ||||||
| Angelina | 46.39 | B a | 41.34 | B b | 35.49 | B c |
| Autumn Joy | 56.65 | A a | 53.38 | A ab | 48.26 | A b |
| Blue Daze | 33.20 | D a | 19.07 | C b | 13.53 | C c |
| Blue Spruce | 38.97 | C a | 36.79 | B a | 32.34 | B b |
| K+/Na+ ratio | ||||||
| Angelina | 279.23 | A a | 3.73 | B b | 1.02 | B b |
| Autumn Joy | 304.20 | A a | 6.75 | A b | 2.99 | A b |
| Blue Daze | 73.91 | B a | 1.42 | C b | 0.52 | C b |
| Blue Spruce | 262.03 | A a | 4.19 | B b | 1.01 | B b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooks, T.; Niu, G. Relative Salt Tolerance of Four Herbaceous Perennial Ornamentals. Horticulturae 2019, 5, 36. https://doi.org/10.3390/horticulturae5020036
Hooks T, Niu G. Relative Salt Tolerance of Four Herbaceous Perennial Ornamentals. Horticulturae. 2019; 5(2):36. https://doi.org/10.3390/horticulturae5020036
Chicago/Turabian StyleHooks, Triston, and Genhua Niu. 2019. "Relative Salt Tolerance of Four Herbaceous Perennial Ornamentals" Horticulturae 5, no. 2: 36. https://doi.org/10.3390/horticulturae5020036
APA StyleHooks, T., & Niu, G. (2019). Relative Salt Tolerance of Four Herbaceous Perennial Ornamentals. Horticulturae, 5(2), 36. https://doi.org/10.3390/horticulturae5020036






